Abstract
The serotonin transporter length polymorphism (5-HTTLPR) short allele (5-HTTLPR-s) has been associated with differential susceptibility for anxiety and depression in multiple psychiatric disorders. 5-HTTLPR-s modifies the serotonergic systems that support emotion and behavioral regulation by reducing gene expression, which slows the reuptake of serotonin, and is associated with distinct morphological and functional effects. Serotonergic systems are also shown to be dysfunctional in behavioral variant frontotemporal dementia (bvFTD), a disease characterized by marked socioemotional dysfunction. However, studies of 5-HTTLPR-s effects in bvFTD have been inconsistent. Our objective was to investigate the patterns of gray matter volume by 5-HTTLPR-s genotype in both healthy older controls and bvFTD patients. We performed voxel-based morphometry of 179 cognitively normal older adults and 24 bvFTD cases to determine brain changes associated with dose (0/1/2) of 5-HTTLPR-s allele. 5-HTTLPR-s frequency did not differ between controls and bvFTD. We found a significant interaction effect whereby carrying more 5-HTTLPR-s alleles in bvFTD was associated with smaller volume in left inferior frontal gyrus (T = 4.86, PFWE = 0.03) and larger volume in right temporal lobe (T = 5.01, PFWE = 0.01). These results suggest that the 5-HTTLPR-s allele differentially influences brain morphology in bvFTD. We propose that patients with bvFTD and 5-HTTLPR-s have altered volumes in regions that support socioemotional behavior, which may be a developmental or disease-related compensation for altered serotonergic activity.
Keywords: Serotonin transporter gene, Frontotemporal dementia, Structural MRI, Emotion, Amygdala, Neurodegeneration
Highlights
-
•
5-HTTLPR-s correlates with greater right medial temporal lobe (R MTL) volume in FTD.
-
•
5-HTTLPR-s correlates with lower left inferior frontal gyrus (L IFG) volume in FTD.
-
•
R MTL and L IFG volumes are associated with neuropsychiatric symptom severity.
-
•
5-HTTLPR-s effects on R MTL and L IFG volumes occur independently of disease severity.
1. Introduction
Behavioral variant frontotemporal dementia (bvFTD) is a neurodegenerative disease that affects the frontal and temporal lobes of the brain. bvFTD is characterized by marked socioemotional dysfunction and progressive changes in behavior and personality, including disinhibition, social inappropriateness, empathy deficits, and reckless or impulsive actions (Rascovsky et al., 2007). There are both genetic and sporadic forms of bvFTD, and up to 40% of individuals have a family history of dementia or other related neurodegenerative or psychiatric disorder (Rohrer et al., 2009; Woolley et al., 2011). Patients with bvFTD often receive early misdiagnoses of psychiatric disorders such as depression or bipolar given behavioral symptoms such as apathy, irritability, compulsions, euphoria and dietary changes (Woolley et al., 2011). Since a clinical hallmark of bvFTD is change in emotion, it is possible that genetic variation that modifies the development or function of the neuroanatomical substrates of emotion could also impact disease processes. The effect of neuromodulatory genetic variation on neurodegenerative processes can inform our understanding of biological mechanisms of disease and highlight potential therapeutic targets.
Serotonin (5-hydroxytryptamine, 5-HT) was originally identified in 1948 as the brain substance that caused peripheral vasoconstriction (Rapport et al., 1948) and has since been described for its diverse roles as a neurotransmitter, affecting a broad range of behaviors including food intake, sensory processing, motor activity, cognition and emotion (reviewed in Canli and Lesch, 2007). Serotonin has been studied widely as a modulator of emotional reactivity, particularly in the context of anxiety, aggression and impulse control where regulating behavior by tuning brain reactivity to negative stimuli is critical for maintaining a balance between adaptive and maladaptive reactions. It is mainly synthesized in the dorsal raphe nucleus of the midbrain. Serotonergic neurons project throughout cortical and subcortical regions, and prominent terminals include the hypothalamus, prefrontal cortex, hippocampus, amygdala, and striatum (Lucki, 1998).
The serotonin transporter (5-HTT, SERT, SLC6A4) is responsible for recycling serotonin from the synaptic cleft to the presynaptic neuron, terminating its effects and enabling its reuse (Murphy et al., 2004). A length polymorphism in its gene promoter (5-HTTLPR) results in differing levels of transporter expression. The ‘long’ allele is more common, and the ‘short’ allele (5-HTTLPR-s) is associated with reduced gene and protein expression. This results in less efficient transporter function: serotonin remains in the synapse longer and is recycled more slowly, resulting in a net reduction in circulating serotonin (Heils et al., 1996). It has been proposed that this net reduction in circulating serotonin has important downstream effects in the circuitry between the ventromedial prefrontal cortex (vmPFC), amygdala, and dorsal raphe (Jasinska et al., 2012).
The 5-HTTLPR-s allele is widely studied and has been associated with risk for multiple psychiatric disorders such as anxiety and depression (Caspi et al., 2010; Gallinat et al., 2008; Homberg and Lesch, 2011), an effect that may be stronger in the context of a negative life event (Caspi et al., 2003). It has been suggested that 5-HTTLPR-s may exert its risk effects by impacting brain morphology (Gallinat et al., 2008; Jedema et al., 2010), and that heightened neural activity in individuals with lower circulating serotonin levels may both increase emotional reactivity (Dannlowski et al., 2010; Homberg and Lesch, 2011) and improve cognition (Homberg and Lesch, 2011). In addition to associations with increased negative emotion (Gyurak et al., 2013), 5-HTTLPR-s has also been associated with increased positive emotion (Gyurak et al., 2013; Haase et al., 2015). These findings are consistent with the idea that this functional polymorphism results in overall increased levels of reactivity regardless of emotional valence. In the healthy brain, 5-HTTLPR-s has been associated with reduced volume in the middle frontal gyrus, gyrus rectus, anterior cingulate cortex and amygdala in healthy subjects (Gallinat et al., 2008). bvFTD is associated with degeneration of these and other paralimbic structures, as well as serotonergic neuron loss. However, studies of 5-HTTLPR-s association with FTD risk have been inconsistent (Albani et al., 2008; Borroni et al., 2010; Lorenzi et al., 2010).
Given that it reduces circulating serotonin levels and is associated with volume loss in frontal and limbic brain regions, 5-HTTLPR-s could be a risk factor for bvFTD, at least in the sense that it might exacerbate symptoms or progression. Conversely, since 5-HTTLPR-s is associated with heightened emotional reactivity, it may bolster the otherwise blunted emotional responsiveness in patients with bvFTD. In this study we sought to characterize the effects of 5-HTTLPR-s on neurodegeneration in bvFTD by addressing two questions: 1) Is 5-HTTLPR-s associated with similar or different patterns of gray matter volume in healthy older controls and patients with bvFTD? 2) Do these genotype-associated brain regions relate to neuropsychiatric symptomatology?
2. Materials and methods
2.1. Study participants
Two hundred three participants (179 healthy controls and 24 individuals with bvFTD) participated in the present study. Individuals were broken into two groups. The first group was a cohort of healthy, cognitively normal older adults between ages 49 and 87 (n = 70 males, n = 109 females) recruited from the San Francisco community for studies of healthy aging at the Memory and Aging Center (MAC) of the University of California, San Francisco (UCSF). The second group was a cohort of patients diagnosed with bvFTD between ages 29 and 83 (n = 16 males, n = 8 females). All adults from both cohorts were Caucasian (self-described). All participants and surrogates provided written informed consent, and the University of California, San Francisco institutional review board, approved all aspects of the study.
2.2. Clinical assessment
All participants underwent a multi-step screening process. Study participants were evaluated during an in-person visit to the UCSF MAC. Individuals underwent a neurological examination, cognitive assessment, and medical history. Each individual had a study partner who was interviewed to evaluate his or her functional abilities. A multidisciplinary team composed of a neurologist, neuropsychologist, and nurse then reviewed all potential participants. All participants were clinically diagnosed as normal controls or as having bvFTD. All control individuals had a Mini-Mental State Examination (Folstein et al., 1975) score ≥25, normal neurological and neuropsychological examinations, no psychiatric diagnosis, and a consensus diagnosis of healthy control. Patients with bvFTD were diagnosed using consensus criteria (Neary et al., 2005).
2.3. Genotyping
Genotyping was conducted according to standard protocols. HTTLPR genotypes were determined according to the procedures outlined in Assal et al. (2004) with slight modifications (Gyurak et al., 2013). In short, DNA was extracted from de-identified peripheral blood samples and stored in a −70 °C freezer. A PCR product was amplified with primers (5′-GGCGTTGCCGCTCTGAATGC-3′) and (5′-GAGGGACTGAGCTGGACAACCA-3′) flanking the region containing the variation of interest. The PCR conditions consisted of a 2-min denaturation step at 94 °C, 35 cycles of 30-s denaturation at 95 °C, 30-s annealing at 60 °C, and 30-s extension at 72 °C, and a final 7-min extension step at 72 °C. Individuals with 16 repeats are referred to as carrying the long (L) allele and individuals with 14 repeats carry the short (S) allele.
2.4. Image acquisition
Participants underwent structural T1-weighted MR imaging on a 3 T (n = 145 controls, n = 5 bvFTD) scanner at the Neuroscience Imaging Center at UCSF and a 1.5 T (n = 29 controls, n = 17 bvFTD) or 4 T (n = 5 controls, n = 2 bvFTD) scanner at the San Francisco Veterans Affairs Medical Center with acquisition protocols very similar to the ADNI standards, as described in more detail elsewhere (Sturm et al., 2013). MRI scans from all healthy controls were acquired within 1 year of the clinical visit and neuropsychological evaluation. MRI scans from all individuals diagnosed with bvFTD were acquired within 6 months of the clinical visit and neuropsychological evaluation.
2.5. Image preprocessing
Structural T1-weighted MR images were visually inspected for movement artifacts and were bias corrected; and segmented into gray matter (GM), white matter, and cerebrospinal fluid using Statistical Parametric Mapping (SPM) 8 default preprocessing parameters, with the exception of light clean-up of isolated voxels. The Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) tool box (Ashburner, 2007) was used to warp each participant's image to a custom healthy-aging template to optimize intersubject registration. The template was composed of 144 healthy aging controls and derived using DARTEL. The cohort's DARTEL-processed GM images were smoothed with an 8 mm full-width at half-maximum kernel prior to analyses.
2.6. Neuroimaging analyses
We conducted whole-brain voxel-based morphometry (VBM) analyses to assess the correlation of 5-HTTLPR-s dosage on GM volumes in healthy controls and patients with bvFTD. All VBM analyses were performed using the VLSM2.55 toolbox (Bates et al., 2003). A permutation based analysis (1000 permutations) was used to establish a study-specific error distribution and determine a one-tailed T threshold for multiple comparisons at PFWE < 0.05 (Hayasaka and Nichols, 2004). Resampling the data in a permutation analysis enabled us to compare our results to a null distribution derived from the original data set, thus providing an accurate representation of type 1 error at P < 0.05 across the entire brain (Kimberg et al., 2007).
The main analysis of interest examined the correlation/anticorrelation interaction effects of bvFTD × dose (0/1/2) of the 5-HTTLPR-s allele on GM and included the following covariates: age, sex, years of education, scan type, GM volume (to account for individual variability in disease severity), and total intracranial volume (TIV; to account for individual variability in head size). In addition to this main analysis, VBM was also performed to assess the main effect of 5-HTTLPR-s dose in healthy controls and to identify the morphological differences between healthy controls and bvFTD patients (both including the same covariates as above). Results were reported when at a cluster size greater than 0.5 cm3 and an uncorrected P < 0.001.
2.7. Neuropsychiatric assessment
The Neuropsychiatric Inventory Questionnaire (NPI) (Cummings et al., 1994) or NPI-Q (Kaufer et al., 2000) was administered within 180 days of image acquisition by informant interview and was available for analysis in a subset of individuals (165 controls and 15–20 patients with bvFTD). The presence and measurement of neuropsychiatric syndromes measured by the NPI is well established in Alzheimer's disease and FTD (Aalten et al., 2007; Banks and Weintraub, 2008; Bozeat et al., 2000; Chow and Binns, 2009). When absent, NPI-Q scores were generated from available NPI data in order to maximize the number of individuals with analyzable information. Frequency (presence or absence in the month prior to administration) × Severity [ranging from 1 to 3] NPI-Q subscale scores were averaged to represent a mean score for each of four syndromes: hyperactivity (average from: agitation, euphoria, disinhibition, irritability, and aberrant motor behavior), psychosis (average from: delusions, hallucinations, and night-time behavior disturbances), affective (average from: depression and anxiety), and apathy (average from: apathy and appetite/eating abnormalities) based on previous work using NPI data (Borroni et al., 2006; Hollingworth et al., 2006). NPI-Q data has been shown to be highly correlated with NPI data (Kaufer et al., 2000). To validate our modified syndrome scoring method, we also assessed syndrome scores based on full NPI data from the subset of individuals for which these data were available (165 controls and 7 patients with bvFTD).
2.8. Statistical analysis
Linear regression models were used to examine the relationship between HTTLPR genotype with GM volume and neuropsychiatric symptoms in bvFTD. As in the VBM analysis, HTTLPR genotype was scored as dose of short allele (0/1/2). Participant-specific volumes adjusted by the covariates included for each VBM analysis were created as best linear unbiased estimates. These volumes were used in secondary analyses to further examine the relationship of volume and neuropsychiatric syndromes with HTTLPR genotype in both bvFTD and healthy controls. Multiple testing corrections were implemented using the Benjamini & Yekutieli method (Benjamini and Yekutieli, 2005). All regression analyses and statistical tests, including interactions, were conducted using Stata10.1/MP (Stata Corp. LP, College Station, TX). Linear regression lines as well as slope and intercept comparisons were calculated in Prism 6 via two-tailed F-statistics (GraphPad Software, Inc., La Jolla, CA).
3. Results
3.1. Participant characteristics
A cohort of 203 adults (86 males and 117 females) were analyzed, including 24 individuals with a clinical diagnosis of bvFTD and 179 individuals clinically screened as cognitively healthy controls. Detailed cohort characteristics, by dose of 5-HTTLPR-s risk allele, are provided in Table 1. On average, the entire cohort had 17.2 years of education (range 11–21 years). The control cohort's ages ranged from 49 to 87 years (mean 68.5 years). The patient cohort ranged from 29 to 83 years of age (mean 60.6 years). There were two individuals, aged 29 and 34, who were included in the bvFTD cohort. Both were pathologically diagnosed with frontotemporal lobar degeneration with FUS pathology and were negative for FUS mutations. The next youngest individual after these two was 47 years old. In order to test whether the inclusion of these two younger individuals influenced our main and/or secondary findings, all analyses were performed with the two youngest individuals removed. We found that our findings were consistent irrespective of the inclusion or exclusion of these two individuals and therefore chose to report findings from the larger cohort in order to maximize the patient sample size.
Table 1.
Descriptive cohort information by allele count.
| Dose of short allele |
P-val | |||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| Age (mean ± SE) | 67.5 ± 1.0 | 66.9 ± 0.9 | 69.2 ± 1.3 | 0.37 |
| Edu (mean ± SE) | 17.4 ± 0.3 | 17.2 ± 0.2 | 17.2 ± 0.3 | 0.86 |
| Sex (M/F) | 23/36 | 51/52 | 12/29 | 0.07 |
| Scan type (1.5 T/3 T/4 T) | 11/47/1 | 19/81/3 | 16/22/3 | 0.02 |
Demographic summary of cohort by dose of the 5-HTTLPR-s allele. SE — standard error, M — male, F — female, and T — Tesla.
There were no significant differences in age, education, or sex between 5-HTTLPR-s dosage groups, though scanner type did differ across genotypes (P = 0.02; Table 1). In the bvFTD group, 27% of individuals had no copies of the short variant, 36% of individuals had one copy of the short variant, and 36% of individuals had two copies of the short variant (Table 2). In the control group, 30% of individuals had no copies of the short variant, 52% of individuals had one copy of the short variant, and 18% of individuals had two copies of the short variant. There were no significant differences between diagnostic groups when compared by genotype (L/L, S/L, and S/S) or by allele count (L or S). When broken down by diagnosis and sex within each genotype, there was a significant difference in genotype distribution between male and female controls (P = 0.04). Further information on the distribution of genotypes by diagnosis and sex is provided in Inline Supplementary Table S1.
Table 2.
5-HTTLPR-s allele count by diagnostic group.
| Genotype count | Control |
bvFTD |
P-val | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| L/L | 53 | 29.6% | 6 | 25.0% | 0.23 |
| S/L | 93 | 52.0% | 10 | 41.7% | |
| S/S | 33 | 18.4% | 8 | 33.3% | |
| Allele count | N | % | N | % | P-val |
| L | 199 | 55.6% | 22 | 45.8% | 0.20 |
| S | 159 | 44.4% | 26 | 54.2% | |
Demographic summary of cases and controls by genotype and by allele count. bvFTD — behavioral variant frontotemporal dementia.
Inline Supplementary Table S1.
Table S1.
Genotypes and allele counts by diagnosis and gender. Summary of genotypes and allele counts for each diagnosis. Genotypes and allele counts are further broken down by gender within each diagnosis, with percent of total by diagnosis and count type. There was no significant interaction between gender × short allele count on bvFTD risk (P = 0.76) after accounting for age, years of education and main effects.
| Genotype count | Control |
Within control P-val | bvFTD |
Within bvFTD P-val | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male |
Female |
Male |
Female |
|||||||
| N | % total | N | % total | N | % total | N | % total | |||
| 16/16 | 18 | 10.0% | 35 | 19.6% | 0.043 | 5 | 20.8% | 1 | 4.2% | 0.41 |
| 14/16 | 44 | 24.6% | 49 | 27.4% | 7 | 29.2% | 3 | 12.5% | ||
| 14/14 | 8 | 4.5% | 25 | 14.0% | 4 | 16.7% | 4 | 16.7% | ||
| Allele count | N | % total | N | % total | N | % total | N | % total | ||
| 16 | 80 | 22.3% | 119 | 33.2% | 0.64 | 17 | 35.4% | 5 | 10.4% | 0.15 |
| 14 | 60 | 16.8% | 99 | 27.7% | 15 | 31.3% | 11 | 22.9% | ||
3.2. Neuroimaging analysis
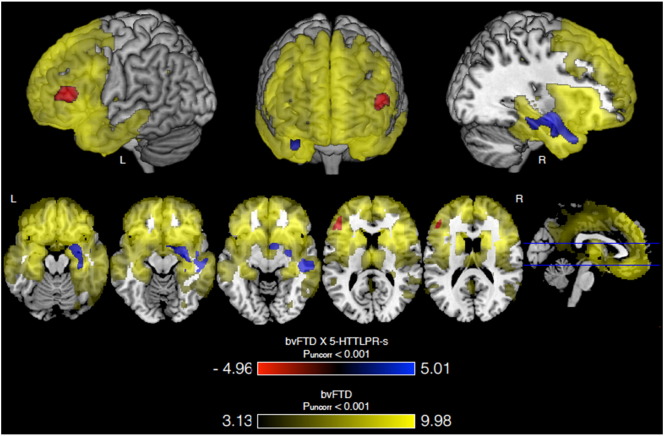
Using whole-brain VBM we first confirmed the expected volumetric atrophy in the frontal and temporal lobes in bvFTD patients versus controls (PFWE < 0.001, Max T = 9.98, MNI = 12, 18, 9, cluster = 272.5 cm3; Fig. 1, in yellow).
Fig. 1.
VBM results for bvFTD, 5-HTTLPR-s dose and their interaction. Carrying more 5-HTTLPR-s alleles and having bvFTD is associated with greater volume in right medial temporal lobe (blue) and lower volume in left inferior frontal gyrus (red) compared to controls. Both regions are associated with atrophy in bvFTD (versus controls; yellow). Three T-Maps are overlaid on a normal template brain in MRIcron and all are thresholded as stated. First, areas of greater volume loss associated with the bvFTD × 5-HTTLPR-s interaction are shown in red. Second, areas of lesser volume loss associated with the bvFTD × 5-HTTLPR-s interaction are shown in blue. Third, areas of volume loss associated with bvFTD versus controls are shown in yellow. Images are in neurological orientation.
We next tested for an interaction effect of 5-HTTLPR-s allele count with diagnosis to assess whether carrying more short alleles was associated with greater or lesser volume loss in patients with bvFTD when compared to controls. The interaction was associated with greater volume in the right medial temporal lobe (R MTL), with the finding extending through portions of the amygdala, hippocampus, and temporal pole (PFWE = 0.006, Max T = 5.01, MNI = 30, −7, −15, cluster = 7.3 cm3; Fig. 1, in blue; Table 3). The interaction was also associated with lower volume in the left inferior frontal gyrus (L IFG) (PFWE = 0.032, Max T = 4.86, MNI = −46, 36, 15, cluster = 3.1 cm3; Fig. 1, in red; Table 3). We had 51% power to detect an interaction effect of this magnitude and significance. Additional regions of association at uncorrected P < 0.001 and with cluster size larger than 0.5 cm3 are provided in Inline Supplementary Table S2.
Table 3.
Interaction effect of bvFTD × 5-HTTLPR-s dose on brain volume.
| Variable | Left inferior frontal gyrus |
Right medial temporal lobe |
||
|---|---|---|---|---|
| β ± SE | P-value | β ± SE | P-value | |
| Age | 1.21 × 10−3 ± 3.05 × 10−3 | 0.69 | −2.03 × 10−3 ± 6.08 × 10−3 | 0.74 |
| Sex | −0.033 ± 0.046 | 0.48 | −0.070 ± 0.092 | 0.45 |
| Education | −0.010 ± 9.48 × 10−3 | 0.29 | 4.81 × 10−3 ± 0.019 | 0.80 |
| Scan type | −0.12 ± 0.046 | 0.011 | 0.11 ± 0.091 | 0.22 |
| TIV | −1.42 × 10−5 ± 1.64 × 10−5 | 0.39 | −5.81 × 10−6 ± 3.27 × 10−6 | 0.86 |
| GM vol. | 5.78 × 10−4 ± 5.02 × 10−5 | 1.11 × 10−23 | 1.28 × 10−3 ± 1.00 × 10−4 | 1.57 × 10−27 |
| bvFTD diagnosis | 0.22 ± 0.13 | 0.076 | −2.06 ± 0.25 | 2.44 × 10−14 |
| Short allele dose | −2.82 × 10−3 ± 0.032 | 0.93 | 0.023 ± 0.063 | 0.71 |
| bvFTD × 5-HTTLPR-s interaction | −0.45 ± 0.085 | 3.52 × 10−7 | 0.84 ± 0.17 | 1.42 × 10−6 |
Summary of regression analyses by region of interest. The estimated β coefficient and accompanying standard error are summarized for each independent variable used in the model. TIV — total intracranial volume, GM vol. — gray matter volume, β — beta coefficient, SE — standard error.
Inline Supplementary Table S2.
Table S2.
Regions of interest in bvFTD × 5-HTTLPR-s interaction analyses. Summary of findings for bvFTD × 5-HTTLPR-s interaction analyses are displayed above. The direction of the effect in each cluster is specified — either towards increased volume or decreased volume. For each finding the volume of the cluster and coordinates of the voxel with the highest T-score for each cluster are provided as X, Y, and Z coordinates in the MNI152 coordinate system. The maximum T-score within each cluster, unadjusted P-value, and corrected P-value are provided. L — left, R — right.
| bvFTD × 5-HTTLPR-s interaction analyses | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect direction | Region | L/R | Volume (cm3) | X | Y | Z | Max T | Unadjusted P | Corrected P |
| Greater volume | Medial temporal | R | 7.3 | 30 | −7 | −15 | 5.01 | <0.001 | 0.0060 |
| Middle temporal gyrus | R | 3.9 | 57 | −24 | −6 | 4.53 | <0.001 | 0.031 | |
| Inferior temporal gyrus | R | 1.5 | 45 | −1 | −39 | 3.84 | <0.001 | 0.21 | |
| Postcentral gyrus | R | 0.6 | 40 | −31 | 49 | 3.74 | <0.001 | 0.56 | |
| Lesser volume | Inferior frontal gyrus | L | 3.1 | −47 | 36 | 15 | 4.86 | <0.001 | 0.032 |
| Middle frontal gyrus | L | 1.3 | −36 | 60 | −9 | 4.96 | <0.001 | 0.14 | |
Given that there was a significant difference in scan type by genotype, we retested our analyses in the 47 individuals with available 1.5 T scans. Despite the smaller sample size, our findings were largely unchanged.
To further clarify this interaction effect, we next explored the main effects of 5-HTTLPR-s dose in patients and controls separately. No results met our predetermined significance threshold of PFWE < 0.05. In controls, we did not find regions of greater or lesser volume at unadjusted P < 0.001 with cluster size larger than 0.5 cm3. In patients with bvFTD, carrying more risk alleles was associated with greater volume in the right angular gyrus (P < 0.001, unadjusted, Max T = 6.07, MNI = 48, −66, 48, cluster = 0.6 cm3) and smaller volume in the left superior temporal gyrus (P < 0.001, unadjusted, Max T = 5.48, MNI = −38, −39, 22, cluster = 1.6 cm3). These findings were consistent with the fact that the magnitude of the interaction effect was much greater than that of the main 5-HTTLPR-s effect in the primary analysis (Table 3).
3.3. Clinical severity and volumetric correlations
When volume in the R MTL and L IFG was plotted by disease severity in the bvFTD patients, volume decreased with increasing severity across all genotype groups (no significant interaction of 5-HTTLPR-s genotype × CDR sum of boxes, P = 0.76 for R MTL, P = 0.69 for L IFG; no significant difference in slopes, P = 0.95 in R MTL and L IFG; Fig. 2), arguing against a disease-modifying genotype effect. There was, however, a significant difference between intercepts for each group (P = 0.03 for R MTL, P = 0.0002 for L IFG), suggesting 5-HTT genotype may alter baseline volume in these individuals.
Fig. 2.
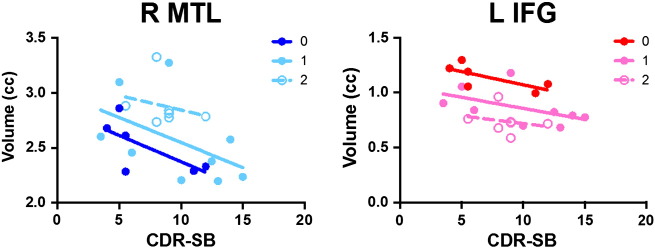
Volume plotted by disease severity in bvFTD patients. Adjusted volume plotted by CDR sum of boxes (CDR-SB) in bvFTD patients by dose of 5-HTTLPR-s allele. In R MTL, baseline is higher with more alleles (significant difference in intercepts but not slopes, P = 0.03). In L IFG, baseline is lower with more risk alleles (P = 0.0002).
3.4. Neuropsychiatric syndromes and volumetric correlations
We next examined each of the four NPI syndrome scores to test whether the regions of interest we found in the primary bvFTD × 5-HTTLPR-s dose interaction analysis correlated with behavioral features. NPI-Q data were available in a subset of 165 controls and 15–20 bvFTD patients. As expected, the great majority of controls had NPI-Q scores of 0, while average scores for bvFTD patients were significantly higher (P < 0.0001 for each syndrome; Inline Supplementary Table S3). We found that larger volumes in the R MTL were negatively correlated with mean psychosis (ρ = −0.29), affect (ρ = −0.34), apathy (ρ = −0.45), and hyperactivity (ρ = −0.39) syndrome scores across both groups after correction for multiple testing (Table 4, Supplementary Fig. 1). Larger volumes in the L IFG were negatively correlated with mean apathy (ρ = −0.21) syndrome scores across both patients and controls after correction for multiple testing (Table 4, Supplementary Fig. 1). Analysis of syndrome measures calculated from NPI data in a smaller subset of individuals rendered similar results (Inline Supplementary Table S4). These data suggest that greater volume in R MTL and L IFG are associated with fewer (or less severe) behavioral changes.
Inline Supplementary Table S3.
Table S3.
Mean NPI-Q scores by neuropsychiatric syndrome. Summary of mean NPI-Q score for each of the four neuropsychiatric syndromes examined. Participants with a neuropsychiatric syndrome score of zero are reported as a percentage. bvFTD — behavioral variant frontotemporal dementia, SE — standard error.
| Diagnosis | Hyperactivity |
Psychosis |
Affect |
Apathy |
||||
|---|---|---|---|---|---|---|---|---|
| Control | bvFTD | Control | bvFTD | Control | bvFTD | Control | bvFTD | |
| N | 165 | 19 | 165 | 15 | 165 | 16 | 165 | 20 |
| Mean ± SE | 0.063 ± 0.013 | 1.42 ± 0.16 | 0.055 ± 0.014 | 0.67 ± 0.20 | 0.048 ± 0.016 | 0.88 ± 0.23 | 0.064 ± 0.015 | 2.15 ± 0.15 |
| % zero | 83.0% | 5.3% | 88.5% | 26.6% | 92.7% | 37.5% | 89.10% | 0.0% |
Table 4.
Correlation between volume and neuropsychiatric syndromes in bvFTD and controls.
| Syndrome | N | Left inferior frontal gyrus |
Right medial temporal lobe |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Coef. ± SE | Raw P-value | Adj. P-value | ρ | Coef. ± SE | Raw P-value | Adj. P-value | ρ | ||
| Psychosis | 180 | −0.02 ± (0.09) | 0.79 | NS | −0.02 | −0.86 ± (0.21) | 9.00E−05 | <0.001 | −0.29 |
| Affective | 181 | −0.12 ± (0.07) | 0.11 | NS | −0.12 | −0.83 ± (0.17) | 2.00E−06 | <0.001 | −0.34 |
| Apathy | 185 | −0.12 ± (0.04) | 0.004 | <0.05 | −0.21 | −0.61 ± (0.09) | 1.98E−10 | <0.001 | −0.45 |
| Hyperactivity | 184 | −0.05 ± (0.06) | 0.38 | NS | −0.07 | −0.74 ± (0.13) | 4.99E−08 | <0.001 | −0.39 |
Summary of regression and correlation analyses by region of interest and neuropsychiatric syndrome, derived from NPI-Q scores. The estimated β coefficient and accompanying standard error are summarized for each independent variable used in the model. N — total number of individuals with data available for analysis, ρ — correlation coefficient, Adj. — adjusted, β — beta coefficient, SE — standard error.
Inline Supplementary Table S4.
Table S4.
Correlation between volume and neuropsychiatric syndromes in bvFTD and controls. Summary of regression and correlation analyses by region of interest and neuropsychiatric syndrome. The estimated β coefficient and accompanying standard error are summarized for each independent variable used in the model. ρ — correlation coefficient, Adj. — adjusted, β — beta coefficient, SE — standard error.
| Syndrome | Left inferior frontal gyrus |
Right medial temporal lobe |
||||||
|---|---|---|---|---|---|---|---|---|
| Coef. ± SE | Raw P-value | Adj. P-value | ρ | Coef. ± SE | Raw P-value | Adj. P-value | ρ | |
| Psychosis | −0.31 ± 0.14 | 0.026 | NS | −0.16 | −0.18 ± 0.056 | 2.16 × 10−3 | <0.05 | −0.23 |
| Affective | −0.071 ± 0.12 | 0.57 | NS | −0.044 | −0.11 ± 0.050 | 0.027 | NS | −0.17 |
| Apathy | −0.63 ± 0.20 | 1.74 × 10−3 | <0.05 | −0.24 | −0.45 ± 0.076 | 2.72 × 10−8 | <0.001 | −0.41 |
| Hyperactivity | −0.43 ± 0.31 | 0.17 | NS | −0.11 | −0.55 ± 0.12 | 9.91 × 10−6 | <0.001 | −0.33 |
4. Discussion
In this study we found that patients with bvFTD who carry a particular variant in the serotonin transporter that is associated with heightened affect have greater volume in emotion-generating and lower volume in emotion-regulating regions of the brain. In a subset of the cohort, the volume of these regions correlated with less severe behavioral symptoms including apathy, hyperactivity/disinhibition, affect and psychosis. Genotype-specific differences in baseline brain volume may underlie this effect.
The amygdala is located in MTL and is well characterized as redirecting attention towards personally salient cues, triggering quick enactment of physiological and behavioral changes in response to emotional material (Adolphs, 2008) and modulating the encoding of emotional memories (Davis and Whalen, 2001; Phelps and LeDoux, 2005). The IFG is involved in cognitive control of emotional information (Ochsner and Gross, 2005; Ochsner et al., 2002), acting upon amygdala to moderate behavioral response during cognitive reappraisal of emotion information (Wager et al., 2008).
Although traditionally studied as risk variant for psychiatric disease, there may be some benefits to carrying the 5-HTTLPR-s allele. Specifically, carriers of this allele are generally considered more sensitive to their environment — both emotionally (Beevers et al., 2010) and physiologically (Papousek et al., 2013). Although heightened sensitivity to both self and others can be detrimental in the context of negative life experiences (Caspi et al., 2003) or other significant stressors (Belsky et al., 2009), it can also be beneficial for prosocial situations that require attention to emotional cues (Bakermans-Kranenburg and van Ijzendoorn, 2008; Cents et al., 2014; Mileva-Seitz et al., 2011). Increased self-monitoring can also improve cognitive functioning (Homberg and Lesch, 2011). Because of this, some argue that the gene should not be considered a “risk” gene, but rather a “plasticity” gene, which makes an individual's behavior more modifiable in response to environmental factors (van IJzendoorn et al., 2012). Jasinska and colleagues proposed that this sensitivity stems from the short allele's influence on the dynamics of serotonergic activity in the brain (Jasinska et al., 2012).
Given this framework, there are two distinct hypotheses that could explain the mechanism by which 5-HTTLPR genotypes affect brain atrophy in bvFTD. The first is that altered levels and function of 5-HTT modify the way the brain atrophies in bvFTD, such that after disease onset there arises differential patterns of degeneration based on the ability of serotonergic transmission to adapt to pathological processes occurring in the brain. The second is that 5-HTTLPR genotype may alter neurodevelopment, such that the brain is structured differently and thus functions in an altered state throughout life and is primed to respond to both environmental and pathological stresses in different ways. The latter developmental theory has been proposed previously (Hariri et al., 2006; Jonassen and Landrø, 2014), and puts forth a model by which genetic differences in corticolimbic reactivity predispose individuals to affective illnesses such as depression and anxiety in the context of stressful events (Hariri et al., 2006). Because of the critical roles serotonin plays in regulating synaptic plasticity and neuronal activity, it would make sense that life-long differences in 5-HTT efficiency could result in differential neural circuitry that responds to stimuli and injury with varying capacity.
The latter neurodevelopmental model is supported by literature in the rhesus macaque — which carries a functional ortholog of the 5-HTTLPR-s variant — that suggests the polymorphism exerts a developmental effect on cortical structures, rather than causing a specific difference in serotonergic signaling (Jedema et al., 2010). Mice lacking 5-HTT also support this model, demonstrating altered neuronal architecture and cortical function that results in lasting emotional abnormalities (Hariri et al., 2006). In the context of bvFTD, we venture to speculate that neurodevelopmental differences due to 5-HTTLPR-s extend beyond an individual's response to the external environment to the internal, physiological environment. Perhaps, the same mechanistic processes underlying genotype-specific susceptibility to environmental impacts is also applicable in neurodegenerative disease. Individuals carrying the ‘sensitivity’ allele could be uniquely resilient or vulnerable to neurodegenerative processes in different areas of the brain, thereby promoting an adaptive preservation of emotional experience despite pathological changes in corticolimbic brain regions. Conceptually, this framework which links 5-HTTLPR-s to neurodegeneration in bvFTD may also apply to behavior as previously posited by the theory of “behavioral reserve” (Premi et al., 2013). 5-HTTLPR-s status could potentially confer unique resilience to behavioral deficits in bvFTD and represent a route towards future therapeutic interventions.
The laterality of the bvFTD × 5-HTTLPR-s dose interaction effects, with greater volume in R MTL and lower volume in L IFG, are intriguing in the context of emotion generation and regulation, respectively, since bvFTD is characterized by deficits in both. Greater atrophy of R MTL has been associated with more disinhibition — a component of the hyperactivity syndrome scale — in bvFTD (Rosen et al., 2005; Zamboni et al., 2008). The L IFG has been identified previously as a regulator in the reappraisal of positive emotions (Grecucci et al., 2013; Sturm et al., 2015). Our VBM results propose reduced capacity to regulate emotion in the left hemisphere and greater capacity to generate emotion in the right hemisphere in 5-HTTLPR-s carriers with bvFTD. Although more emotion generation and less emotion regulation could contribute to disinhibition, it could also be advantageous in the context of bvFTD where patients progressively lose emotional responsiveness. Smaller volume in these regions was also associated with higher apathy in bvFTD, however, which suggests that this pattern of brain atrophy may relate to affective symptom severity and disease progression in bvFTD more broadly.
This study's findings are strengthened by its use of a clinically well-characterized cohort and multiple information modalities to study the effects of 5-HTTLPR-s in bvFTD. Limitations of the present study include its use of MR images from multiple scanners, age difference between the control and bvFTD groups, limited size of the overall cohort, and limited neuropsychiatric syndrome data. To minimize the impact of using MR images from different scanners, we included scan-type as a covariate in all analyses. Other groups have found that combining scans from multiple scanners can provide robust results (Fennema-Notestine et al., 2007; Stonnington et al., 2008). Similarly, we included age as a covariate in all analyses to control for age differences between healthy controls and bvFTD. Likewise, we only included the 29 and 34 year-old individuals in the bvFTD cohort after obtaining pathological confirmation of each individual's clinical diagnosis. Although we strove to maximize the number of individuals in our study, we were unable to detect morphological differences by genotype that survived permutation testing at a threshold of PFWE < 0.05 within the control and bvFTD groups separately, limiting our ability to further dissect the genotype-disease interaction. Others have identified differences by 5-HTTLPR-s, primarily in younger healthy adults (Jonassen and Landrø, 2014); it is possible that aging minimizes these differences due to normal cortical thinning, making them difficult to detect in whole brain analysis in elderly populations. Although plotting individuals by disease severity is an imperfect proxy for identifying genotype-specific changes in atrophy over time, our data suggest that 5-HTTLPR-s dose alters baseline volume in L IFG and R MTL. Studies of longitudinal change in bvFTD patients are required to confirm this observation directly.
5. Conclusion
In conclusion, our study suggests that variation in the serotonin transporter modifies atrophy of brain regions critical for emotion regulation and generation in patients with bvFTD. This study provides unique insight into the genetic modifiers of a devastating neurodegenerative disease and extends associations between 5-HTTLPR and psychiatric disease to a degenerative condition characterized by emotional dysfunction. To better understand the effects of 5-HTTLPR-s in bvFTD, future studies will need to replicate this finding in an independent, larger, cohort. Additionally, longitudinal analyses may clarify whether the effects of 5-HTTPLR-s reflect baseline differences in volume rather than differences in serotonergic signaling. Taken together, these results support the notion that 5-HTTLPR genotype modifies the brain's response to neurodegenerative insult and promotes increased emotionality.
The following is the supplementary data related to this article.
Syndromic scores are plotted by region for tests which survived multiple testing correction. All volumes are given in cubic centimeters. MTL – medial temporal lobe, IFG – inferior frontal gyrus, ρ – correlation coefficient.
Acknowledgments
We gratefully acknowledge DW Sirkis and KL Karunungan for valuable scientific discussions and literature suggestions. We thank all research participants for their longstanding dedication to our studies. Primary analysis was supported by funding from the Larry L. Hillblom Foundation (2012-A-015-FEL, JSY), the Association for Frontotemporal Degeneration Susan Marcus Memorial Fund Clinical Research Grant (JSY), and the NIH-NI (K01-AG049152 (JSY); Diversity Supplement to P50-AG03006 (to JSY; PI: BLM). Support for data collection was provided by the NIH-NIA, Alzheimer's Disease Research Center of California, ALS Association, Tau Consortium, Consortium for Frontotemporal Dementia Research, Larry L. Hillblom Foundation, and the Association for Frontotemporal Degeneration.
References
- Aalten P., Verhey F.R.J., Boziki M., Bullock R., Byrne E.J., Camus V., Caputo M., Collins D., De Deyn P.P., Elina K., Frisoni G., Girtler N., Holmes C., Hurt C., Marriott A., Mecocci P., Nobili F., Ousset P.J., Reynish E., Salmon E., Tsolaki M., Vellas B., Robert P.H. Neuropsychiatric syndromes in dementia. Results from the European Alzheimer Disease Consortium: part I. Dement. Geriatr. Cogn. Disord. 2007;24(6):457–463. doi: 10.1159/000110738. 17986816 [DOI] [PubMed] [Google Scholar]
- Adolphs R. Fear, faces, and the human amygdala. Curr. Opin. Neurobiol. 2008;18(2):166–172. doi: 10.1016/j.conb.2008.06.006. 18655833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albani D., Prato F., Fenoglio C., Batelli S., Dusi S., De Mauro S., Polito L., Lovati C., Galimberti D., Mariani C., Scarpini E., Forloni G. Association study to evaluate the serotonin transporter and apolipoprotein E genes in frontotemporal lobar degeneration in Italy. J. Hum. Genet. 2008;53(11–12):1029–1033. doi: 10.1007/s10038-008-0344-5. 19020798 [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. 17761438 [DOI] [PubMed] [Google Scholar]
- Assal F., Alarcón M., Solomon E.C., Masterman D., Geschwind D.H., Cummings J.L. Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer disease. Arch. Neurol. 2004;61(8):1249–1253. doi: 10.1001/archneur.61.8.1249. 15313842 [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M.J., van Ijzendoorn M.H. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc. Cogn. Affect. Neurosci. 2008;3(2):128–134. doi: 10.1093/scan/nsn004. 19015103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks S.J., Weintraub S. Neuropsychiatric symptoms in behavioral variant frontotemporal dementia and primary progressive aphasia. J. Geriatr. Psychiatry Neurol. 2008;21(2):133–141. doi: 10.1177/0891988708316856. 18474722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E., Wilson S.M., Saygin A.P., Dick F., Sereno M.I., Knight R.T., Dronkers N.F. Voxel-based lesion symptom mapping. Nat. Neurosci. 2003;6(5):448–450. doi: 10.1038/nn1050. 12704393 [DOI] [PubMed] [Google Scholar]
- Beevers C.G., Clasen P., Stice E., Schnyer D. Depression symptoms and cognitive control of emotion cues: a functional magnetic resonance imaging study. Neuroscience. 2010;167(1):97–103. doi: 10.1016/j.neuroscience.2010.01.047. 20116416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J., Jonassaint C., Pluess M., Stanton M., Brummett B., Williams R. Vulnerability genes or plasticity genes? Mol. Psychiatry. 2009;14(8):746–754. doi: 10.1038/mp.2009.44. 19455150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Yekutieli D. Quantitative trait loci analysis using the false discovery rate. Genetics. 2005;171(2):783–790. doi: 10.1534/genetics.104.036699. 15956674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B., Grassi M., Agosti C., Costanzi C., Archetti S., Franzoni S., Caltagirone C., Di Luca M., Caimi L., Padovani A. Genetic correlates of behavioral endophenotypes in Alzheimer disease: role of COMT, 5-HTTLPR and APOE polymorphisms. Neurobiol. Aging. 2006;27(11):1595–1603. doi: 10.1016/j.neurobiolaging.2005.09.029. 16257094 [DOI] [PubMed] [Google Scholar]
- Borroni B., Grassi M., Agosti C., Premi E., Archetti S., Alberici A., Bellelli G., Caimi L., Di Luca M., Padovani A. Establishing short-term prognosis in frontotemporal Lobar degeneration spectrum: role of genetic background and clinical phenotype. Neurobiol. Aging. 2010;31(2):270–279. doi: 10.1016/j.neurobiolaging.2008.04.004. 18495299 [DOI] [PubMed] [Google Scholar]
- Bozeat S., Gregory C.A., Ralph M.A., Hodges J.R. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? J. Neurol. Neurosurg. Psychiatry. 2000;69(2):178–186. doi: 10.1136/jnnp.69.2.178. 10896690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T., Lesch K.-P. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 2007;10(9):1103–1109. doi: 10.1038/nn1964. 17726476 [DOI] [PubMed] [Google Scholar]
- Caspi A., Sugden K., Moffitt T.E., Taylor A., Craig I.W., Harrington H., McClay J., Mill J., Martin J., Braithwaite A., Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. 12869766 [DOI] [PubMed] [Google Scholar]
- Caspi A., Hariri A.R., Holmes A., Uher R., Moffitt T.E. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am. J. Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cents R.A., Kok R., Tiemeier H., Lucassen N., Székely E., Bakermans-Kranenburg M.J., Hofman A., Jaddoe V.W., van IJzendoorn M.H., Verhulst F.C., Lambregtse-van den Berg M.P. Variations in maternal 5-HTTLPR affect observed sensitive parenting. J. Child Psychol. Psychiatry Allied Discip. 2014;55(9):1025–1032. doi: 10.1111/jcpp.12205. 24484301 [DOI] [PubMed] [Google Scholar]
- Chow T.W., Binns M.A., Cummings J.L., Lam I., Black S.E., Miller B.L., Freedman M., Stuss D.T., van Reekum R. Apathy symptom profile and behavioral associations in frontotemporal dementia vs dementia of Alzheimer type. Arch. Neurol. 2009;66(7):888–893. doi: 10.1001/archneurol.2009.92. 19597092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. 7991117 [DOI] [PubMed] [Google Scholar]
- Dannlowski U., Konrad C., Kugel H., Zwitserlood P., Domschke K., Schöning S., Ohrmann P., Bauer J., Pyka M., Hohoff C., Zhang W., Baune B.T., Heindel W., Arolt V., Suslow T. Emotion specific modulation of automatic amygdala responses by 5-HTTLPR genotype. Neuroimage. 2010;53(3):893–898. doi: 10.1016/j.neuroimage.2009.11.073. 19962442 [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. 11244481 [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C., Gamst A.C., Quinn B.T., Pacheco J., Jernigan T.L., Thal L., Buckner R., Killiany R., Blacker D., Dale A.M., Fischl B., Dickerson B., Gollub R.L. Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. Neuroinformatics. 2007;5(4):235–245. doi: 10.1007/s12021-007-9003-9. 17999200 [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. 1202204 [DOI] [PubMed] [Google Scholar]
- Gallinat J., Bauer M., Heinz A. Genes and neuroimaging: advances in psychiatric research. Neurodegener. Dis. 2008;5(5):277–285. doi: 10.1159/000135612. 18520162 [DOI] [PubMed] [Google Scholar]
- Grecucci A., Giorgetta C., Bonini N., Sanfey A.G. Reappraising social emotions: the role of inferior frontal gyrus, temporo-parietal junction and insula in interpersonal emotion regulation. Front. Hum. Neurosci. 2013;7:523. doi: 10.3389/fnhum.2013.00523. 24027512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A., Haase C.M., Sze J., Goodkind M.S., Coppola G., Lane J., Miller B.L., Levenson R.W. The effect of the serotonin transporter polymorphism (5-HTTLPR) on empathic and self-conscious emotional reactivity. Emotion. 2013;13(1):25–35. doi: 10.1037/a0029616. 22906085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase C.M., Beermann U., Saslow L.R., Shiota M.N., Saturn S.R., Lwi S.J., Casey J.J., Nguyen N.K., Whalen P.K., Keltner D., Levenson R.W. Short alleles, bigger smiles? The effect of 5-HTTLPR on positive emotional expressions. Emotion. 2015;15(4):438–448. doi: 10.1037/emo0000074. 26029940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Drabant E.M., Weinberger D.R. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol. Psychiatry. 2006;59(10):888–897. doi: 10.1016/j.biopsych.2005.11.005. 16442081 [DOI] [PubMed] [Google Scholar]
- Hayasaka S., Nichols T.E. Combining voxel intensity and cluster extent with permutation test framework. Neuroimage. 2004;23(1):54–63. doi: 10.1016/j.neuroimage.2004.04.035. 15325352 [DOI] [PubMed] [Google Scholar]
- Heils A., Teufel A., Petri S., Stöber G., Riederer P., Bengel D., Lesch K.P. Allelic variation of human serotonin transporter gene expression. J. Neurochem. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. 8632190 [DOI] [PubMed] [Google Scholar]
- Hollingworth P., Hamshere M.L., Moskvina V., Dowzell K., Moore P.J., Foy C., Archer N., Lynch A., Lovestone S., Brayne C., Rubinsztein D.C., Lawlor B., Gill M., Owen M.J., Williams J. Four components describe behavioral symptoms in 1,120 individuals with late-onset Alzheimer's disease. J. Am. Geriatr. Soc. 2006;54(9):1348–1354. doi: 10.1111/j.1532-5415.2006.00854.x. 16970641 [DOI] [PubMed] [Google Scholar]
- Homberg J.R., Lesch K.-P. Looking on the bright side of serotonin transporter gene variation. Biol. Psychiatry. 2011;69(6):513–519. doi: 10.1016/j.biopsych.2010.09.024. 21047622 [DOI] [PubMed] [Google Scholar]
- Jasinska A.J., Lowry C.A., Burmeister M. Serotonin transporter gene, stress and raphe-raphe interactions: a molecular mechanism of depression. Trends Neurosci. 2012;35(7):395–402. doi: 10.1016/j.tins.2012.01.001. 22301434 [DOI] [PubMed] [Google Scholar]
- Jedema H.P., Gianaros P.J., Greer P.J., Kerr D.D., Liu S., Higley J.D., Suomi S.J., Olsen A.S., Porter J.N., Lopresti B.J., Hariri A.R., Bradberry C.W. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol. Psychiatry. 2010;15(5):512–522. doi: 10.1038/mp.2009.90. 19721434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen R., Landrø N.I. Serotonin transporter polymorphisms (5-HTTLPR) in emotion processing. Implications from current neurobiology. Prog. Neurobiol. 2014;117:41–53. doi: 10.1016/j.pneurobio.2014.02.003. 24548605 [DOI] [PubMed] [Google Scholar]
- Kaufer D.I., Cummings J.L., Ketchel P., Smith V., MacMillan A., Shelley T., Lopez O.L., DeKosky S.T. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J. neuropsychiatry clin. neurosci. 2000;12(2):233–239. doi: 10.1176/jnp.12.2.233. 11001602 [DOI] [PubMed] [Google Scholar]
- Kimberg D.Y., Coslett H.B., Schwartz M.F. Power in voxel-based lesion-symptom mapping. J. Cogn. Neurosci. 2007;19(7):1067–1080. doi: 10.1162/jocn.2007.19.7.1067. 17583984 [DOI] [PubMed] [Google Scholar]
- Lorenzi C., Marcone A., Pirovano A., Marino E., Cordici F., Cerami C., Delmonte D., Cappa S.F., Bramanti P., Smeraldi E. Serotonin transporter and saitohin genes in risk of Alzheimer's disease and frontotemporal lobar dementia: preliminary findings. Neurol. Sci. 2010;31(6):741–749. doi: 10.1007/s10072-010-0400-8. 20852909 [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol. Psychiatry. 1998;44(3):151–162. doi: 10.1016/s0006-3223(98)00139-5. 9693387 [DOI] [PubMed] [Google Scholar]
- Mileva-Seitz V., Kennedy J., Atkinson L., Steiner M., Levitan R., Matthews S.G., Meaney M.J., Sokolowski M.B., Fleming A.S. Serotonin transporter allelic variation in mothers predicts maternal sensitivity, behavior and attitudes toward 6-month-old infants. Genes Brain Behav. 2011;10(3):325–333. doi: 10.1111/j.1601-183X.2010.00671.x. 21232011 [DOI] [PubMed] [Google Scholar]
- Murphy D.L., Lerner A., Rudnick G., Lesch K.-P. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol. Interv. 2004;4(2):109–123. doi: 10.1124/mi.4.2.8. 15087484 [DOI] [PubMed] [Google Scholar]
- Neary D., Snowden J., Mann D. Frontotemporal dementia. Lancet Neurol. 2005;4(11):771–780. doi: 10.1016/S1474-4422(05)70223-4. 16239184 [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D.E. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. 12495527 [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. (Regul. Ed.) 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. 15866151 [DOI] [PubMed] [Google Scholar]
- Papousek I., Reiser E.M., Schulter G., Fink A., Holmes E.A., Niederstätter H., Nagl S., Parson W., Weiss E.M. Serotonin transporter genotype (5-HTTLPR) and electrocortical responses indicating the sensitivity to negative emotional cues. Emotion. 2013;13(6):1173–1181. doi: 10.1037/a0033997. 24040881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. 16242399 [DOI] [PubMed] [Google Scholar]
- Premi E., Garibotto V., Gazzina S., Grassi M., Cosseddu M., Paghera B., Turla M., Padovani A., Borroni B. Beyond cognitive reserve: behavioural reserve hypothesis in frontotemporal dementia. Behav. Brain Res. 2013;245:58–62. doi: 10.1016/j.bbr.2013.01.030. 23380679 [DOI] [PubMed] [Google Scholar]
- Rapport M.M., Green A.A., Page I.H. Crystalline serotonin. Science. 1948;108(2804):329–330. doi: 10.1126/science.108.2804.329. 17748034 [DOI] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Kipps C.M., Johnson J.K., Seeley W.W., Mendez M.F., Knopman D., Kertesz A., Mesulam M., Salmon D.P., Galasko D., Chow T.W., Decarli C., Hillis A., Josephs K., Kramer J.H., Weintraub S., Grossman M., Gorno-Tempini M.L., Miller B.M. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis. Assoc. Disord. 2007;21(4):S14–S18. doi: 10.1097/WAD.0b013e31815c3445. 18090417 [DOI] [PubMed] [Google Scholar]
- Rohrer J.D., Guerreiro R., Vandrovcova J., Uphill J., Reiman D., Beck J., Isaacs A.M., Authier A., Ferrari R., Fox N.C., Mackenzie I.R., Warren J.D., de Silva R., Holton J., Revesz T., Hardy J., Mead S., Rossor M.N. The heritability and genetics of frontotemporal lobar degeneration. Neurology. 2009;73(18):1451–1456. doi: 10.1212/WNL.0b013e3181bf997a. 19884572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H.J., Allison S.C., Schauer G.F., Gorno-Tempini M.L., Weiner M.W., Miller B.L. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128(11):2612–2625. doi: 10.1093/brain/awh628. 16195246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonnington C.M., Tan G., Klöppel S., Chu C., Draganski B., Jack C.R., Chen K., Ashburner J., Frackowiak R.S. Interpreting scan data acquired from multiple scanners: a study with Alzheimer's disease. Neuroimage. 2008;39(3):1180–1185. doi: 10.1016/j.neuroimage.2007.09.066. 18032068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm V.E., Yokoyama J.S., Eckart J.A., Zakrzewski J., Rosen H.J., Miller B.L., Seeley W.W., Levenson R.W. Damage to left frontal regulatory circuits produces greater positive emotional reactivity in frontotemporal dementia. Cortex. 2015;64:55–67. doi: 10.1016/j.cortex.2014.10.002. 25461707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm V.E., Yokoyama J.S., Seeley W.W., Kramer J.H., Miller B.L., Rankin K.P. Heightened emotional contagion in mild cognitive impairment and Alzheimer's disease is associated with temporal lobe degeneration. Proc. Natl. Acad. Sci. U. S. A. 2013;110(24):9944–9949. doi: 10.1073/pnas.1301119110. 23716653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van IJzendoorn M.H., Belsky J., Bakermans-Kranenburg M.J. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Transl. Psychiatry. 2012;2:e147. doi: 10.1038/tp.2012.73. 22872162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Prefrontal–subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. 18817740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley J.D., Khan B.K., Murthy N.K., Miller B.L., Rankin K.P. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J. Clin. Psychiatry. 2011;72(2):126–133. doi: 10.4088/JCP.10m06382oli. 21382304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni G., Huey E.D., Krueger F., Nichelli P.F., Grafman J. Apathy and disinhibition in frontotemporal dementia: insights into their neural correlates. Neurology. 2008;71(10):736–742. doi: 10.1212/01.wnl.0000324920.96835.95. 18765649 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Syndromic scores are plotted by region for tests which survived multiple testing correction. All volumes are given in cubic centimeters. MTL – medial temporal lobe, IFG – inferior frontal gyrus, ρ – correlation coefficient.