Abstract
Accumulation of unesterified cholesterol-rich lipid vesicles in the subendothelial space contributes to atherogenesis. Transport of cholesterol from the subendothelial intima back to the circulating blood inhibits atherosclerosis development; however, the mechanism for this process has not been fully defined. Using cultured mouse aortic endothelial cells (MAECs), we observed that unesterified cholesterol can be transported across the endothelial cell monolayer from the basolateral to the apical compartment. Administration of high-density lipoprotein (HDL) or apolipoprotein AI (apoAI) to the apical compartment enhanced transendothelial cholesterol transport in a concentration-dependent manner. Knockdown of ATP-binding cassette transporter G1 (ABCG1) or scavenger receptor class B type I (SR-B1), or inhibition of SR-B1 diminished HDL-induced transendothelial cholesterol transport; while knockdown of ABCA1 reduced apoAI-mediated cholesterol transport. HDL enhanced phosphorylation of phosphatidylinositol 3-kinase (PI3K) and Akt in MAECs. However, inhibition PI3K or Akt did not reduce HDL-induced transendothelial cholesterol transport. These results suggest that HDL enhances transendothelial cholesterol transport by activation of a mechanism involving ABCA1, ABCA1 and SR-B1 but not involving PI3K and Akt.
Keywords: transendothelial cholesterol transport, high-density lipoprotein, scavenger receptor class B type I, ATP-binding cassette transporter
1. Introduction
Accumulation of cholesterol, especially unesterified cholesterol-rich lipid vesicles, in the extracellular subendothelial space occurs at the early and late stages of atherosclerosis [1; 2]. Removal of cholesterol from the subendothelial intima by reverse cholesterol transport (RCT) protects against atherosclerosis development [3]. A critical step of RCT is the transfer of cholesterol from the arterial wall to high density lipoprotein (HDL). It has been suggested that HDL crosses endothelial cells into the subendothelial space, where it accepts cholesterol from the lipid-laden macrophages, and then reenters the bloodstream for delivery of cholesterol to the liver [4]. In agreement with this theory, previous studies have shown that HDL particles could be transported from the apical to the basolateral compartment in cultured endothelial cells [4], and that apolipoprotein (apo) AI exists in atherosclerotic lesions [5]. These observations imply that plasma HDL are able to infiltrate into the tunica intima. However, the path for transport of subendothelial HDL back to the bloodstream has not been fully defined. Recent studies suggest that the HDL particles accumulated in the atherosclerotic plaques likely travel back to the bloodstream via lymphatic vessels [6]. Additional evidence is required to prove this concept. Lymphatic vessels are not present in the arterial intima, except in the most advanced plaques, where lymphatic capillaries invade the intima along with the vasa vasorum [7].
Besides controlling the transendothelial trafficking of HDL particles, endothelial cells are also able to efficiently efflux cholesterol to apoAI and HDL [8; 9], which could be a step of the RCT. There are at least four distinct cholesterol efflux pathways, i.e.cholesterol transports mediated by ATP-binding cassette transporter A1 (ABCA1), ABCG1 and scavenger receptor class B type I (SR-B1), as well as passive cholesterol diffusion [10]. The ABCA1-mediated pathway transports cholesterol from the cell membrane to lipid-free apoAI, whereas the other three pathways employ HDL as cholesterol acceptors. O'Connell et al. observed that inhibition of ABC transporters and SR-B1 did not significantly diminish endothelial cholesterol efflux to HDL, and suggested that efflux of cholesterol to HDL occurs simply through passive diffusion [8]. However, data from other studies suggested that the other three pathways also play a part in endothelial cholesterol efflux, and the one mediated by ABCG1 appears to be predominant, as knockout of ABCG1 induced more severe oxysterol accumulation in mouse aortas than knockout of ABCA1 did [9]. Recent studies also addressed the importance of endothelial ABCA1 in vascular health. Specifically, endothelial-specific overexpression of ABCA1 was found to elevate plasma HDL and reduced atherosclerosis in mice fed a high-fat/high-cholesterol diet [11]. In addition, endothelial cells obtained from ABCA1 transgenic mice showed increased cholesterol efflux [12]. The expression level of SR-B1 may not be high enough to make it a major pathway for cholesterol efflux in endothelial cells [8]. However, knockout of this receptor has been shown to induce lipid deposition in mouse aortas and atherosclerotic lesions [13].
Data from the current report suggest that mouse aortic endothelial cells (MAECs) were able to transfer cholesterol from the basolateral to the apical compartment. In this process, apoAI and HDL in the apical compartment do not cross endothelial cells but accept cholesterol transported by ABCA1, ABCG1 and SR-B1.
2. Materials and Methods
2.1. Preparation of HDL
Human serum obtained from Leico Technologies (St. Louis, MO) was overlaid with a potassium bromide (KBr) gradient solution at a density of 1.063 g/ml. Low- and very low-density lipoproteins were removed from these samples by ultracentrifugation at 35,000 rpm for 18 h. The infranatant was adjusted to 1.21 g/ml with solid KBr and mixed with 1.21 g/ml buffered KBr solution, and then centrifuged at 48,000 rpm for 48 h. HDL was collected and dialysed against three changes of phosphate buffer saline (PBS) with 1 mM EDTA at 4°C for 48 h. Final dialysis was against PBS without EDTA for 8 h, followed by filtration through a 0.22-µm filter
2.2. Preparation of Liposomes
It has been suggested that the cholesterol-rich liposome vesicles isolated from the atherosclerotic lesions contain cholesterol (>90% in unesterified form) and phospholipids (mainly phosphatidylcholine and sphingomyelin), in a molar ratio of 2:1 [2]. In this study, a mixture containing 0.1 µM distearoyl phosphotidylcholine, 0.1 µM sphingomyelin and 20 µCi [3H]-cholesterol (~450,000 dpm/µCi) or 0.4 µM unlabeled cholesterol was dissolved in chloroform/methanol 1:1 (v/v) and dried using a Labconco CentriVap concentrator, as described previously [14]. The pellet was resuspended in 10 ml of DMEM by sonication. The liposome solution was passed through a 0.22-µm filter and diluted 10 times with DMEM when used in experiments. The final concentration of cholesterol in the working solutions was 20 µCi/ml or 4 nM.
2.3. Transcellular Cholesterol Transport
MAECs were isolated from C57BL mice using an outgrowth technique as described previously [15]. All procedures for handling animals were conducted following protocols approved by the Institutional Animal Care and Use Committee at Meharry Medical College. The 8th and 9th passages of MAECs were seeded in 0.4 µm transwell inserts, and cultured in 24-well plates in 10% FBS to confluence. In experiments using siRNAs, MAECs seeded in transwell inserts were transfected with siRNAs, as previously described [16]. Scrambled control siRNA (ASO0YEDZ) and the siRNAs targeting mouse ABCA1 (162501), ABCG1 (162293) or SR-B1 (ASO0Z7EF) were purchased from Life Technologies (Carlsbad, CA).
For determination of basolateral-apical cholesterol transport, the basolateral medium was replaced with 1.2 ml of 0.2 µCi/ml [3H]-cholesterol liposome solution supplemented with 0.1% bovine serum albumin (BSA), while the apical medium was replaced with 200 µl of DMEM supplemented with 10% FBS ± human HDL or human apoAI at the concentrations as indicated in figure legends. In experiments using SR-B1, PI3K and Akt inhibitors, 1 µM of block lipid transport-1 (BLT-1), 20 of µM LY294002 or 600 nM of Akt inhibitor XI was added into the apical medium. After an 18 h incubation, the apical medium was centrifuged at 16,000×g for 10 min. The supernatant was mixed with scintillation fluid to analyze radioactivity using a Tri-Carb 2300TR Liquid Scintillation Analyzer (PerkinElmer, Waltham, MA). Transcellularly transported [3H]-cholesterol was determined by the radioactivity in the medium. For determination of apical-basolateral cholesterol transport, the apical medium was replaced with 200 µl of 0.2 µCi/ml [3H]-cholesterol liposome solution supplemented with 10% FBS, while the basolateral medium was replaced with 1.2 ml of DMEM supplemented with 0.1% BSA ± 50 µg/ml HDL or 20 µg/ml apoAI. After 18 h incubation, radioactivity in the basolateral medium was analyzed as abovementioned.
2.4. Cholesterol Efflux
To study the effect of HDL and apoAI on cholesterol efflux, MAECs grown in 0.4 µm transwell inserts were incubated with 1.2 ml of DMEM containing 0.2 µCi/ml [3H]-cholesterol and 0.1% BSA in the basolateral side, and with 200 µl of DMEM supplemented with 10% FBS in the apical side. After an 18 h incubation, both the apical and basolateral compartments were washed thrice with DMEM. The cells were then incubated with DMEM containing 0.1% BSA ± 50 µg/ml HDL or 20 µg/ml of apoAI in both the apical and basolateral sides for 4 h. The culture media were collected, and the cell pellet was lysed with 10 volumes of cell lysis buffer (50 mM NaCl, 0.5% Triton X-100, and 20 mM Tris-HCl, pH 7.4). The lysate, and the apical and basolateral media were each mixed with scintillation fluid to analyze radioactivity. Cholesterol efflux to the apical or basolateral compartment was expressed as the percentage of radioactivity in the apical or basolateral medium compared to the total radioactivity (cells plus media), as described previously [17].
2.5. Western Blot Analysis
MAECs were lysed using M-PER mammalian protein extraction reagent. Cell lysates were resolved on 10% SDS-PAGE gels. Proteins were transferred to a PVDF membrane. After blocking with 3% fat-free milk, the membranes were incubated sequentially with primary and second antibodies. The antibodies against ABCA1 (sc-20794), ABCG1 (sc-20795), SR-B1 (sc-67099), Akt (sc8312) and phosphorylated Akt (sc7985-R) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies against phosphorylated PI3K (4228S) and PI3K (4292) were purchased from Cell Signaling (Billerica, MA). Immunoreactive bands were visualized using ECL-plus chemiluminescence reagent (GE Healthcare–Amersham) and analyzed with a GS-700 Imaging Densitometer (Bio-Rad) [18].
2.6. Statistical Analysis
Data are reported as the mean ± SEM. Differences among control and treatment groups were analyzed by Student’s unpaired t-test (for two groups) and one-way or multiple factor analysis of variance (for more than two groups) followed by Tukey’s post-hoc test. Statistical significance was considered when P was less than 0.05. VassarStats (vassarstats.net) software was used for statistical analysis.
3. RESULTS
3.1. HDL and apoAI enhance cholesterol transport across MAECs
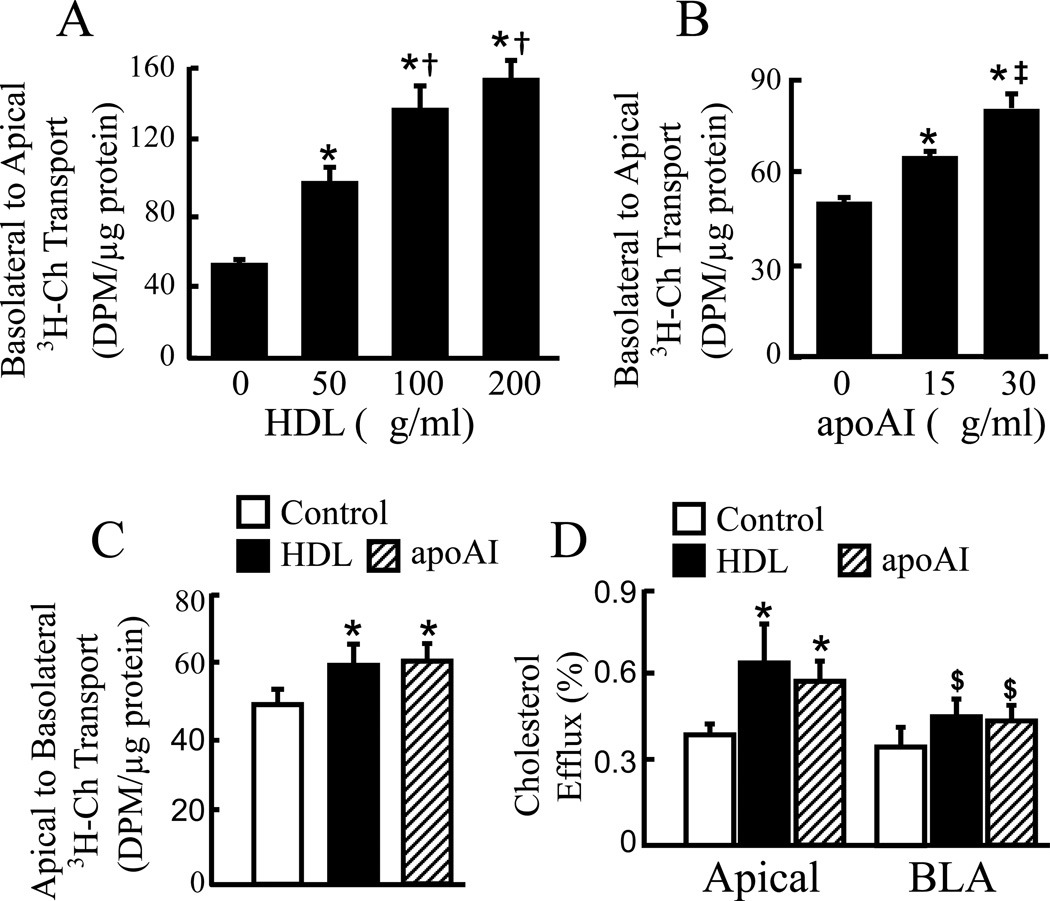
Transport of cholesterol across a MAEC monolayer was studied in a culture system mimicking the in vivo environment surrounding the vascular endothelial cells. Specifically, the apical side was incubated with 10% FBS, while the basolateral side was incubated with protein-poor medium. The data in Fig. 1A–B show that cholesterol can be transported across the endothelial cell monolayer from the basolateral to the apical compartment. Administration of HDL or apoAI into the apical medium induced a dose-dependent increase in transendothelial cholesterol transport. Specifically, the radioactivity in the apical medium was ~93, 173 and 209% higher in cells treated with 50, 100 and 200 µg/ml HDL than in the untreated control cells, respectively (Fig. 1A). Administration of 15 and 30 µg/ml apoAI to the apical medium also induced ~31 and 72% increase in apical radioactivity (Fig. 1B). Further, data in Fig. 1C show that cholesterol also can be transported from the apical to the basolateral compartment. Administration of 50 µg/ml HDL or 20 µg/ml apoAI to the basolateral medium enhanced the apical-basolateral transcellular cholesterol transport by ~21 and 24%, respectively. The rates of HDL- and apoAI-induced apical-basolateral cholesterol transport was lower than the basolateral-apical transport.
Fig. 1.
HDL and apoAI enhance cholesterol efflux and transendothelial cholesterol transport. (A–B) MAECs grown in transwell inserts were incubated for 18 h with [3H]-cholesterol (3H-Ch) liposome solution in the basolateral compartment, and with the indicated concentrations of HDL (A) and apoAI (B) in the apical compartment. The radioactivity in the apical medium was counted for determination of basolateral-apical transcellular cholesterol transport. (C) MAECs grown in transwell inserts were incubated for 18 h with 3H-Ch liposome solution in the apical compartment, and with 50 µg/ml HDL, 20 µg/ml apoAI or control medium in the basolateral compartment. The radioactivity in the basolateral medium was counted for determination of apical-basolateral transcellular cholesterol transport. (D) MAECs grown in transwell inserts were labeled with 3H-Ch, and then incubated with 50 µg/ml HDL, 20 µg/ml apoAI or control medium in both apical and basolateral (BLA) compartments. After a 4 h incubation, the rate of cholesterol efflux into the apical or BLA medium was determined, respectively, by counting the radioactivity in these media. Values represent the mean ± SEM of 4–5 independent experiments. * P<0.05 vs. cells without HDL or apoAI treatment (control), † P< 0.05 vs. 50 µg/ml HDL, ‡ P<0.05 vs. 15 µg/ml apoAI, and $ P< 0.05 vs. HDL or apoAI treatment in the apical compartment.
Transcellular passage of cholesterol includes at least two major steps, i.e., cholesterol influx and efflux. Data in Fig. 1D show that MAECs were able to efflux cholesterol to the apical and basolateral sides by both HDL/apoAI-dependent and -independent pathways. The rates of HDL- and apoAI-induced cholesterol efflux to the basolateral side was lower than that to the apical side. Specifically, ~0.63, 0.57 and 0.39% of the cell-incorporated radioactive cholesterol was exported into the apical medium within 4 h in MAECs treated with HDL, apoAI and control medium, respectively. Namely, treatment of MAECs with 50 µg/ml HDL or 20 µg/ml apoAI induced an ~1.5 fold increase in efflux of cholesterol to the apical medium. In contrast, the same dose of HDL or apoAI did not significantly increase cholesterol efflux to the basolateral medium.
3.2. ABCG1, ABCA1 and SR-B1 contributes to transendothelial cholesterol transport
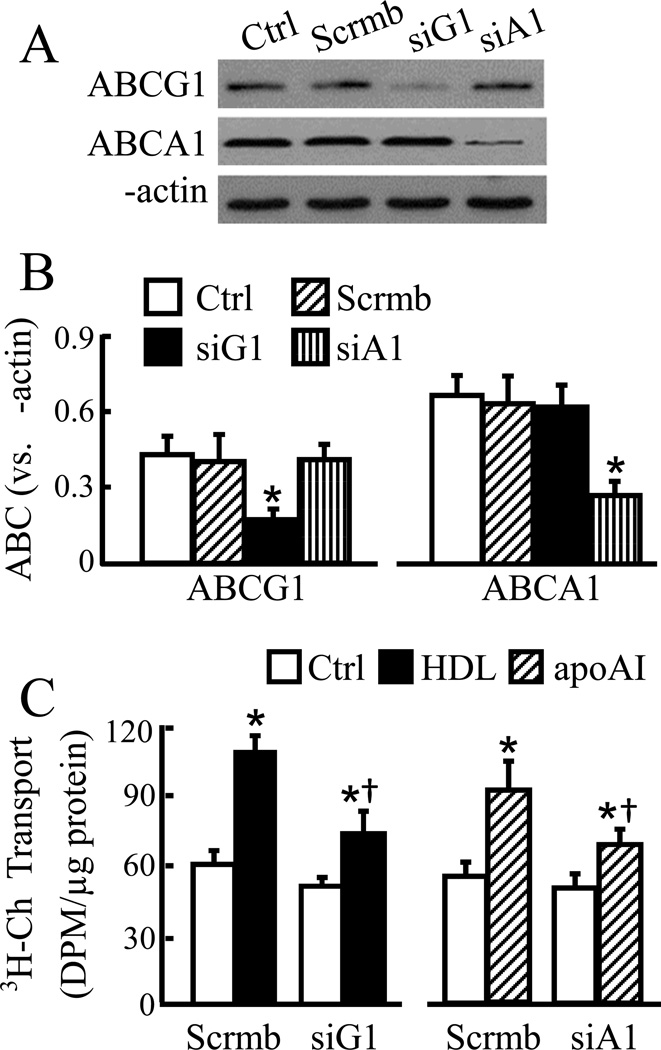
ABCA1, ABCG1 and SR-B1 have been implicated in endothelial cholesterol efflux [9; 13]. Here we studied the effect of ABCA1, ABCG1 and SR-B1 underexpression on apoAI- and HDL-induced transendothelial cholesterol transport. Figs. 2A–B illustrates that transfection of MAECs with scrambled siRNA did not significantly affect the protein level of ABCG1 and ABCA1. In contrast, transfection with siRNAs specifically targeting ABCG1 and AGCA1 reduced the protein level of these genes by ~57 and 60%, respectively. These levels of ABCG1 and ABCA1 underexpression diminished HDL- and apoAI-induced transcellular cholesterol transport in MAECs (Fig. 2C). Specifically, treatment of scrambled siRNA-transfected cells with 50 µg/ml HDL or 20 µg/ml apoAI increased transendothelial cholesterol transport by ~80 and 67%, respectively (Fig. 2C). These agree with the results obtained from cells without siRNA transfection (Fig. 1A–B). The data in Fig. 2C also show that the levels of HDL- and apoAI-increased transcellular cholesterol transport in cells transfected with ABCG1 and ABCA1 siRNAs were ~54 and 50% less, respectively, than in those transfected with scrambled siRNA.
Fig. 2.
Knockdown of ABCG1 or ABCA1 diminishes transendothelial cholesterol transport. (A–B) MAECs were transfected with scrambled (scrmb), ABCG1 (siG1) or ABCA1 (siA1) siRNAs, or untransfected with siRNA as a control (ctrl). The protein levels of ABCG1 and ABCA1 were determined by western blot analysis and expressed relative to β-actin. (C) Cells transfected with siRNAs were incubated for 18 h with [3H]-cholesterol (3H-Ch) liposome solution in the basolateral compartment and with 50 µg/ml HDL, 20 µg/ml apoAI or culture medium alone as a control (Ctrl) in the apical compartment. Transendothelial cholesterol transport was determined by the radioactivity in the apical medium. Data represent the mean ± SEM of 4–5 independent experiments. * P<0.05 vs. control; and † P<0.05 vs. cells transfected scrmb siRNA plus treated with HDL or apoAI.
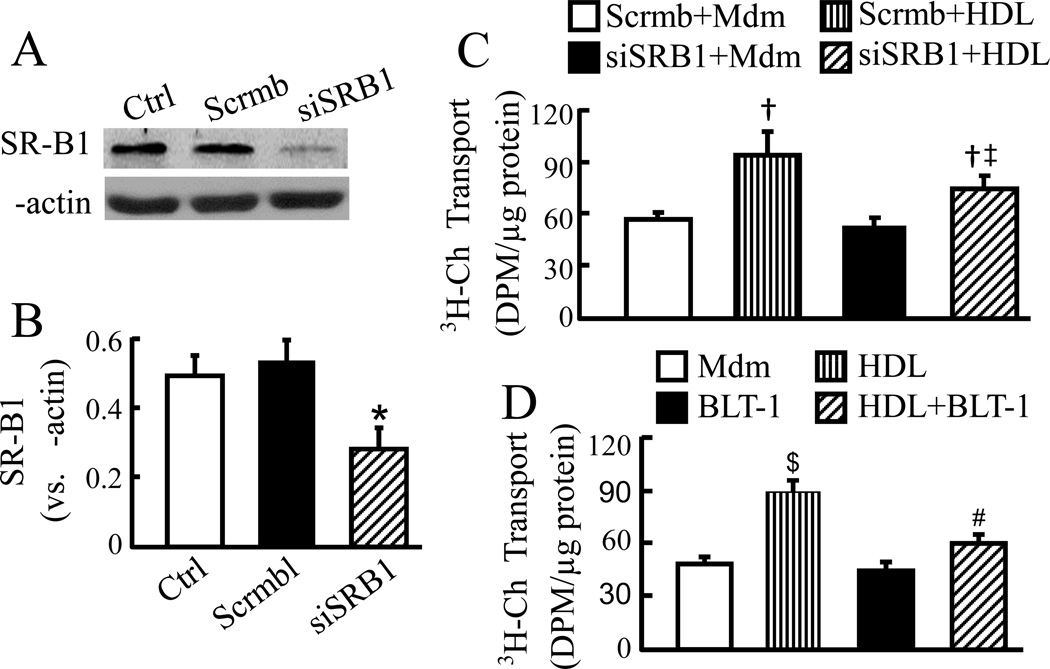
Fig. 3A–B shows ~47% reduction in SR-B1 protein in cells transfected with SR-B1 siRNA compared to the cells transfected with scrambled control siRNA. This level of SR-B1 underexpression diminished HDL-induced transcellular cholesterol transport by ~41% (Fig. 3C). The regulatory role of SR-B1 in transendothelial cholesterol transport was confirmed by inhibition of SR-B1. As the data in Fig. 3D show, the transcellular cholesterol transport was ~33% less in MAECs treated with both HDL and BLT-1 than in those only treated with HDL.
Fig. 3.
Knockdown or inhibition of SR-B1 diminishes transendothelial cholesterol transport. (A–B) MAECs were transfected with scrambled siRNA (Scrmb) or siRNAs specific for SR-B1 (siSRB1). Cells without siRNA transfection were used as a control (Ctrl). The protein level of SR-B1 was determined by western blot analysis and expressed relative to β-actin. (C–D) The siRNA-transfected (C) and untransfected (D) MAECs were incubated for 18 h with [3H]-cholesterol (3H-Ch) liposome solution in the basolateral compartment, and with culture medium alone (Mdm) or 50 µg/ml HDL ± 1 µM BLT-1 in the apical compartment. Transcellular cholesterol transport was determined by the radioactivity in the apical medium. Data represent the mean ± SEM of 4–5 independent experiments. * P<0.05 vs. control; † P<0.05 vs. cells transfected with same siRNAs and untreated with HDL; ‡ P<0.05 vs. cells transfected with scrambled siRNA plus treated with HDL; $ P<0.05 vs. cells treated with medium alone; and # P<0.05 vs. cells treated with HDL or BLT-1 alone.
The data in Figs. 2 and 3 also show that in the absence of apoAI or HDL treatment the level of transcellular cholesterol transport in MAECs transfected with ABCA1, ABCG1 or SR-B1 siRNAs is, respectively, comparable to that in cells transfected with scrambled siRNA. These results suggest that ABCA1, ABCG1 and SR-B1 do not affect the basal transcellular cholesterol transport in MAECs.
3.3. Induction of PI3K and Akt phosphorylation is not a mechanism for HDL-induced transendothelial cholesterol transport
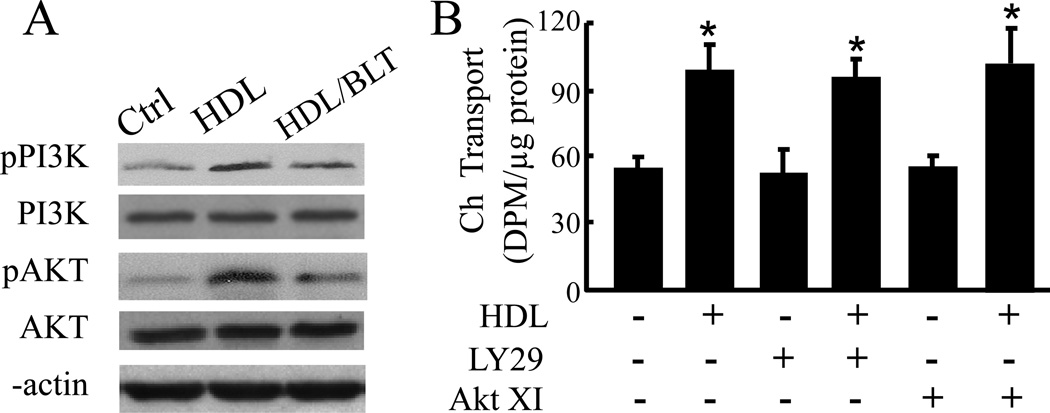
Besides functioning as a cholesterol transporter, SR-B1 also activates a plethora of signaling molecules, including PI3K and Akt [19]. To explore the mechanism responsible for HDL-induced transcellular cholesterol transport, we determined the impact of HDL and SR-B1 inhibitor BLT-1 on PI3K and Akt expression and phosphorylation, and the effect of inhibition of PI3K and Akt on HDL-induced transcellular cholesterol transport in MAECs. As shown in Fig. 4A, HDL treatment did not alter the levels of total PI3K and Akt proteins, but enhanced the phosphorylation of these two proteins. Treatment of MAECs with BLT-1 reduced HDL-induced PI3K and Akt phosphorylation. However, inhibition of PI3K by LY294002 or inhibition of Akt by Akt inhibitor XI did not diminish HDL-induced cholesterol transport (Fig. 4B).
Fig. 4.
HDL induces transendothelial cholesterol transport via PI3K/Akt-independent mechanisms. (A) MAECs grown in transwell inserts were treated with 50 µg/ml HDL ± 1 µM BLT-1 M or medium alone as a control (ctrl) in the apical compartment for 2 h. The protein levels of total and phosphorylated PI3K and Akt were determined by western blot analysis. (B) MAECs were incubated for 18 h with [3H]-cholesterol (3H-Ch) liposome solution in the basolateral compartment, and with culture medium alone or 50 µg/ml HDL ± 20 µM LY294002 (LY29) or 600 nM Akt inhibitor XI (XI) in the apical compartment. Transendothelial cholesterol transport was determined by the radioactivity in the apical medium. Values represent the mean ± SEM of 5 independent experiments. * P<0.05 vs. control or cells treated with LY294002 or Akt inhibitor XI alone.
4. DISCUSSION
The current report clearly demonstrated a bidirectional cholesterol transport across endothelial cells. Namely, cholesterol unassociated with lipoproteins can be transferred by endothelial cells from the basolateral to the apical compartment or from the apical to the basolateral compartment. If the bidirectional transendothelial cholesterol transport also occurs in the arterial wall, then the lipoprotein-unassociated cholesterol could be transported between the circulating blood and subendothelial space. Transport of lipoprotein-unassociated cholesterol from the bloodstream to the subendothelial space might be negligible, as cholesterol in the blood is associated with various lipoproteins and transported to the arterial wall with lipoprotein particles. In contrast, cholesterol accumulation in the subendothelial extracellular space is largely present in lipid vesicles [1; 2], which might be a product of LDL that has been taken up by cells, processed and re-secreted in forms of liposomes [20]. Thus, transport of cholesterol from the subendothelial space to the circulating blood could be a mechanism for endothelial cells to protect the arterial wall from cholesterol accumulation and inhibit the onset and development of atherosclerosis.
Transport of cholesterol from the subendothelial space to circulating blood (i.e., from the basolateral to the apical compartment) is an integrated process, including at least two major steps. i.e., taking up cholesterol from the basolateral compartment and exporting internalized cholesterol to the apical compartment. Data from the current report showed that administration of HDL or apoAI to the apical compartment enhanced the basolateral-apical transendothelial cholesterol transport. This report has not investigated the mechanism how endothelial cells take up cholesterol from the basolateral compartment. However, our data demonstrated that apoAI and HDL enhanced endothelial cholesterol efflux. The mechanism underlying the regulation of apoAI and HDL on cholesterol efflux has been extensively studied in various cell types. It has been suggested that apoAI enhances cholesterol efflux by interaction with ABCA1, while HDL does this by interaction with SR-B1 and ABCG1 [10]. Data from this report demonstrated that knockdown of ABCA1 diminished apoAI-mediated transcellular cholesterol transport in MAECs, while knockdown of ABCG1 or SR-B1, or inhibition of SR-B1 attenuated HDL-mediated transendothelial cholesterol transport. These results suggest that export of cholesterol by ABCA1, ABCG1 and SR-B1 is a step in apoAI- and HDL-mediated transcellular cholesterol transport in MAECs.
It has been suggested that activation of SR-B1 by HDL triggers PI3K/Akt signaling, which in turn regulates endothelial functions [21]. Data from the current report demonstrated that treatment of MAECs with HDL augmented PI3K and Akt phosphorylation, and that inhibition of SR-B1 attenuated HDL-induced PI3K and Akt phosphorylation in these cells. However, inhibition of either PI3K or Akt did not reduce HDL-induced transendothelial cholesterol transport. These observations suggest that HDL activates PI3K and Akt through a mechanism involving SR-B1. However, both PI3K and Akt are not involved in the pathway through which SR-B1 regulates transcellular cholesterol transport in endothelial cells. It has been reported that interaction of HDL with SR-B1 is able to regulate other signaling molecules, such as inhibiting the protein complex of phosphatidic acid phosphatase type 2A (PPAP2A)/hematopoietic tyrosine phosphatase (HePTP), and activating the multivalent PDZ (PSD-95, Drosophila discs-large protein, Zonula occludens protein 1) domain-containing protein (PDK1), the Gi-coupled sphingosine 1-phosphate (S1P) receptor-3, and extracellular signal-regulated kinases (ERK ½) [21]. Further studies are required in order to understand whether these signaling molecules regulate HDL-induced transendothelial cholesterol transport.
In summary, the current report demonstrated that cultured MAECs are able to transport cholesterol from the basolateral to the apical compartment. HDL and apoAI enhance this transendothelial cholesterol transport. Knockdown of ABCAI, ABCG1 or SR-B1, or inhibition of SR-B1 diminishes apoAI- and HDL-mediated cholesterol transport. These results suggest that cholesterol efflux mediated by ABCA1, ABCG1 and SR-B1 is a step in the path that endothelial cells transport cholesterol from the basolateral to the apical compartment. Further studies are needed to assess the mechanism by which endothelial cells take up cholesterol from the basolateral compartment. Considering that there exist cholesterol-rich liposomes in the subendothelial extracellular space [1; 2], increase in transendothelial transport of cholesterol from the subendothelial intima to the circulating blood could be a mechanism by which HDL promotes cholesterol removal from the arterial wall.
Highlights.
Cholesterol can be transported across the endothelial cell monolayer.
HDL and apoE enhance transendothelial cholesterol transport.
ABCA1, ABCG1 and SR-B1 contribute to apoAI- and HDL-mediated transendothelial cholesterol transport.
Acknowledgments
This study was supported by NIH grants: SC1HL101431, G12 MD007586, the Vanderbilt CTSA grant UL1 TR000445 from NCATS/NIH, and Meharry MeTRC grant U54MD0007593.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts to disclose.
References
- 1.Chao FF, Amende LM, Blanchette-Mackie EJ, Skarlatos SI, Gamble W, Resau JH, Mergner WT, Kruth HS. Unesterified cholesterol-rich lipid particles in atherosclerotic lesions of human and rabbit aortas. Am.J.Pathol. 1988;131:73–83. [PMC free article] [PubMed] [Google Scholar]
- 2.Chao FF, Blanchette-Mackie EJ, Dickens BF, Gamble W, Kruth HS. Development of unesterified cholesterol-rich lipid particles in atherosclerotic lesions of WHHL and cholesterol-fed NZW rabbits. J.Lipid Res. 1994;35:71–83. [PubMed] [Google Scholar]
- 3.Cucuianu M, Coca M, Hancu N. Reverse cholesterol transport and atherosclerosis. A mini review. Rom.J.Intern.Med. 2007;45:17–27. [PubMed] [Google Scholar]
- 4.Rohrer L, Ohnsorg PM, Lehner M, Landolt F, Rinninger F, von EA. High-density lipoprotein transport through aortic endothelial cells involves scavenger receptor BI and ATP-binding cassette transporter G1. Circ.Res. 2009;104:1142–1150. doi: 10.1161/CIRCRESAHA.108.190587. [DOI] [PubMed] [Google Scholar]
- 5.DiDonato JA, Huang Y, Aulak KS, Even-Or O, Gerstenecker G, Gogonea V, Wu Y, Fox PL, Tang WH, Plow EF, Smith JD, Fisher EA, Hazen SL. Function and distribution of apolipoprotein A1 in the artery wall are markedly distinct from those in plasma. Circulation. 2013;128:1644–1655. doi: 10.1161/CIRCULATIONAHA.113.002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, Kreisel D, Swartz MA, Sorci-Thomas MG, Randolph GJ. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J.Clin.Invest. 2013;123:1571–1579. doi: 10.1172/JCI63685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kholova I, Dragneva G, Cermakova P, Laidinen S, Kaskenpaa N, Hazes T, Cermakova E, Steiner I, Yla-Herttuala S. Lymphatic vasculature is increased in heart valves, ischaemic and inflamed hearts and in cholesterol-rich and calcified atherosclerotic lesions. Eur.J.Clin.Invest. 2011;41:487–497. doi: 10.1111/j.1365-2362.2010.02431.x. [DOI] [PubMed] [Google Scholar]
- 8.O'Connell BJ, Denis M, Genest J. Cellular physiology of cholesterol efflux in vascular endothelial cells. Circulation. 2004;110:2881–2888. doi: 10.1161/01.CIR.0000146333.20727.2B. [DOI] [PubMed] [Google Scholar]
- 9.Terasaka N, Yu S, Yvan-Charvet L, Wang N, Mzhavia N, Langlois R, Pagler T, Li R, Welch CL, Goldberg IJ, Tall AR. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J.Clin.Invest. 2008;118:3701–3713. doi: 10.1172/JCI35470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J.Intern.Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 11.Vaisman BL, Demosky SJ, Stonik JA, Ghias M, Knapper CL, Sampson ML, Dai C, Levine SJ, Remaley AT. Endothelial expression of human ABCA1 in mice increases plasma HDL cholesterol and reduces diet-induced atherosclerosis. J.Lipid Res. 2011 doi: 10.1194/jlr.M018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao H, Langmann T, Schmitz G, Zhu Y. Native LDL upregulation of ATP-binding cassette transporter-1 in human vascular endothelial cells. Arterioscler.Thromb.Vasc.Biol. 2002;22:127–132. doi: 10.1161/hq1201.101772. [DOI] [PubMed] [Google Scholar]
- 13.Van EM, Twisk J, Hoekstra M, Van Rij BT, Van der Lans CA, Bos IS, Kruijt JK, Kuipers F, Van Berkel TJ. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J.Biol.Chem. 2003;278:23699–23705. doi: 10.1074/jbc.M211233200. [DOI] [PubMed] [Google Scholar]
- 14.Iqbal J, Anwar K, Hussain MM. Multiple, independently regulated pathways of cholesterol transport across the intestinal epithelial cells. J.Biol.Chem. 2003;278:31610–31620. doi: 10.1074/jbc.M301177200. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Shi MJ, Richardson A, Vijg J, Guo ZM. Attenuation of leukocyte-endothelium interaction by antioxidant enzymes. Free Radic.Biol.Med. 2003;35:266–276. doi: 10.1016/s0891-5849(03)00277-6. [DOI] [PubMed] [Google Scholar]
- 16.Lin X, Yang H, Zhang H, Zhou L, Guo Z. A novel transcription mechanism activated by ethanol: induction of Slc7a11 gene expression via inhibition of the DNA-binding activity of transcriptional repressor octamer-binding transcription factor 1 (OCT-1) J.Biol.Chem. 2013;288:14815–14823. doi: 10.1074/jbc.M113.466565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okoro EU, Zhao Y, Guo Z, Zhou L, Lin X, Yang H. Apolipoprotein E4 is deficient in inducing macrophage ABCA1 expression and stimulating the Sp1 signaling pathway. PLoS.One. 2012;7:e44430. doi: 10.1371/journal.pone.0044430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Yang H, Ramesh A, Roberts LJ, Zhou L, Lin X, Zhao Y, Guo Z. Overexpression of Cu/Zn-superoxide dismutase and/or catalase accelerates benzo(a)pyrene detoxification by upregulation of the aryl hydrocarbon receptor in mouse endothelial cells. Free Radic.Biol.Med. 2009;47:1221–1229. doi: 10.1016/j.freeradbiomed.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang QH, Zu XY, Cao RX, Liu JH, Mo ZC, Zeng Y, Li YB, Xiong SL, Liu X, Liao DF, Yi GH. An involvement of SR-B1 mediated PI3K-Akt-eNOS signaling in HDLinduced cyclooxygenase 2 expression and prostacyclin production in endothelial cells. Biochem.Biophys.Res.Commun. 2012;420:17–23. doi: 10.1016/j.bbrc.2012.02.103. [DOI] [PubMed] [Google Scholar]
- 20.Ong DS, Anzinger JJ, Leyva FJ, Rubin N, Addadi L, Kruth HS. Extracellular cholesterol-rich microdomains generated by human macrophages and their potential function in reverse cholesterol transport. J.Lipid Res. 2010;51:2303–2313. doi: 10.1194/jlr.M005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Jarallah A, Trigatti BL. A role for the scavenger receptor, class B type I in high density lipoprotein dependent activation of cellular signaling pathways. Biochim.Biophys.Acta. 2010;1801:1239–1248. doi: 10.1016/j.bbalip.2010.08.006. [DOI] [PubMed] [Google Scholar]