Abstract
Objective
To assess improvements in sound source localization and speech understanding in complex listening environments following unilateral cochlear implantation for single-sided deafness (SSD).
Study Design
Non-randomized, open, prospective case series
Setting
Tertiary referral center
Patients
Nine subjects with a unilateral cochlear implant (CI) for SSD (SSD-CI) were tested. Reference groups for the task of sound source localization included young (n=45) and older (n=12) normal hearing (NH) subjects and 27 bilateral CI (BCI) subjects.
Intervention
Unilateral cochlear implantation
Main outcome measures
Sound source localization was tested with 13 loudspeakers in a 180 arc in front of the subject. Speech understanding was tested with the subject seated in an 8-loudspeaker sound system arrayed in a 360-degree pattern. Directionally appropriate noise, originally recorded in a restaurant, was played from each loudspeaker. Speech understanding in noise was tested using the Azbio sentence test and sound source localization quantified using root mean square error.
Results
All CI subjects showed poorer-than-normal sound source localization. SSD-CI subjects showed a bimodal distribution of scores - six subjects had scores near the mean of those obtained by BCI subjects, while three had scores just outside the 95th percentile of NH listeners. Speech understanding improved significantly in the restaurant environment when the signal was presented to the side of the CI.
Conclusions
Cochlear implantation for SSD can offer improved speech understanding in complex listening environments and improved sound source localization in both children and adults. On tasks of sound source localization, SSD-CI patients typically perform as well as BCI patients and, in some cases, achieve scores at the upper boundary of normal performance.
INTRODUCTION
In one of the newest applications of cochlear implants, single-sided deaf (SSD) patients, i.e., individuals with one normal hearing ear and one deafened ear, have been fit with a cochlear implant (CI). Following implantation, SSD-CI patients experience a reduction in tinnitus strength, a large improvement in sound source localization and, in some test environments, an improvement in speech understanding (1–6). These improvements, in combination with a greatly expanded sense of auditory space, underlie an improved health-related quality of life (1,7,8).
In a previous paper we described the results of an experiment using a small sample (n=4) in which we probed the information that underlies sound source localization by SSD-CI patients (9). Using high- and low-pass noise bands to restrict the patients’ access to interaural level difference (ILD) cues and to interaural time difference (ITD) cues, we inferred that sound source localization in SSD-CI patients is based primarily on ILD cues. This is a reasonable outcome given that fine temporal information is not well transmitted by cochlear implants (10).
We also reported that the sound source localization performance of SSD-CI patients, while poorer than normal, was superior to that of bimodal CI patients, i.e., patients with a CI in one ear and a traditional hearing aid in the contralateral ear with low-frequency (under 500 Hz) residual hearing. We rationalized this outcome by noting that bimodal patients have relatively good access to timing information from the ear with low-frequency acoustic hearing and have relatively good access to signal level information from the ear fit with a CI. Neither timing nor level information is well represented at both ears. For that reason, sound source localization is very poor.
Cochlear implant signal processing severely compresses signal level information due to the automatic gain control (AGC) function at the front end of the signal processing chain and the logarithmic compression of acoustic signals into the electric dynamic range at the back end (10). For bilateral cochlear implant (BCI) patients, this signal level compression should be reasonably symmetrical between ears given similar settings of the independent signal processors for each ear. However, for SSD-CI patients the normal hearing ear will experience relatively large signal levels while the CI ear will experience much reduced signal levels. The magnitude of the difference is shown in the following example [taken from Dorman et al. (in press)]: for normal hearing listeners the ILD at 3 kHz for a sound source at 45 degrees azimuth is approximately 10 dB; at 15 degrees azimuth, the ILD is approximately 3dB. Following CI signal processing, at 45 degrees azimuth, the ILD is 1.6 dB and at 15 degrees it is 0.4 dB (9,11). Thus, SSD-CI patients should experience a distorted representation of signal level as a function of signal azimuth when listening with one normal hearing ear and one deaf ear fitted with a CI. Based on the peripheral representation of signal amplitude, we should expect different levels of sound source localization for BCI and SSD-CI patients.
As noted above, SSD-CI patients have been found to have improved speech understanding but the magnitude of the improvement is critically contingent on the test environment. For example, Arndt et al. reported no benefit in speech understanding in the normal-hearing ear plus CI condition vs. the normal-hearing ear alone condition when both the signal and the noise were presented from a single speaker at 0 degrees azimuth, i.e., in a standard audiometric test environment. However, when the signal was at 45 degrees azimuth on the side of the CI and the noise was at 45 degrees azimuth on the side of the normal hearing ear, then a large improvement (approximately 28 percentage points) was observed in the normal-hearing ear plus CI condition vs. the normal-hearing ear alone condition (1).
In this paper we compare the sound source localization performance of SSD-CI patients to that of BCI patients. The relative performance of the SSD-CI and BCI patients is of interest because both groups rely on ILDs for sound source localization. However, in contradistinction to the BCI group that receives reasonably symmetrical signal levels at the two ears, the SSD-CI group does not. Furthermore, we expand the environments in which SSD-CI patients have been tested and ask whether the benefit to speech understanding extends to a situation in which directionally appropriate restaurant noise is presented from an array of 8 loudspeakers surrounding the listener. In our simulated restaurant test environment, the target sentences were presented on the side of the CI in two conditions, normal-hearing ear only and normal-hearing ear plus CI.
METHODS
Forty-five young NH listeners, 12 older NH listeners, 27 BCI patients, and 9 SSD-CI patients who underwent unilateral CI for SSD from 2011 to 2014 served as subjects. The young NH listeners ranged in age from 21–40 years and were recruited from the undergraduate and graduate student populations at Arizona State University. All had pure tone thresholds of 20 dB or less at octave frequencies from .125 to 4 kHz (12). The older NH listeners ranged in age from 51 to 70 years. All but one had pure tone thresholds of 20 dB or less through 2 kHz. One had a 30 dB threshold at 2 kHz. The BCI sample consisted of 16 subjects fit with Med El implants (as described in Dorman et al., 2014) and 11 subjects fit with Cochlear Corporation devices (11). These patients ranged in age from 32 to 79 years. For the SSD-CI population, all subjects had a pure tone average (0.5, 1, 2, and 4 kHz) in the normal range in the contralateral, normal-hearing ear, but one of the nine subjects (S5) had a mild-to-moderate neurosensory loss at 4, 6, and 8 kHz. The patients ranged in age from 12 to 63 years. All subjects received full consent of the study procedures. This project was reviewed and approved by Arizona State University’s Institutional Review Board.
Surgery was carried out in all cases using a standard transmastoid, facial recess approach. All electrode arrays were implanted either through a round window or cochleostomy approach depending on the intraoperative anatomy encountered.
Sound source localization testing
Test signal
The stimulus was a wideband (WB) noise signal band-pass filtered between 125 and 6000 Hz. The filter roll-offs were 48-dB/octave. The overall signal level was 65 dBA.
Test environment
As described in previous publications (11,12), the stimuli were presented from 11 of 13 loudspeakers arrayed within an arc of 180 degrees on the frontal plane. The speakers were 15 degrees apart. An additional speaker was appended to each end of the 11-loudspeaker array but was not used for signal delivery. The room was lined with acoustic foam. Subjects sat in a chair at a distance of 1.67 m from the loudspeakers. Loudspeakers were located at the height of the listeners’ pinna.
Test conditions
Stimulus presentation was controlled by Matlab. Each stimulus was presented 4 times from each loudspeaker. The presentation level was 65 dBA with a 2-dB rove in level. Level roving was used to reduce any cues that might be provided by the acoustic characteristics of the loudspeakers. Subjects were instructed to look at the midline (center loudspeaker) until a stimulus was presented. They entered the number of the loudspeaker (1–13) on a keypad.
Speech understanding in noise testing
Speech understanding was tested in the R-Space™ test environment (13). The listener was seated in the middle of an 8-loudspeaker sound system arrayed in a 360-degree pattern around the listener. Directionally appropriate noise, originally recorded in a restaurant, was played from each loudspeaker. The test stimuli were sentences from the AzBio test corpus (14). The sentences were always played from the loudspeaker at 0 degrees azimuth to the CI, i.e., from the loudspeaker closest to the CI. There were two test conditions. In one, the CI was not activated. In this condition, the sentences were at 180 degrees to the normal hearing ear. In this condition, for each patient, the signal-to-noise ratio (with the signal level fixed at 60 dB SPL) was adjusted to produce performance between 20 and 60 % correct. This signal-to-noise ratio was then used for the second condition in which the CI was activated (in addition to the normal hearing ear). Two lists of 20 sentences were used in each condition. Performance was scored in terms of percent words correct. Six of the nine listeners tested in the localization experiment were tested in this experiment.
RESULTS
Demographic data for the 9 SSD-CI listeners are shown in Table 1. The mean age of the SSD-CI patients was 43 years (range, 12–63 yrs.). Four of the included subjects were female. The patients had 1–6 years of severe-to-profound hearing loss prior to receiving the CI. The mean duration of CI experience at the time of testing was 8.6 months (range, 2–33 mo.). Eight patients received a Med-El Cochlear Implant System (Innsbruck, Austria) and one received an Advanced Bionics Cochlear Implant System (Valencia, CA, USA). All patients had a full insertion of the electrode array and there were no surgical complications.
Table 1.
Biographical data for SSD-CI patients.
| Age (yr) | Age at profound HL | Etiology | Time since activation (mo) | Implant | Electrode | Speech: CI only | Localization CI + NH ear (RMS error) | |
|---|---|---|---|---|---|---|---|---|
| S1 | 39 | 34 | MD | 2 | Advanced Bionics | Mid-scala | 77% | 38 ° |
| S2 | 38 | 36 | ISSNHL | 9 | Med-El | Flex 28 | 96% | 37 ° |
| S3 | 48 | 42 | ISSNHL | 3 | Med-El | Flex 28 | 76% | 39 ° |
| S4 | 49 | 48 | ISSNHL | 16 | Med-El | Standard | 60% | 11 ° |
| S5 | 63 | 63 | Iatrogenic* | 4 | Med-El | Flex 28 | 53% | 40 ° |
| S6 | 12 | 5 | Idiopathic progressive | 33 | Med-El | Standard | 95% | 14 ° |
| S7 | 39 | 37 | ISSNHL | 6 | Med-El | Flex 24 | DNT | 33 ° |
| S8 | 50 | 45 | ISSNHL | 2.5 | Med-El | Flex 28 | 67% | 41 ° |
| S9 | 49 | 43 | ISSNHL | 2 | Med-El | Flex 28 | DNT | 16 ° |
HL = hearing loss; Speech: CI only = AzBio sentences in quiet. WB = wideband noise; MD = Meniere’s Disease; ISSNHL = idiopathic sudden sensorineural hearing loss;
hearing loss occurred during microvascular decompression for trigeminal neuralgia;
NH = normal hearing; RMS = root mean square
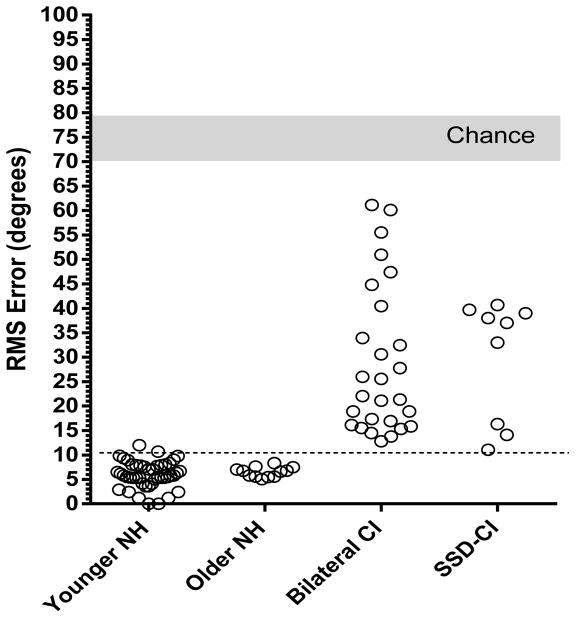
Localization accuracy was calculated in terms of root mean square (RMS) error using the D statistic of Rakerd and Hartman (15). Chance performance, calculated using a Monte Carlo method, was 73.5 degrees (SD, 3.2). Localization accuracy for all listeners is summarized in Figure 1.
Figure 1.
RMS error for sound source localization to a wide band noise stimulus for young NH listeners, older NH listeners, patients fit with bilateral CIs and SSD patients fit with a CI. Each open circle indicates the performance of one listener. The light grey area indicates chance performance. The dotted line indicates the 95th percentile for scores from the young NH sample.
RMS error for the young NH group was 6.0 degrees (SD, 2.7); for the older NH group, 6.5 degrees (SD, 1.0), for the BCI group, 29.0 degrees (SD, 15) and for the SSD-CI group, 30.0 degrees (SD, 12). The distribution of scores for the SSD-CI patients was clearly bimodal with a cluster of six scores between 33–40 degrees RMS error and another cluster of three scores between 11–16 degrees RMS error. There was no correlation between any of the studied demographic variables and performance on the localization testing. There was also no difference in RMS error based on the time between implantation and testing.
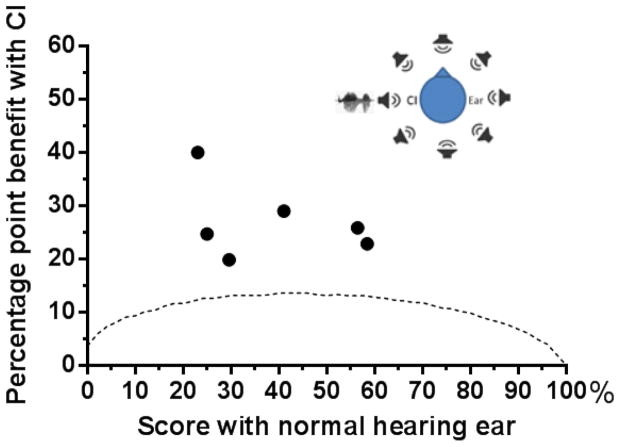
The results for speech understanding in noise in the combined normal-hearing-ear plus CI condition are summarized in Figure 2. All listeners showed a significant benefit in speech understanding, i.e., for each patient scores in the combined condition were higher than the 95 percent critical difference scores for the AzBio sentences (14) in the normal-hearing ear alone condition.
Figure 2.
Percentage point change in performance in the NH ear plus CI condition as a function of the score (percent correct) for the NH ear alone. Each filled circle shows the performance of one SSD-CI patient. The dotted line indicates the 95 percent critical difference scores for the test material. The listening environment is illustrated at top right. Noise was presented from all loudspeakers and speech was presented to the side of the CI.
DISCUSSION
In the introduction we pointed out that the peripheral representation of ILDs should be very different for NH listeners, BCI patients and SSD-CI patients. For any patient with a CI, signal levels at the ear with the CI will be compressed due to CI signal processing. We suppose that for BCI patients the compression will be relatively symmetrical – at least to the degree that the two independent signal processors are set in similar fashion. This symmetry should be lost for SSD-CI patients for whom only one ear receives a compressed signal. As a consequence we speculated that sound source localization based on ILD cues would likely be poorer for SSD-CI patients than for bilateral CI patients.
Localization by normal-hearing listeners and bilateral CI patients
The RMS error for the NH listeners as a whole in this study was 6.1 degrees with a standard deviation of 2.5 degrees. Grantham et al. reported a mean error score for normal-hearing listeners of 6.7 degrees with a standard deviation of 1.1 degrees (16). The mean error score for our sample of BCI patients was 29 degrees with a standard deviation of 15 degrees. Grantham et al. reported a mean score of 31 degrees with a standard deviation of 10 degrees (16). The similarity of our data to that of Grantham et al. suggests that our data for normal-hearing listeners and bilateral CI patients are a reasonable reference for the sound source localization abilities of SSD-CI patients.
Localization accuracy was highly variable across the sample of bilateral CI patients. One account of the variability of scores revolves around deviations from bilateral matching in electrode location (17) and a host of signal processor settings, e.g., (i) AGC settings; (ii) frequency allocation tables; (iii) electrode pitch; (iv) numbers of activated electrodes, (v) electrode dynamic ranges; (vi) output compression settings; and (vii) processor volumes (11,18). It may be the case that the patients with the better localization scores are the ones for whom electrode locations across ears are well matched and the effective signal compression of the two processors is well matched.
Localization by SSD-CI patients
The error scores for the SSD-CI patients were clearly bimodal. Six patients had scores that were toward the upper end of the distribution for bilateral CI patients and three had error scores that were similar to the best scores from patients in the bilateral CI group. Given the different signal levels between ears for the SSD-CI group, the relatively poor scores for six of the patients is not unexpected.
On the other hand, the outcome of three scores equal to that obtained by the best BCI patients and just above the upper end of the distribution of scores for NH listeners is surprising – the more so because of the short interval between device turn-on and testing for two of the three patients. One of these patients was tested at 2 months and obtained an error score of 16 degrees. As we noted in Dorman et al. (in press), the patient with 11 degrees of error when tested in our laboratory at 16 months after device turn-on, had been tested at another laboratory at 1 month post CI hookup and obtained an RMS error score of 13 degrees (9). Thus, one of the critical problems confronting SSD-CI patients in sound source localization, a large asymmetry in signal level at the two ears, can be at least partially resolved by central processing mechanisms very soon after device turn-on. Tavora-Vieira et al., using a virtual loudspeaker array and a high frequency, narrow-band stimulus, also report a small number SSD-CI patients with error scores that are at the upper edge of error scores for normal hearing listeners. The listeners in that study, however, had more experience with their CIs than the patients in our study (6).
Speech understanding by SSD-CI patients
As we noted in the Introduction, one of our aims was to assess the value of a CI for SSD patients when the listening environment simulated a ‘real world’ situation, i.e., listening in a restaurant when the talker was on the side of the CI. In this environment each patient exhibited a large and significant improvement in speech understanding. This outcome documents a real-world environment in which a CI significantly aids a listener who has normal hearing in one ear. Although we did not evaluate alternatives to a CI in our listening environment, e.g., a CROS hearing aid, or a BAHA device, others, have shown much better performance with a CI than with a CROS aid or a BAHA in similar environments (1).
Conclusion
The provision of a CI to the deaf ear of SSD patients allows for significant improvements in sound source localization and speech understanding in complex listening environments. However, there is a significant amount of variance between patients regarding their performance on these tasks, and the variance does not appear to be predicated on any of the studied demographic variables or length of CI usage. Future studies should further attempt to account for this observed variance among individuals undergoing CI for SSD, as well as to optimize CI signal processing to improve performance on these tasks.
Acknowledgments
The research reported here was supported by grants from the NIDCD to MFD and RHG (R01-DC010821) and from the AFOSR to WAY (FA9550-12-1-0312).
References
- 1.Arndt S, Aschendorff A, Laszig R, et al. Comparison of pseudo-binaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2010;32:39–47. doi: 10.1097/MAO.0b013e3181fcf271. [DOI] [PubMed] [Google Scholar]
- 2.Firszt JB, Holden LK, Reeder RM, Waltzman SB, Arndt S. Auditory abilities after cochlear implantation in adults with unilateral deafness: a pilot study. Otol Neurotol. 2012;33(8):1339–46. doi: 10.1097/MAO.0b013e318268d52d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassepass F, Aschendorff A, Wesarg T, et al. Unilateral deafness in children: audiologic and subjective assessment of hearing ability after cochlear implantation. Otol Neurotol. 2013;34:53–60. doi: 10.1097/MAO.0b013e31827850f0. [DOI] [PubMed] [Google Scholar]
- 4.Jacob R, Stelzig Y. The Koblenz experience in treating single-sided deafness with cochlear implants. Audiol Neurotol. 2011;16(suppl 1):6–8. [Google Scholar]
- 5.Nawaz S, McNeill C, Greenberg S. Improving sound localization after cochlear implantation and auditory training for the management of single-sided deafness. Otol Neurotol. 2014;35(2):271–6. doi: 10.1097/MAO.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 6.Tavora-Vieira D, Marino R, Acharya A, Rajan GP. The impact of cochlear implantation on speech understanding, subjective hearing performance and tinnitus perception in patients with unilateral severe to profound hearing loss. Otol Neurotol. 2015;36:430–6. doi: 10.1097/MAO.0000000000000707. [DOI] [PubMed] [Google Scholar]
- 7.Tavora-Vieira D, Marino R, Krishnaswamy J, Kuthbutheen J, Rajan GP. Cochlear implantation for unilateral deafness with and without tinnitus: a case series. Laryngoscope. 2013;123:1251–5. doi: 10.1002/lary.23764. [DOI] [PubMed] [Google Scholar]
- 8.Vermeire K, Van de Heyning P. Binaural hearing after cochlear implantation in subjects with unilateral sensorineural deafness and tinnitus. Audiol Neurotol. 2009;14:163–71. doi: 10.1159/000171478. [DOI] [PubMed] [Google Scholar]
- 9.Dorman MF, Zeitler DM, Cook SJ, et al. Interaural level difference cues (ILDs) determine sound source localization by single-sided deaf patients fit with a cochlear implant. Audiol Neurotol. 2015 doi: 10.1159/000375394. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson B, Dorman MF. The design of cochlear implants. In: Niparko J, editor. Cochlear Implants, Principals and Practices. Philadelphia: Lippincott; 2009. pp. 95–136. [Google Scholar]
- 11.Dorman MF, Loiselle L, Yost WA, et al. Interaural level differences and sound source localization for bilateral cochlear implant patients. Ear Hear. 2014;35(6):633–40. doi: 10.1097/AUD.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yost W, Loiselle L, Dorman M, Brown C, Burns J. Sound source localization of filtered noises by listeners with normal hearing: A statistical analysis. J Acoust Soc Am. 2013;133(5):2876–82. doi: 10.1121/1.4799803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compton-Conley CL, Neuman AC, Killion MC, Levitt H. Performance of directional microphones for hearing aids: real-world versus simulation. J Am Acad Audiol. 2004;15:440–55. doi: 10.3766/jaaa.15.6.5. [DOI] [PubMed] [Google Scholar]
- 14.Spahr AJ, Dorman MF, Litvak LF, et al. Development and validation of the AzBio sentence lists. Ear Hear. 2012;33(1):112–17. doi: 10.1097/AUD.0b013e31822c2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakerd B, Hartmann WM. Localization of sound in rooms, III: Onset and duration effects. J Acoust Soc Am. 1986;80:1695–1706. doi: 10.1121/1.394282. [DOI] [PubMed] [Google Scholar]
- 16.Grantham W, Ashmead D, Ricketts T, Labadie R, Haynes D. Horizontal-plane localization of noise and speech signals by postlingually deafened adults fitted with bilateral cochlear implants. Ear Hear. 2007;28:524–541. doi: 10.1097/AUD.0b013e31806dc21a. [DOI] [PubMed] [Google Scholar]
- 17.Kan A, Litovsky R, Goupell M. Effects of interaural pitch matching and auditory image centering on binaural sensitivity in cochlear implant users. Ear Hear. 2015 doi: 10.1097/AUD.0000000000000135. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Hoesel RJM, Ramsden R, O’Driscoll M. Sound-direction identification, interaural time delay discrimination, and speech intelligibility advantages in noise for a bilateral cochlear implant user. Ear Hear. 2002;23:137–49. doi: 10.1097/00003446-200204000-00006. [DOI] [PubMed] [Google Scholar]