Abstract
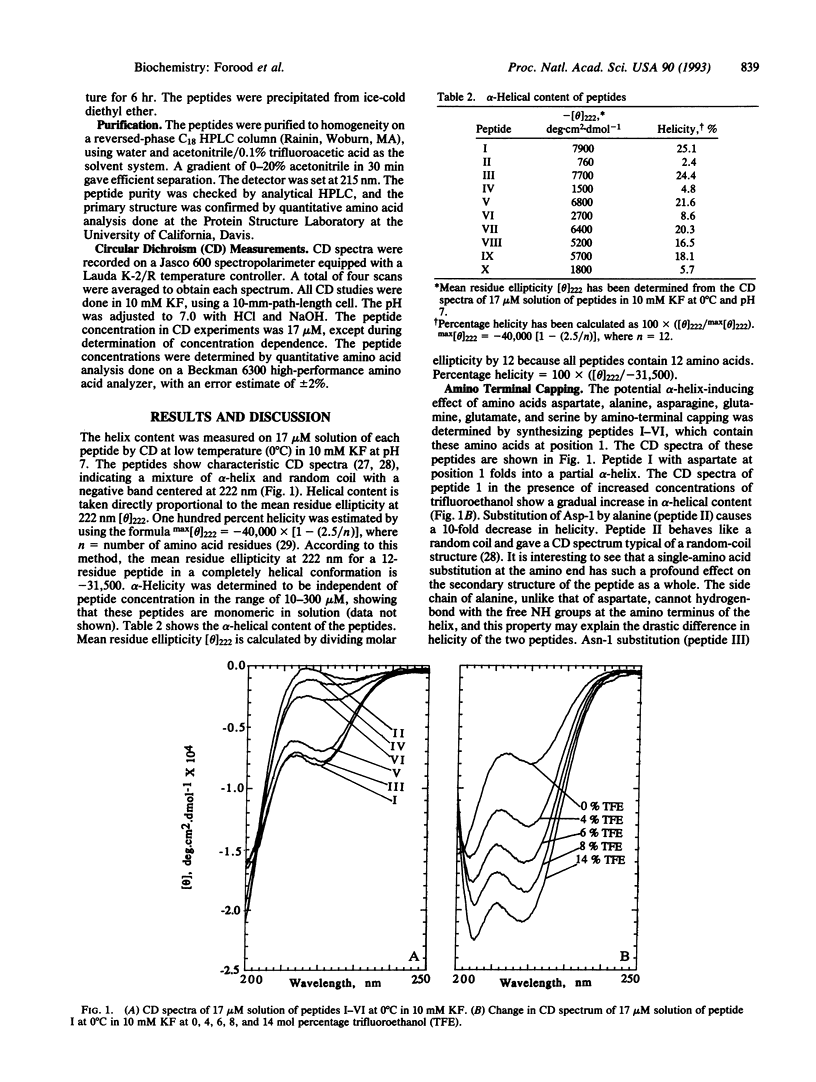
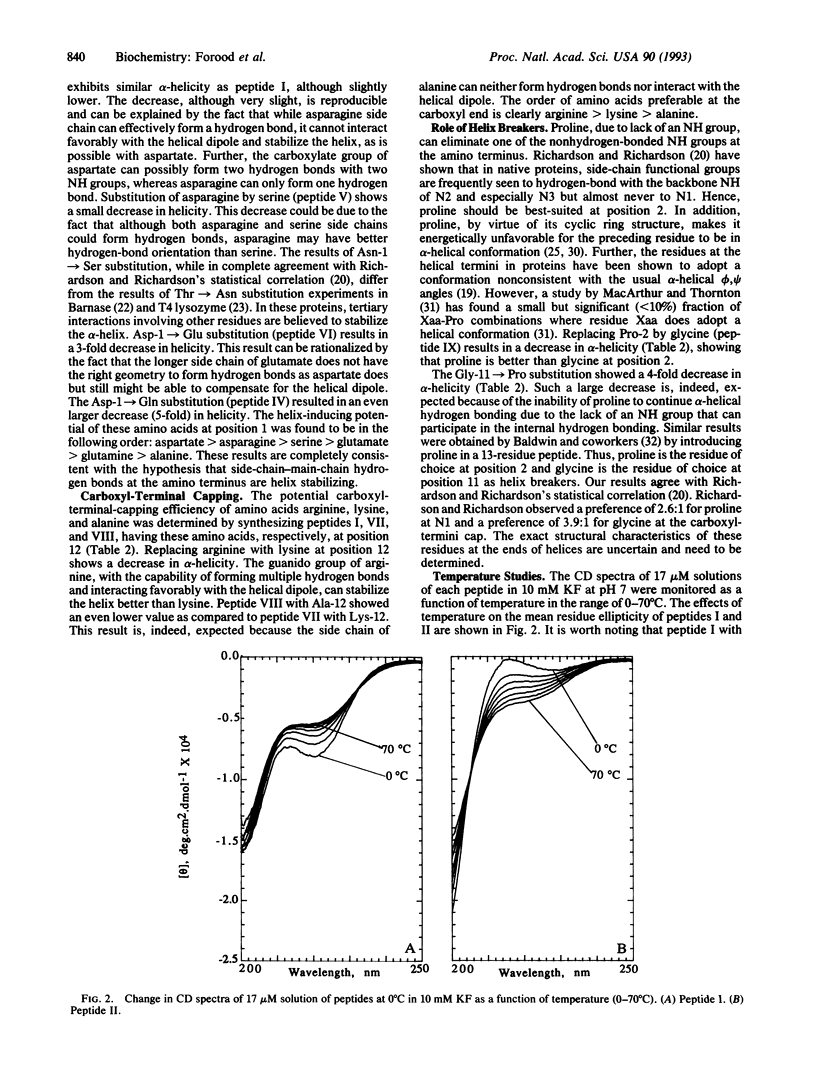
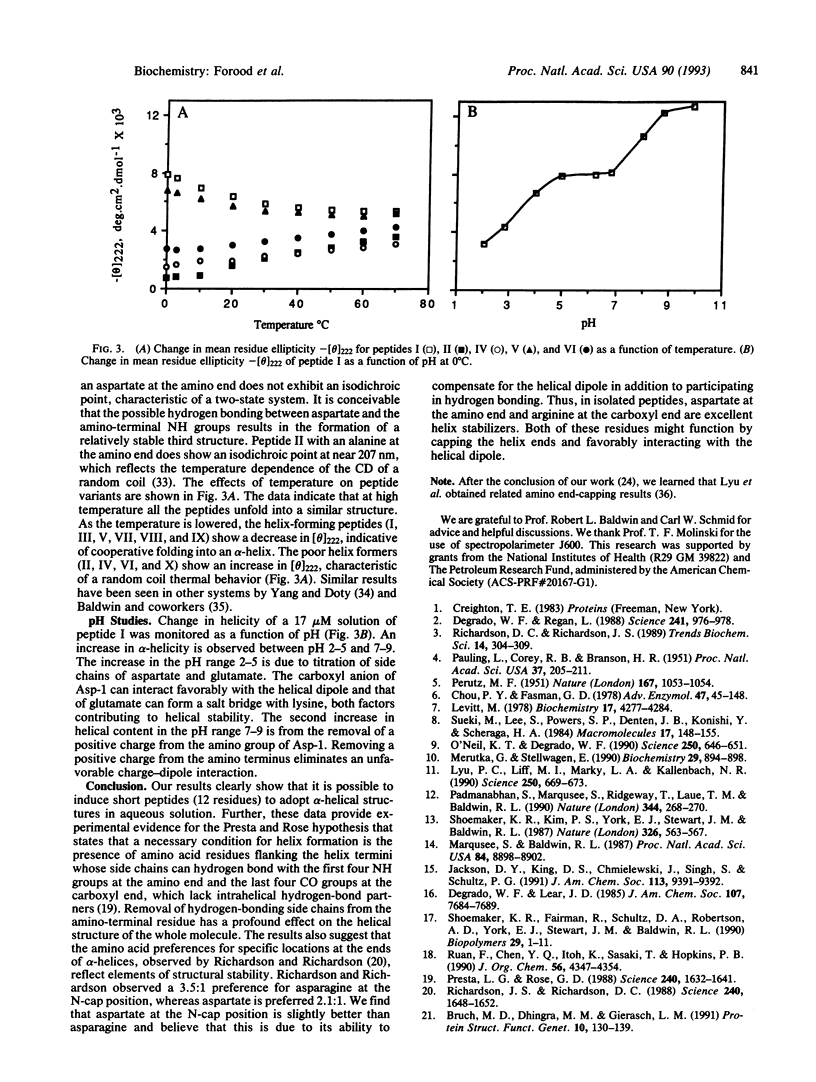
The alpha-helix-stabilizing effect of different amino acid residues at the helical termini of short peptides in aqueous solution has been determined. Several dodecapeptides containing alanine, asparagine, aspartate, glutamine, glutamate, and serine at the amino terminus and arginine, lysine, and alanine at the carboxyl terminus were synthesized, and the alpha-helical content of each peptide was measured by using circular dichroism spectroscopy. The trend in alpha-helix-inducing ability of these amino acids was found to be as follows: aspartate > asparagine > serine > glutamate > glutamine > alanine at the amino terminus and arginine > lysine > alanine at the carboxyl terminus. Our results agree with the Presta and Rose hypothesis [Presta, L. G. & Rose, G. D. (1988) Science 240, 1632-1641] on the role of end capping in helix stabilization.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. A., Becktel W. J., Sauer U., Baase W. A., Matthews B. W. Dissection of helix capping in T4 lysozyme by structural and thermodynamic analysis of six amino acid substitutions at Thr 59. Biochemistry. 1992 Apr 14;31(14):3590–3596. doi: 10.1021/bi00129a006. [DOI] [PubMed] [Google Scholar]
- Brahms S., Brahms J. Determination of protein secondary structure in solution by vacuum ultraviolet circular dichroism. J Mol Biol. 1980 Apr;138(2):149–178. doi: 10.1016/0022-2836(80)90282-x. [DOI] [PubMed] [Google Scholar]
- Bruch M. D., Dhingra M. M., Gierasch L. M. Side chain-backbone hydrogen bonding contributes to helix stability in peptides derived from an alpha-helical region of carboxypeptidase A. Proteins. 1991;10(2):130–139. doi: 10.1002/prot.340100206. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Levitt M. Conformational preferences of amino acids in globular proteins. Biochemistry. 1978 Oct 3;17(20):4277–4285. doi: 10.1021/bi00613a026. [DOI] [PubMed] [Google Scholar]
- Lyu P. C., Liff M. I., Marky L. A., Kallenbach N. R. Side chain contributions to the stability of alpha-helical structure in peptides. Science. 1990 Nov 2;250(4981):669–673. doi: 10.1126/science.2237416. [DOI] [PubMed] [Google Scholar]
- MacArthur M. W., Thornton J. M. Influence of proline residues on protein conformation. J Mol Biol. 1991 Mar 20;218(2):397–412. doi: 10.1016/0022-2836(91)90721-h. [DOI] [PubMed] [Google Scholar]
- Marqusee S., Baldwin R. L. Helix stabilization by Glu-...Lys+ salt bridges in short peptides of de novo design. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8898–8902. doi: 10.1073/pnas.84.24.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqusee S., Robbins V. H., Baldwin R. L. Unusually stable helix formation in short alanine-based peptides. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5286–5290. doi: 10.1073/pnas.86.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merutka G., Stellwagen E. Positional independence and additivity of amino acid replacements on helix stability in monomeric peptides. Biochemistry. 1990 Jan 30;29(4):894–898. doi: 10.1021/bi00456a007. [DOI] [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science. 1990 Nov 2;250(4981):646–651. doi: 10.1126/science.2237415. [DOI] [PubMed] [Google Scholar]
- PAULING L., COREY R. B., BRANSON H. R. The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proc Natl Acad Sci U S A. 1951 Apr;37(4):205–211. doi: 10.1073/pnas.37.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERUTZ M. F. New x-ray evidence on the configuration of polypeptide chains. Nature. 1951 Jun 30;167(4261):1053–1054. doi: 10.1038/1671053a0. [DOI] [PubMed] [Google Scholar]
- Padmanabhan S., Marqusee S., Ridgeway T., Laue T. M., Baldwin R. L. Relative helix-forming tendencies of nonpolar amino acids. Nature. 1990 Mar 15;344(6263):268–270. doi: 10.1038/344268a0. [DOI] [PubMed] [Google Scholar]
- Piela L., Némethy G., Scheraga H. A. Proline-induced constraints in alpha-helices. Biopolymers. 1987 Sep;26(9):1587–1600. doi: 10.1002/bip.360260910. [DOI] [PubMed] [Google Scholar]
- Presta L. G., Rose G. D. Helix signals in proteins. Science. 1988 Jun 17;240(4859):1632–1641. doi: 10.1126/science.2837824. [DOI] [PubMed] [Google Scholar]
- Regan L., DeGrado W. F. Characterization of a helical protein designed from first principles. Science. 1988 Aug 19;241(4868):976–978. doi: 10.1126/science.3043666. [DOI] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C. Amino acid preferences for specific locations at the ends of alpha helices. Science. 1988 Jun 17;240(4859):1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C. The de novo design of protein structures. Trends Biochem Sci. 1989 Jul;14(7):304–309. doi: 10.1016/0968-0004(89)90070-4. [DOI] [PubMed] [Google Scholar]
- Scholtz J. M., Qian H., York E. J., Stewart J. M., Baldwin R. L. Parameters of helix-coil transition theory for alanine-based peptides of varying chain lengths in water. Biopolymers. 1991 Nov;31(13):1463–1470. doi: 10.1002/bip.360311304. [DOI] [PubMed] [Google Scholar]
- Shoemaker K. R., Fairman R., Schultz D. A., Robertson A. D., York E. J., Stewart J. M., Baldwin R. L. Side-chain interactions in the C-peptide helix: Phe 8 ... His 12+. Biopolymers. 1990 Jan;29(1):1–11. doi: 10.1002/bip.360290104. [DOI] [PubMed] [Google Scholar]
- Shoemaker K. R., Kim P. S., York E. J., Stewart J. M., Baldwin R. L. Tests of the helix dipole model for stabilization of alpha-helices. Nature. 1987 Apr 9;326(6113):563–567. doi: 10.1038/326563a0. [DOI] [PubMed] [Google Scholar]
- Strehlow K. G., Robertson A. D., Baldwin R. L. Proline for alanine substitutions in the C-peptide helix of ribonuclease A. Biochemistry. 1991 Jun 11;30(23):5810–5814. doi: 10.1021/bi00237a026. [DOI] [PubMed] [Google Scholar]



