Abstract
Background:
Cumulative data indicate that the endocannabinoid system plays a major role in feeding behavior and energy balance. Genetic silencing of cannabinoid receptor type 1 (CB1) reduces body weight gain, independently of food intake.
Methods:
In this work, we investigated whether the hypothalamic neuropeptide expression pattern supports the absence of the anorexigenic response observed under constitutive CB1 ablation, by using neuronal CB1 conditional null mice (CamK-CB1-KO) and whole body CB1 null mice (CB1-KO).
Results:
Our data showed that both CB1 null models display a marked decrease in proopiomelanocortin (POMC) and cocaine-amphetamine-regulated transcript (CART) expression in the arcuate nucleus of the hypothalamus (ARC).
Conclusions:
This evidence suggests that a lack of hypophagia is associated with the suppression of ARC anorexigenic neuropeptides and that behavioral changes in food intake (or lack thereof) after constitutive CB1 ablation are likely mediated by impaired melanocortin and CART signaling in the hypothalamus.
Keywords: CART, CB1, food intake, hypothalamus, POMC
Introduction
The endocannabinoid system (ECS), consists of endogenous cannabinoids, the enzymatic machinery involved in their synthesis and degradation, and the G-protein-coupled cannabinoid receptors type 1 and 2 (CB1 and CB2; Quarta et al., 2011). This system has emerged as a major regulatory mechanism of several physiological and pathophysiological processes, including feeding behavior and energy balance, where ECS activation promotes energy storage (Bermudez-Silva et al., 2010, 2012; Quarta et al., 2010). Central administration of cannabinoids increases food intake through CB1 activation (Jamshidi and Taylor, 2001; Verty et al., 2005). It is also known that feeding suppression induced by a cannabinoid receptor blockade has a major peripheral component through the involvement of either peripheral nerve terminals, a direct participation of sympathetic nervous system, ghrelin, or leptin (Alen et al., 2013; Silvestri and Di Marzo, 2013). Moreover, rodents with diet-induced or genetic obesity show increased endocannabinoid hypothalamic levels (Quarta et al., 2011). Conversely, genetic or pharmacological CB1 silencing (Verty et al., 2009; Quarta et al., 2010; Rorato et al., 2013) supresses food intake in lean and obese starved animals (Quarta et al., 2011). However, studies have shown that CB1 blockade decreases fat mass and body weight gain independently of changes in food intake, mainly through increased sympathetic outflow, mitochondrial biogenesis, and heat production, as well as fatty acid mobilization and oxidation (Jbilo et al., 2005; Nogueiras et al., 2008; Quarta et al., 2010). As a result, CB1 blockade ameliorates obesity-related metabolic disorders (Cota et al., 2003; Ravinet Trillou et al., 2004; Quarta et al., 2011).
The endocannabinoid system is thought to regulate food intake, essentially by affecting eating motivation through the mesolimbic pathway (Di Marzo et al., 2009) and the hypothalamus (Cota et al., 2003; Verty et al., 2005, 2009; Rorato et al., 2013). Pharmacological modulation of the ECS regulates neuronal activity (in parallel to feeding behavior) in the paraventricular (PVH), ventromedial, and arcuate (ARC) nuclei of the hypothalamus, as well as in the dorsomedial nucleus and lateral hypothalamic area (Verty et al., 2009; Soria-Gomez et al., 2014). Also, the endogenous cannabinoid ligand anandamide (arachidonylethanolamide) increases food intake through CB1 receptor activation when infused into the ventromedial nculei of the hypothalamus (Jamshidi and Taylor, 2001). Within the hypothalamus, the CB1 receptor has been shown to colocalize with corticotropin-releasing hormone (CRH) and cocaine-amphetamine-regulated transcript (CART) neurons in the PVH (Cota et al., 2003), suggesting a direct influence of endocannabinoids on hypothalamic neuropeptide expression. Accordingly, different studies have shown that the decrease in food intake induced by CB1 receptor antagonism is associated with increased CRH (Verty et al., 2009; Rorato et al., 2013), α-melanocyte-stimulating hormone (Verty et al., 2009), and CART (Verty et al., 2009; Rorato et al., 2013) expression, as well as decreased neuropeptide Y (NPY) levels (Verty et al., 2009; Rorato et al., 2013).
Interestingly, it has been demonstrated that chronic pharmacological treatment with CB1 receptor antagonists renders animals tolerant to their anorectic action (Quarta et al., 2010; Rorato et al., 2013). Thus, chronic CB1 receptor antagonism might lead to neuronal and neuropeptide changes different from those observed after acute treatments in rats (Rorato et al., 2013). Accordingly, previous data have shown no changes in feeding behavior in adult conditional mutants lacking CB1 receptor neuronal expression (CamK-CB1-KO) or in total CB1 receptor knockout (CB1-KO) adult mice (Quarta et al., 2010). In contrast, food intake is reduced in young CB1-KO mice (Cota et al., 2003). Here, we analyzed the role of the CB1 receptor on feeding behavior and hypothalamic neuropeptides, using CamK-CB1-KO and CB1-KO mutant adult male mice fed a standard (SD) or high fat diet (HFD). Our data suggest that the lack of decreased food intake in both models, which are in contrast to those observed after CB1 receptor acute antagonism (Rorato et al., 2013), further highlights the relevance of neuronal plasticity in energy homeostasis. These data provide new clues for understanding how the cannabinoid system is involved in the regulation of food intake, which should aid further developments of new CB1 antagonists.
Methods
Animals
We used adult (8 weeks) male CB1-KO mice (Cota et al., 2003; Ravinet Trillou et al., 2004) and CaMK-CB1-KO mice and their wildtype (WT) littermates. In CaMK-CB1-KO mice, the CB1 receptor was deleted in forebrain neurons expressing the Ca2+/calmodulin dependent kinase IIa, while CB1 expression is maintained in cortical GABAergic interneurons and in cerebellar neurons (Marsicano et al., 2003). Mutant animals were in a mixed genetic background, with a predominant C57BL/6N contribution (seven backcrosses). The experiments were performed in agreement with the International Law on Animal Experimentation and were approved by the University of Bologna and USC Local Ethical Committee and the USC Ethical Committee (Project ID 15010/14/006). We used 6–10 animals per group. Animals were fed either a SD containing 12.3 KJ/g (11% fat, 19% protein, 70% carbohydrate) or a HFD having 18.9 KJ/g (40% fat, 15% protein, 45% carbohydrate; both diets from Dottor Piccioni Lab).
Peripheral Treatment with CB1 Antagonist
C57BL6 mice were treated intraperitoneally (i.p.; 4 hours) with either the CB1 antagonist AM281 (Tocris Bioscience; 3mg/Kg) or vehicle (dimethyl sulfoxide) after overnight fasting.
Serum Analyses
Serum leptin concentrations were determined using a Mouse Leptin ELISA kit EZML-82K (Millipore). Ghrelin concentrations were measured using a Rat/Mouse Ghrelin (Total) ELISA kit EZRGRT-91K (Millipore) and peptide YY (PYY3-36) concentrations were determined using a Peptide YY (3-36; Rat, Mouse, Porcine, Canine) EIA Kit EK-059-04 (Phoenix Pharmaceuticals, Inc.).
In Situ Hybridization
Specific oligonucleotides for agouti-related peptide (AgRP), CART, CRH, NPY, and proopiomelanocortin (POMC) detection were used (Supplementary Table S1). These probes were 3’-end labeled with 35S-α-dATP using terminal deoxynucleotidyl transferase (Amersham Biosciences). In situ hybridizations were performed as previously published (Lopez et al., 2008, 2010; Lage et al., 2010; Martinez de Morentin et al., 2014). The slides from all experimental groups from the same experiment (wildtype vs. knockout for each genotype and diet) were exposed to the same autoradiographic film. All sections (16 μm) were scanned and the specific hybridization signal was quantified by densitometry (ImageJ 1.33, National Institutes of Health). The optical density of the hybridization signal was determined and subsequently corrected by the optical density of its adjacent background. We used 6–10 animals/group and 16–20 sections/animal (4–5 slides with four sections/slide).
Statistical Analysis
Data are expressed as mean ± standard error of the mean. Statistical significance was determined by student’s t-test. A p-value < 0.05 was considered significant.
Results
Food Intake, Body Weight, and Hormonal Plasma Levels in Adult CB1-KO Mice
When compared to WT littermates fed the SD (Figure 1A) or HFD (Figure 1B), CB1-KO mice showed a sustained decrease in body weight. Still, no differences in food intake were found between CB1-KO mice and their respective WT littermates during this period on either the SD (Figure 1C) or HFD (Figure 1D). The HFD did not impact circulating levels of ghrelin or PYY3-36, althought differences in ghrelin levels become significant when both genotypes are compared on a HFD. In keeping with the body weight data, a HFD did not induce a further increase in leptin levels in CB1-KO mice (Supplementary Table S2).
Figure 1.
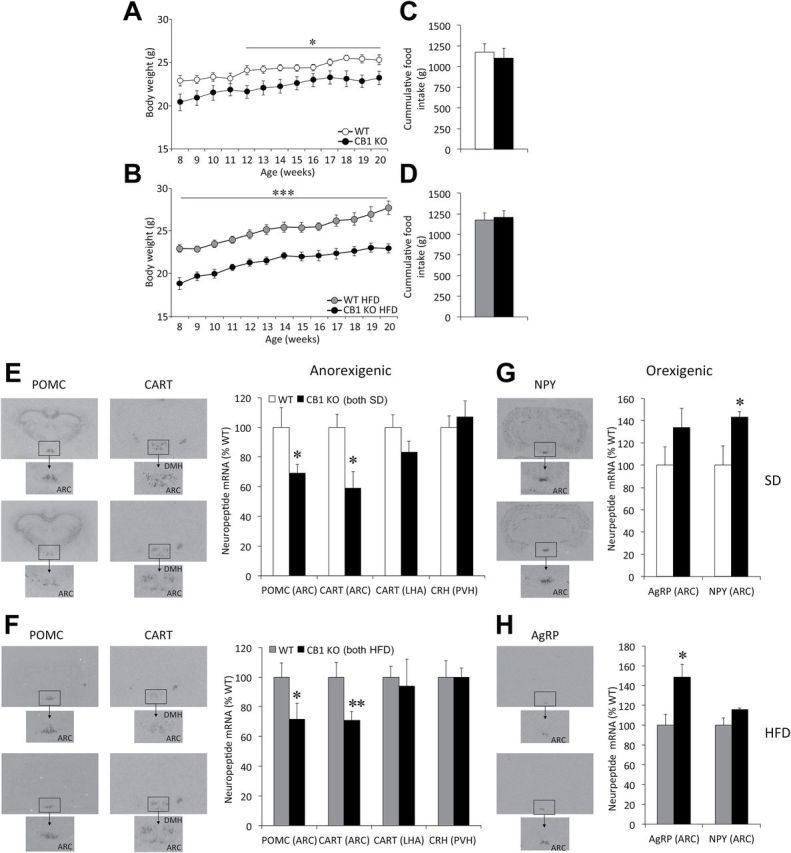
Food intake, body weight, and hypothalamic neuropeptide expression in adult whole body CB1 null mice (CB1-KO) mice. (A and B) Body weight gain and (C and D) cumulative food intake between 8–20 weeks in CB1-KO mice under either a standard diet (SD; A and C) or high-fat diet (HFD; B and D). (E–H) Representative in situ hybridization autoradiographic images (left panels) and neuroptide mRNA levels (right panels) of anorexigenic (E and F) and orexigenic (G and H) neuropeptides, in CB1-KO mice under SD (E and G) and HFD (F and H). AgRP, agouti-related peptide; ARC, arcuate nucleus of the hypothalamus; CART, cocaine-amphetamine-regulated transcript; CRH, corticotropin-releasing hormone; DMH, dorsomedial nucleus of the hypothalamus; LHA, lateral hypothalamic area; NPY, neuropeptide Y; POMC, proopiomelanocortin; PVH, paraventricular nuclei of the hypothalamus. Data are expressed as mean ± standard error of the mean; n = 7–10 animals per experimental group. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. wildtype (WT).
CB1 Antagonism in Mice Fed HFD
Mice fed a HFD acutely (4 hours) and i.p. treated with the CB1 antagonist AM281 diplayed significantly hypophagia (Supplementary Figure S1A) with concominant increases in the expression of CART and POMC in the ARC (Supplementary Figure S1B).
Hypothalamic Neuropeptide Expression in CB1-KO
A significant decrease in the mRNA levels of CART and POMC was detected in the ARC of CB1-KO mice, both under SD (WT and KO n = 7; CART: p = 0.02; POMC: p = 0.03; Figure 1E) and HFD (WT and KO n = 10; CART: p = 0.008; POMC: p = 0.03; Figure 1F). CB1-KO mice showed a increase in AgRP mRNA levels, regardless of the diet (p = 0.029 in HFD; Figure 1G and 1H), and a significant increase in NPY mRNA in SD (p = 0.027 in HFD; Figure 1G).
Food Intake, Body Weight, and Hormonal Plasma Levels in Adult CaMK-CB1-KO Mice
When compared to WT littermates fed a SD (Figure 2A) or HFD (Figure 2B), CaMK-CB1-KO mice showed a sustained, lower body weight. Similar to CB1-KO mice, no differences in food intake were found between CaMK-CB1-KO mice and their respective WT littermates during this period under either SD (Figure 2C) or HFD (Figure 2D). HFD did not impact PYY3-36 circulating levels but induced a non-significant trend towards decreased ghrelin levels (p = 0.09). In keeping with the body weight data, HFD did not induce increased leptin levels in CaMK-CB1-KO mice (Supplementary Table S2).
Figure 2.
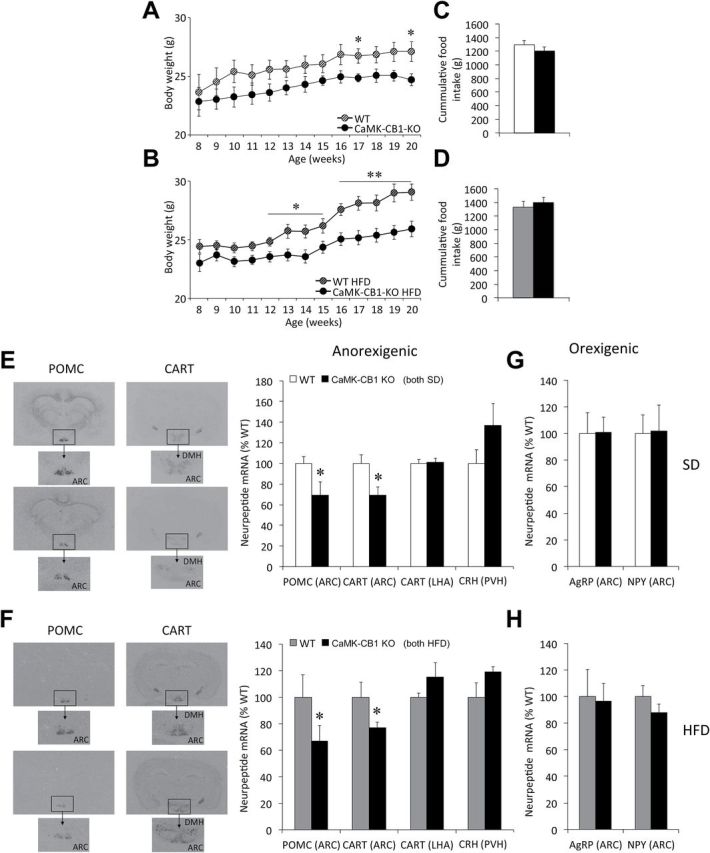
Food intake, body weight, and hypothalamic neuropeptide expression in adult neuronal CB1 conditional null mice (CamK-CB1-KO) mice. (A and B) Body weight gain and (C and D) cumulative food intake between 8–20 weeks in CaMK-CB1-KO mice under either a standard diet (SD; A and C) or high-fat diet (HFD; B and D). (E–H) Representative in situ hybridization autoradiographic images (left panels) and neuroptide mRNA levels (right panels) of anorexigenic (E and F) and orexigenic (G and H) neuropeptides, in CaMK-CB1-KO mice under SD (E and G) and HFD (F and H). ARC, arcuate nucleus of the hypothalamus; LHA, lateral hypothalamic area; DMH, dorsomedial nucleus of the hypothalamus. Data are expressed as mean ± standard error of the mean; n = 6–8 animals per experimental group. *p < 0.05 and **p < 0.01 vs. wildtype.
Hypothalamic Neuropeptide Expression in CaMK-CB1-KO Mice
A significant decrease in CART and POMC mRNA levels was detected in the ARC of CaMK-CB1-KO mice as compared to their WT littermates fed either a SD (WT n = 7; KO n = 6; CART: p = 0.02; POMC: p = 0.04; Figure 2E) or HFD (WT and KO n = 8; CART: p = 0.03; POMC: p = 0.03; Figure 2F). These changes were independent of the diet. No differences were detected in AgRP or NPY mRNA expression in this animal model when subjected to either SD (Figure 2G) or HFD (Figure 2H).
Discussion
Despite the recognition of the hypothalamus as a key structure involved in the regulation of feeding by cannabinoids, the hypothalamic mechanisms mediating this effect are poorly understood. Here, we analyzed hypothalamic neuropeptide expression in CaMK-CB1-KO and CB1-KO adult mice, aiming at clarifying the absence of an anorectic response associated with a prolonged ECS blockade. Our data reveal a significantly decreased expression of anorectic neuropeptides, namely POMC and CART, in both CB1-genetic models, an effect that was independent of the diet. These molecular changes suggest that the lack of hypophagia is mediated by a compensatory mechanism, leading to decreased expression of anorectic signals within the hypothalamus. Thus, hypothalamic neuropeptide regulation would be an adaptative response, secondary to metabolic changes, as a strategy to find and/or keep a new energy balance while avoiding depletion of energetic stores.
As previously reported, our data show a lower body weight gain in both adult CaMK-CB1-KO and CB1-KO mice compared with their respective WT controls, with a more pronounced phenotype in CB1-KO mice (Cota et al., 2003; Quarta et al., 2010). We also observed no effect of CB1 genetic deletion on feeding behavior in adult mice (Cota et al., 2003; Quarta et al., 2010), suggesting that an adaptive tolerance to early hypophagia takes place (Quarta et al., 2010). This tolerance to anorectic stimuli was previously reported in long-term pharmacological studies with CB1 receptor antagonists (Verty et al., 2009; Rorato et al., 2013).
The interaction between CB1 and the melanocortin system in regulating feeding is unclear (Colombo et al., 1998). Our data showed a diet-independent significant decrease in POMC mRNA levels in both CaMK-CB1-KO and CB1-KO mice compared to their respective WT littermates. Although some studies have reported no changes in POMC expression when administering the CB1 antagonist rimonabant (Verty et al., 2004; Sinnayah et al., 2008), these molecular analyses were conducted after long-term rimonabant treatment when no more differences in eating behavior were found, suggesting that tolerance was reached (Rorato et al., 2013). Conversely, in relation to the modulation of hypothalamic neuropeptides under acute CB1 receptor antagonism, it has recently been demonstrated that a significant increase of α-melanocyte-stimulating hormone, causes a molecular change that is in agreement with the anorexia observed (Rorato et al., 2013). Thus, our data suggest that under prolonged CB1 silencing, the significant decrease in POMC mRNA may prevent the anorectic response observed in young animals (Verty et al., 2009). Our data also reveal that, in parallel to the decrease in POMC levels, there is a significant decrease in mRNA expression levels of the anorexigenic precursor CART within the ARC of both CaMK-CB1-KO and CB1-KO mice. Similarity to POMC, CART suppression was observed irrespective of the type of diet. Decreased CART expression has been implicated in the orexigenic response to the CB1 agonist anandamide (Cota et al., 2003). Accordingly, acute CB1 antagonism increases CART levels in the ARC (Osei-Hyiaman et al., 2005), and a pharmacological CB1 blockade is unable to suppress food intake in CART-deficient mice (Verty et al., 2009), suggesting a critical role for CART in mediating the action of the ECS on food intake. Thus, the suppression in CART may contribute to the lack of hypophagia that characterizes these two animal models of genetic CB1 ablation.
We also detected a marked rise in ARC orexigenic neuropeptides, namely AgRP or NPY, in CB1-KO mice. Cumulative studies report conflicting results concerning the interaction between endocannabinoids and NPY. For example, NPY is overexpressed or suppressed in response to anandamide or rimonabant, respectively (Osei-Hyiaman et al., 2005). However, NPY-deficient mice maintain an intact anorectic response when treated acutely with a CB1 receptor antagonist, suggesting that the NPY system plays a minor role in determining the observed anorexia. Notably, the two CB1 null models used in this study display a common loss of anorexigenic neuropeptide expression, independently of ARC orexigenic neuropeptides, suggesting that the changes are mediated by central CB1 receptors. In this regard, it is interesting to note that ghrelin showed a non-significant tendency towards increased levels in total CB1-KO mice when compared with CaMK-CB1-KO mice. This difference became significant when both genotypes were fed a HFD. Given that AgRP and NPY are hypothalamic targets of ghrelin (Lopez et al., 2008; Lage et al., 2010), it is possible that differences in basal ghrelin between genotypes account for the discrepancy in AgRP and NPY expression, a hypothesis that will require further investigation.
Previous studies showed increased PVH neuronal activity and CRH overexpression under acute pharmacological CB1 antagonism (Verty et al., 2009; Rorato et al., 2013). The anorectic role of CRH is widely established, and cumulative data indicate that CRH overexpression could underlie the anorectic effect of acute CB1 antagonist administration (Verty et al., 2009; Rorato et al., 2013). Consistently, hypophagic young CB1-KO mice show increased CRH mRNA levels, suggesting that CB1 receptor activation inhibits CRH expression (Cota et al., 2003; Osei-Hyiaman et al., 2005; Verty et al., 2005, 2009; Rorato et al., 2013). Consistent with the absence of an anorexigenic response in our animals, we failed to observe any substantial change in PVH or CRH expression. Thus, the absence of CRH upregulation, alongside decreased POMC and CART expression, may also contribute to failed hypophagia in adult mice.
In summary, our data indicate that food intake in adult mice under constitutive CB1 ablation is associated with a failure in the activation of anorexigenic signals in ARC neurons, such as POMC and CART, suggesting an adaptative response under sustained negative energy balance that may overrule early anorectic responses. These data identify impaired melanocortin and CART signaling in the hypothalamus as possible mediators of the resistance to hypophagia developed after long-term CB1 ablation. The similarities of the changes exhibited by both models of CB1-ablation clearly indicate that all the changes here reported are mediated by central CB1 receptors.
SUPPLEMENTAL FIGURE S1. Food intake and hypothalamic neuropeptide expression after acute treatment with the CB1 antagonist AM281. (A) Food intake and (B) CART and POMC mRNA levels in the arcuate nucleus of the hypothalamus (ARC) of rats IP-treated with vehicle or the CB1 antagonist AM281. Data are expressed as mean ± SEM; n=6-8 animals per experimental group. * and ** P<0.05 and 0.01 vs. vehicle.
Statement of Interest
None
Supplementary Material
Acknowledgments
The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement nº 281854 of the ObERStress European Research Council Project (Dr López) and 245009 of the Neurofast project (Drs Nogueiras, Diéguez, and López); Xunta de Galicia (Dr Nogueiras: EM 2012/039 and 2012-CP069; Dr López: 2012-CP070); the Frank Mohn Foundation, Bergen (Dr Fernø); Instituto de Salud Carlos III (ISCIII; Dr López: PI12/01814 and PIE13/00024); and MINECO, co-funded by the FEDER Program of EU (Dr Nogueiras: RyC-2008-02219 and BFU2012-35255; Dr Diéguez: BFU2011-29102). CIBER de Fisiopatología de la Obesidad y Nutrición is an initiative of ISCIII.
References
- Alen F, Ramirez-López MT, Gomez de Heras R, Rodriguez de Fonseca F, Orio L. (2013) Cannabinoid receptors and cholecystokinin in feeding inhibition. Vitam Horm 92:165–196. [DOI] [PubMed] [Google Scholar]
- Bermudez-Silva FJ, Viveros MP, McPartland JM, Rodriguez de Fonseca F. (2010) The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacol Biochem Behav 95:375–382. [DOI] [PubMed] [Google Scholar]
- Bermudez-Silva FJ, Cardinal P, Cota D. (2012) The role of the endocannabinoid system in the neuroendocrine regulation of energy balance. J Psychopharmacol 26:114–124. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. (1998) Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci 63:PL113–117. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. (2003) The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 112:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Ligresti A, Cristino L. (2009) The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. Int J Obes (Lond) 33(Supp 2): S18–24. [DOI] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. (2001) Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol 134:1151–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, Penarier G, Soubrie P, Le Fur G, Galiegue S, Casellas P. (2005) The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J 19:1567–1569. [DOI] [PubMed] [Google Scholar]
- Lage R, Vazquez MJ, Varela L, Saha AK, Vidal-Puig A, Nogueiras R, Dieguez C, López M. (2010) Ghrelin effects on neuropeptides in the rat hypothalamus depend on fatty acid metabolism actions on BSX but not on gender. FASEB J 24:2670–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López M, et al. (2008) Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab 7:389–399. [DOI] [PubMed] [Google Scholar]
- López M, et al. (2010) Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med 16:1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, López-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. (2003) CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302:84–88. [DOI] [PubMed] [Google Scholar]
- Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, Lage R, Fernandez-Mallo D, Martinez-Sanchez N, Ruiz-Pino F, Liu J, Morgan DA, Pinilla L, Gallego R, Saha AK, Kalsbeek A, Fliers E, Bisschop PH, Dieguez C, Nogueiras R, Rahmouni K, Tena-Sempere M, López M. (2014) Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab 20:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschop J, Caldwell C, Woods SC, Wittmann G, Watanabe M, Liposits Z, Fekete C, Reizes O, Rohner-Jeanrenaud F, Tschop MH. (2008) Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes 57:2977–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Depetrillo M, Harvey-White J, Bannon AW, Cravatt BF, Kuhar MJ, Mackie K, Palkovits M, Kunos G. (2005) Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of endogenous anandamide. Neuroendocrinology 81:273–282. [DOI] [PubMed] [Google Scholar]
- Quarta C, et al. (2010) CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab 11:273–285. [DOI] [PubMed] [Google Scholar]
- Quarta C, Mazza R, Obici S, Pasquali R, Pagotto U. (2011) Energy balance regulation by endocannabinoids at central and peripheral levels. Trends Mol Med 17:518–526. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. (2004) CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 28:640–648. [DOI] [PubMed] [Google Scholar]
- Rorato R, Miyahara C, Antunes-Rodrigues J, Elias LL. (2013) Tolerance to hypophagia induced by prolonged treatment with a CB1 antagonist is related to the reversion of anorexigenic neuropeptide gene expression in the hypothalamus. Regul Pept 182:12–18. [DOI] [PubMed] [Google Scholar]
- Silvestri C, Di Marzo V. (2013) The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab 17:475–490. [DOI] [PubMed] [Google Scholar]
- Sinnayah P, Jobst EE, Rathner JA, Caldera-Siu AD, Tonelli-Lemos L, Eusterbrock AJ, Enriori PJ, Pothos EN, Grove KL, Cowley MA. (2008) Feeding induced by cannabinoids is mediated independently of the melanocortin system. PLOS ONE 3:e2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria-Gomez E, Massa F, Bellocchio L, Rueda-Orozco PE, Ciofi P, Cota D, Oliet SH, Prospero-Garcia O, Marsicano G. (2014) Cannabinoid type-1 receptors in the paraventricular nucleus of the hypothalamus inhibit stimulated food intake. Neuroscience 263:46–53. [DOI] [PubMed] [Google Scholar]
- Verty AN, McFarlane JR, McGregor IS, Mallet PE. (2004) Evidence for an interaction between CB1 cannabinoid and melanocortin MCR-4 receptors in regulating food intake. Endocrinology 145:3224–3231. [DOI] [PubMed] [Google Scholar]
- Verty AN, McGregor IS, Mallet PE. (2005) Paraventricular hypothalamic CB(1) cannabinoid receptors are involved in the feeding stimulatory effects of Delta(9)-tetrahydrocannabinol. Neuropharmacology 49:1101–1109. [DOI] [PubMed] [Google Scholar]
- Verty AN, Boon WM, Mallet PE, McGregor IS, Oldfield BJ. (2009) Involvement of hypothalamic peptides in the anorectic action of the CB receptor antagonist rimonabant (SR 141716). Eur J Neurosci 29:2207–2216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


