Abstract
Background:
Relapse into drug abuse evoked by reexposure to the drug-associated context has been a primary problem in the treatment of drug addiction. Disrupting the reconsolidation of drug-related context memory would therefore limit the relapse susceptibility.
Methods:
Morphine conditioned place preference (CPP) was used to assess activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) and correlative molecule expression in the Nucleus accumbens (NAc) shell during the reconsolidation of morphine CPP. U0126 and Arc/Arg3.1 antisense oligodeoxynucleotide were adapted to evaluate the role and the underlying mechanism of Arc/Arg3.1 during the reconsolidation.
Results:
The retrieval of morphine CPP in rats specifically increased the Arc/Arg3.1 protein level in the NAc shell, accompanied simultaneously by increases in the phosphorylation of extracellular signal-regulated kinase1/2 (pERK1/2), the phosphorylation of Cyclic Adenosine monophosphate (cAMP) response element-binding (pCREB), and the up-regulation of the membrane α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors GluR1 subunit level. Intra-NAc shell infusion U0126, an inhibitor of the Mitogen-activated protein kinase kinase (MEK), prevented the retrieval-induced up-regulation of pERK1/2, pCREB, Arc/Arg3.1, and membrane GluR1 immediately after retrieval of morphine CPP. The effect of disrupting the reconsolidation of morphine CPP by U0126 could last for at least 14 days, and could not be evoked by a priming injection of morphine. Furthermore, the specific knockdown of Arc/Arg3.1 in the NAc shell decreased the membrane GluR1 level, and impaired both the reconsolidation and the reinstatement of morphine CPP.
Conclusions:
Arc/Arg3.1 in the NAc shell mediates the reconsolidation of morphine-associated context memory via up-regulating the level of membrane of GluR1, for which the local activation of the ERK-CREB signal pathway, as an upstream mechanism of Arc/Arg3.1, is required.
Keywords: Activity regulated cytoskeleton-associated protein/activity-regulated gene 3.1, extracellular signal-regulated kinase, cAMP response element-binding protein, GluR1, conditioned place preference, reconsolidation
Introduction
Repeated drug administration can develop an intense associative memory between drug and drug-related contexts. Once the association is learned, the drug users, whether humans or laboratory animals, can engage in drug-seeking behavior without drugs being present when they are reexposed to the context (O’Brien et al., 1992; Shaham et al., 2003). The contextual cues–induced relapse to drug abuse among addicts has become a major challenge to the treatment of drug addiction. Similar to other types of memories, drug-associated memory can be retrieved or reactivated and return to a labile, sensitive state. This reactivated memory often undergoes another process of consolidation, called reconsolidation, to be restabilized (Misanin et al., 1968; Nader, 2003). One of the animal models often used to study this context-induced drug seeking is morphine conditioned place preference (CPP), analogous to the drug environment associations that could trigger relapse in humans (O’Brien et al., 1992). During the conditioning phase, animals receive a drug in one context and vehicle in another to acquire a drug-associated memory. Following conditioning, memory can be retrieved when the animals are reexposed to the contexts in a drug-free state, and then entered into the process of reconsolidation (Bardo et al., 1995).
In previous studies, the molecular mechanisms underlying memory reconsolidation have been focused on de novo protein-synthesis, transcription factors implication, signal-pathway activation, and immediate-early gene (IEG) expression (Tronson and Taylor, 2007). In comparison, pharmacological options, such as protein synthesis inhibitor anisomycin, administered after reactivation of cocaine or morphine CPP, impaired drug seeking via disruption of the reconsolidation (Milekic et al., 2006; Lin et al., 2014). Apparently, drug-related memory can be inhibited or erased by interrupting its reconsolidation process. Although a growing body of studies have demonstrated that the reconsolidation of addiction memory after recall may rely on common neurobiological mechanisms, including N-methyl-D-aspartic acid-receptor driven protein kinase signal cascades, up-regulation of transcriptional factors, and temporal regulation of mRNA and protein synthesis (Tronson and Taylor, 2007; Nader and Einarsson, 2010), little is known about the specific downstream genes that are critical for the process.
Activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) is a particularly interesting IEG that may be induced by neuronal activity and then impact synaptic plasticity (Lyford et al., 1995; Steward et al., 1998). Previous studies have shown that Arc/Arg3.1 protein is involved in multiple forms of synaptic plasticity, including long-term potentiation, long-term depression, and the stable expansion of dendritic spines (Guzowski et al., 2000; Pandey et al., 2008; Waung et al., 2008). And Arc/Arg3.1 likely underlies the consolidation and reconsolidation of some forms of memory, including spatial memory, fear conditioning, and object recognition (Steward, 2002; Gusev and Gubin, 2010; Chia and Otto, 2013). Recently, the Arc/Arg3.1 protein has been reported to be involved in the molecular mechanisms of drug addiction (Hearing et al., 2011). Also, our previous study showed that Arc/Arg3.1 protein in the NAc shell is specifically involved in mediating the retrieval of morphine CPP, which is also regarded as drug context–induced memory retrieval (Lv et al., 2011).
Based on the above findings, Arc/Arg3.1 could be seen as a candidate for involvement in the reconsolidation of drug-associated memory. Clarification is needed regarding whether Arc/Arg3.1 protein in the NAc shell is required in the reconsolidation of opioid-associated context memory. The present study was designed to explore the effects of NAc-shell Arc/Arg3.1 in the memory reconsolidation of morphine-associated context using morphine-induced CPP.
Materials and Methods
Animals
Male Sprague Dawley rats, weighing 220–240g, were used in this study. These rats were obtained from the Laboratory Animal Center of the Peking University Health Science Center. The rats were housed five per cage in a temperature- (23±2°C) and humidity- (50±5%) controlled animal facility with food and water provided ad libitum and were maintained on a reverse light-dark cycle (12:12h, lights on at 19:00h). Behavioral experiments were conducted during the dark cycle, and rats were handled for 5 days prior to the experiments. All experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the local committee of animal use and protection.
Place Preference Apparatus
The CPP apparatus is a black, rectangular polyvinyl chloride box (79.5cm long × 23.0cm wide × 25.0cm high) containing three chambers (A, B, and C) separated by guillotine doors (Lv et al., 2011). Two large black conditioning chambers (A and C; 280mm × 220mm × 225mm) were separated by a small gray center choice chamber (B; 135mm × 220mm × 225mm). Chamber A had four light-emitting diodes (LEDs) forming a square on the wall and a stainless steel mesh floor (225mm × 225mm), and chamber C had four LEDs forming a triangle on the wall and a stainless-steel rod floor (15mm apart). In contrast, chamber B had a plain floor. Fourteen photobeams were placed across the chambers 47.5mm apart. Using a computer interface, the time spent in each chamber and the numbers of entrances into each of the three compartments were recorded by infrared beam crossings.
Cannula Implantation and Microinfusions
Rats weighing 260–280g received cannula implants and were handled for 2 days prior to surgery. The rats were anesthetized with sodium pentobarbital (40mg/kg, i.p.) and secured in a Kopf stereotaxic apparatus (Kopf Instrument). Stainless steel guide cannulas (0.67mm in outer diameter) were bilaterally implanted 1.5mm above the NAc shell. The coordinates (Paxinos and Watson, 1997) were anterior/posterior, +1.6mm; medial/lateral, ±0.9mm; and dorsal/ventral, −6.5mm. The cannulas were fixed with screws to the skull with dental cement. Internal cannulas were replaced with dummy cannulas, which were 0.5mm longer than the guide cannulas, to keep the cannula patent and prevent infection. Rats were given at least 5–7 days to recover before the conditioning procedures. The intra-nuclear infusions were performed as previously (Lv et al., 2011).
Oligodeoxynucleotide Design and Preparation
Arc/Arg3.1 antisense oligodeoxynucleotide (AS) and scrambled oligodeoxynucleotide (CS) (Shanghai Sangon Synthesis Technology Services Ltd.) were designed as described by Guzowski et al. (2000). The Arc/Arg3.1 AS encoded an antisense sequence for the Arc/Arg.31 mRNA sequence near the translation start site (Lyford et al., 1995). The CS, which does not show significant homology to any sequences in the GenBank database, served as a control. The effectiveness of knocking down Arc/Arg3.1 protein expression and its diffusion in the NAc shell were described in our previous study (Lv et al., 2011).
Conditioned Place Preference
Pretest
On day 0, rats were allowed to freely explore the entire apparatus for 15min to assess the unconditioned chamber preference. The time (in seconds) spent in each compartment and the number of entrances into each of the three compartments were recorded. Fourteen of the initial 280 rats were excluded because of a strong unconditioned preference (>540s). Conditioning was performed using an unbiased, counterbalanced protocol. In the following experiments, rats in every group spent similar amounts of time in both chambers A and C in the pretest.
Conditioning
On day 1, the rats were confined to the corresponding conditioning chambers (A or C) for 30min immediately after the injections of morphine (5mg/kg, i.p.) and then returned to their home cages. On day 2, the rats were injected with saline (1ml/kg, i.p.) and confined to the other conditioning chamber. On the subsequent conditioning days, each rat was trained for 6 consecutive days with alternate injections of morphine and saline. The control group received saline every day. The neutral zone was never used during conditioning and was blocked by guillotine doors.
Test
On day 9, one day after the last conditioning trial, rats were placed in the neutral area with the guillotine doors removed and allowed free access to all compartments in a drug-free state for 15min. The time spent in each compartment and the numbers of entrances into each compartment were recorded. The procedure for the test was the same as the procedure for the initial baseline preference assessment, which also acted as a reactivation session (Robinson et al., 2011). The CPP score was defined as the time (in seconds) spent in the drug-paired chamber minus time spent in the saline-paired chamber during CPP testing.
Experimental Design
After the establishment of CPP, rats were randomly divided into three group sets.
Experiment 1
This experiment measured Arc/Arg3.1, α-amino-3-hydroxy-5-methyl-4-isoxa-zolep-propionate (AMPA) GluR1 cell-surface expression, and extracellular signal-regulated kinase (ERK) cAMP response element-binding (CREB) activation in the NAc shell during the reconsolidation of morphine CPP. For Western blotting, half of the first group of rats was sacrificed by decapitation 2h after the morphine CPP test. The other half, which did not conduct the CPP test, was sacrificed at the corresponding time.
Experiment 2
This experiment measured the effect of the MEK inhibitor, U0126, on the reconsolidation of morphine CPP. Drugs were infused using an infusion pump at 0.25 µl/min. A total volume of 0.5 µl of 2 µg/µl U0126 or vehicle was infused into the NAc shell of both the morphine and saline groups of rats immediately after the CPP test on day 9. Then, half of the rats in each group were sacrificed by decapitation 2h after the microinfusions for Western blotting. The other half had post-CPP tests on day 10 (post-test 1), day 24 (post-test 2), and day 25 (priming test, rats were tested for the reinstatement of CPP immediately after receiving a priming injection of morphine [2.5mg/kg, i.p.] or saline [1ml/kg, i.p.]).
Experiment 3
This experiment measured the effect of Arc/Arg3.1 AS on the reconsolidation of morphine CPP. Both morphine and saline groups received bilateral microinfusions (1 nmol/µl/side) of AS or CS immediately after the CPP test on day 9 (CPP test). Half of the rats in each group were sacrificed by decapitation 2h after the microinfusions for Western blotting. The other half received post-CPP tests on day 10 (post-test 1), day 24 (post-test 2), and day 25 (priming test).
Tissue Preparation and Western Blot
Tissue Preparation
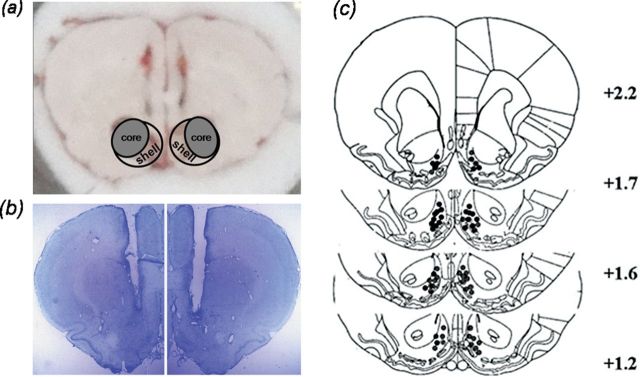
For Western blotting experiments, rats were given an overdose of chloral hydrate (250mg/kg; i.p.), and their brains were quickly removed and frozen in N-hexane (−70°C) for approximately 40 s. Bilateral tissue punches (12/16 gauge) of the NAc shell were obtained from 60-µm thick sections taken on a sliding freezing microtome (Figure 1A). Punches were sonicated in 120–150 µl ice-cold radio-Immunoprecipitation Assay (RIPA) buffer. The homogenate was then centrifuged at 12,000 × g for 5min, and the supernatant was saved for analysis. Protein concentrations were determined using the bicinchoninic acid (BCA) assay (Pierce) and analyzed directly by sodium dodecyl sulfate - Polyacrylamide gel electrophoresis (SDS-PAGE).
Figure 1.
Representative sample punches and cannula placements in the NAc shell. (A) Circles in the coronal section insets indicate the locations of samples punch from the NAc shell. (B) Locations of needle tips for bilateral intra-NAc shell infusions. (C) Distribution of microinjection sites in the NAc shell. Because of extensive overlap between the infusion needle tips, not all tip locations are illustrated in this diagram.
Cell Surface Receptor Cross-Linking Assays
Cell surface expression of GluRs was assayed using the membrane-impermeable cross-linking reagent bis (sulfosuccinimidyl) suberate (BS3) as described previously (O’Brien et al., 1992). Through those bonds, BS3 cross-links GluRs to form high-molecular weight aggregates. Briefly, rats were given an overdose of chloral hydrate (250mg/kg; i.p.) and decapitated. Brains were rapidly removed and sliced into coronal sections (400 μm) with a vibratome. The NAc shell was dissected and added to Eppendorf tubes containing ice-cold artificial cerebrospinal fluid (ACSF) containing 2mM BS3 for incubation with gentle agitation for 30min at 4°C. The cross-linking reaction was terminated by quenching with 20mM glycine. The tissue was then washed four times in ice-cold ACSF (10min each). Samples were moved into another tube containing ice-cold sample buffer and homogenized by sonication to obtain total protein. The total protein was measured for its protein concentration and analyzed directly by SDS-PAGE (4–20% Tris-glycinegels, Bio-Rad Laboratories).
Western Blotting
Total protein extracts (30 µg) were then electrophoresed in SDS-PAGE and transferred to polyvinylidene difluoride membranes. Blots were blocked in tris-buffered saline (TBS) (50mM Tris-HCl, pH 7.5, 150mM NaCl and 0.05% Tween 20) with 5% dry milk and incubated with the following primary antibodies overnight at 4°C: anti-Arc/Arg3.1 antibody (1:800; Santa Cruz Biotechnology), anti-phospho-p44/42 ERK1/2 antibody (1:2000; Cell Signaling), anti-ERK1/2 antibody (1:1000; Cell Signaling), anti-phospho CREBSer133 antibody (1:1000; Cell Signaling), anti-CREB antibody (1:1000; Cell Signaling), anti-GluR1/2/3 antibody (1:1000; Millipore), and anti-β-Actin antibody (1:5000; Sigma). After washing three times for 5min in TBS/0.1% Tween-20, blots were then incubated with anti-rabbit or -mouse secondary antibody conjugated to horseradish peroxidase (1:2000; Zhongshan Biotechnology) and developed using the West Dura chemiluminescent substrate (Pierce Laboratories). Densitometry was determined based on band intensity, and relative protein expression was quantified by densitometry using the Total Lab 2.01 analysis system (Phoretix). To control for inconsistencies in loading, optical densities were normalized to β-actin protein expression. Data for treated animals were normalized to the average value of the naive controls.
Histology
Histological verification of cannula implantation was performed after conditioned place preference testing. Rats were anesthetized and perfused as described above. Coronal sections (30-µm thick) were cut on a cryostat (−20ºC) and mounted on slides coated with gelatin. Cannula placements were assessed by Nissl staining using light microscopy. The locations of the representative cannula tips are shown in Figure 1B and C. Only those rats whose cannulas were correctly placed were used for data analysis.
Drugs
Morphine hydrochloride was purchased from the First Pharmaceutical Factory of Shenyang. U0126 was purchased from Upstate. Morphine was dissolved in 0.9% saline and U0126 was dissolved in dimethyl sulfoxide (DMSO; Sigma) and diluted to 2 µg/µl in 5% DMSO and 6% Tween 80 in 1 × phosphate buffer saline (PBS) before use. BS3 was purchased from Thermo Fisher Scientific Inc.
Statistical Analysis
Data were expressed as the means ± standard error of the mean and analyzed using analyses of variance (ANOVA) with the appropriate between- and within-subjects factors for the different experiments. One-way ANOVAs were used to analyze the Arc/Arg3.1 and GluRs protein levels. Post hoc analyses of significant effects from the ANOVA were performed using the Bonferroni test. The results from the CPP test and other immunoblotting experiments were analyzed with two-way ANOVAs followed by the Bonferroni post-test. Data were processed using Graph Pad Prism 5.0 software. Statistical significance was set to p < 0.05.
Results
Changes of Arc/Arg3.1 Protein, AMPA GluR1 Cell-Surface Expression Level and ERK, CREB Phosphorylation in NAc Shell During the Reconsolidation of Morphine CPP
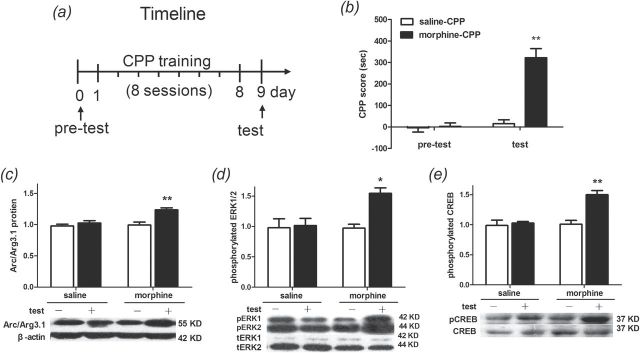
Following 8 days of alternative morphine and saline or saline conditioning alone, rats underwent the CPP expression test on day 9 (Figure 2A). Memory was retrieved when reexposed to the contexts in a drug-free state, and then entered into the process of reconsolidation. Statistical analysis (two-way ANOVA) revealed a significant effect from the interaction [F(1, 36) = 33.39; p < 0.001] between the treatments (saline vs. morphine) and the tests (pretest vs. test). Significant differences were found between saline- and morphine-treated rats [F(1, 36) = 36.24, p < 0.001] and within the pretest and the test [F(1, 36) = 42.49, p < 0.0001]. The Bonferroni post hoc test demonstrated that morphine-conditioned rats spent significantly more time in the drug-paired context during the expression test than saline-treated rats (t = 8.343, p < 0.001; Figure 2B).
Figure 2.
Reexposure to the morphine conditioning context induces drug seeking, increases activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) protein levels, and activates extracellular signal-regulated kinase (ERK) cAMP response element-binding (CREB) in the NAc shell. (A) Diagram outlining the behavioral procedures. (B) After 8 days of drug treatment, rats that received alternating injections of morphine (5mg/kg) and saline (1ml/kg) showed a significant preference for the morphine-paired chamber. Data are expressed as the means ± standard error of the mean (SEM), n = 10. **p < 0.001, compared to the saline-treated group. (C–E) Reexposure to the morphine conditioning context significantly increased Arc/Arg3.1 protein, phosphorylation of extracellular signal-regulated kinase1/2 (pERK1/2), and phosphorylation of CREB (pCREB) levels in the NAc shell of morphine-conditioned rats. Data are expressed as the means ± SEM, n = 5. *p < 0.01; **p < 0.001, compared to the morphine-conditioned group without retrieval. “-” refers to no retrieval, “+” refers to retrieval. CPP, conditioned place preference; KD, kilodalton; tERK, total of extracellular signal-regulated kinase1/2.
To evaluate the Arc/Arg3.1 protein level, AMPA GluR1 cell-surface expression level, and ERK-CREB phosphorylation in the NAc shell during the reconsolidation of morphine CPP, we designated test and no-test as the between-subjects factors, and saline and morphine treatments as within-subjects factors. Rats were sacrificed by decapitation 2h after the test for Western blotting. Statistical analysis (two-way ANOVA) revealed a significant effect from the interaction [F(1, 16) = 6.712, p < 0.05] between the tests (test vs. no-test) and treatments (saline vs. morphine) in Arc/Arg3.1 protein expression. Significant differences were found between tests [F(1, 16) = 15.04, p < 0.01] and within treatments [F(1, 16) = 8.92, p < 0.01]. The Bonferroni post hoc test demonstrated that Arc/Arg3.1 protein expression was increased in rats that received the morphine conditioning and were reexposed to the conditioning context (t = 4.57, p < 0.001; Figure 2C). There were significant interaction effects between tests and treatments [F(1, 16) = 5.958, p < 0.05] when the amount of pERK1/2 was analyzed. Significant differences were found between test factors [F(1, 16) = 7.69, p < 0.05] and within treatment factors [F(1, 16) = 5.66, p < 0.05]. A Bonferroni post hoc analysis confirmed that levels of pERK1/2 were significantly higher in morphine-conditioned rats that were reexposed to the conditioning context than in those not subjected to reexposure (t = 3.69, p < 0.01; Figure 2D). In contrast, total ERK1/2 immunoreactivity showed no significant difference (data not shown). Meanwhile, CREB phosphorylation was analyzed. Statistical analysis revealed a significant effect from the interaction [F(1, 16) = 11.76, p < 0.01] between tests and treatments. Significant differences were found between test factors [F(1, 16) = 16.39, p < 0.001] and within treatment factors [F(1, 16) = 14.25, p < 0.01]. The Bonferroni post hoc test demonstrated that pCREB was increased in rat that underwent morphine conditioning and reexposure to the conditioning context (t = 5.29, p < 0.001; Figure 2E). There were no significant differences in total CREB levels among the groups.
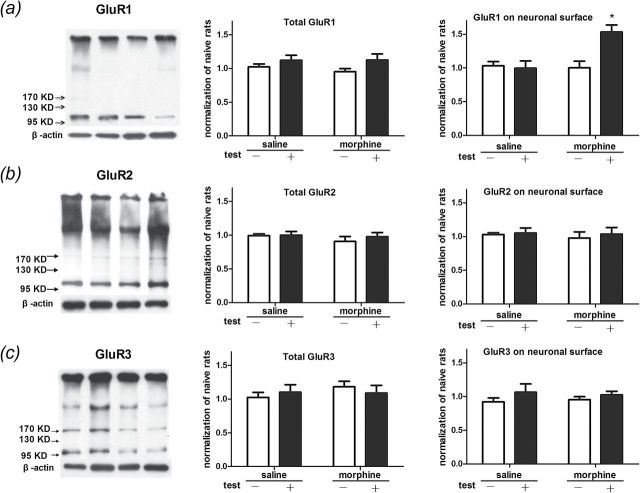
Moreover, we analyzed membrane GluR1, 2, and 3 levels. Statistical analysis revealed a significant interaction effect [F(1, 16) = 8.821, p < 0.01] between the tests and the treatments in protein levels of GluR1 on the membrane. Significant differences were found between test factors [F(1, 16) = 6.77, p < 0.05] and within treatment factors [F(1, 16) = 7.09, p < 0.05]. The Bonferroni post hoc test demonstrated that the membrane protein level of the AMPA receptor GluR1 was increased in rats that underwent morphine conditioning and reexposure to the conditioning context (t = 3.94, p < 0.01; Figure 3A). There were no significant differences in GluR2 and GluR3 levels on membrane surfaces. Statistical analysis revealed no significant difference in the total protein levels of GluR1, GluR2, and GluR3 (Figure 3B and C). These data suggested that Arc/Arg3.1 protein level, AMPA GluR1 cell-surface expression level, and ERK-CREB activity were up-regulated in the NAc shell during the reconsolidation of morphine CPP.
Figure 3.
Reexposure to the morphine conditioning context increases AMPA GluR1 cell-surface in the NAc shell. (A–C) Using bis (sulfosuccinimidyl) suberate (BS3) surface receptor cross-linking assays, we analyze protein levels on the cell surface of GluR1, GluR2, and GluR3 in the NAc shell of morphine-conditioned rats. Through those bonds, BS3 cross-links GluRs to form high-molecular weight aggregates. Data are expressed as the means ± standard error of the mean, n = 5. *p < 0.01, compared to the morphine-conditioned group without retrieval. KD, kilodalton.
Effects of Selective MEK1/2 Inhibitor U0126 on the Changes of Correlative Molecular and the Reconsolidation of Morphine CPP
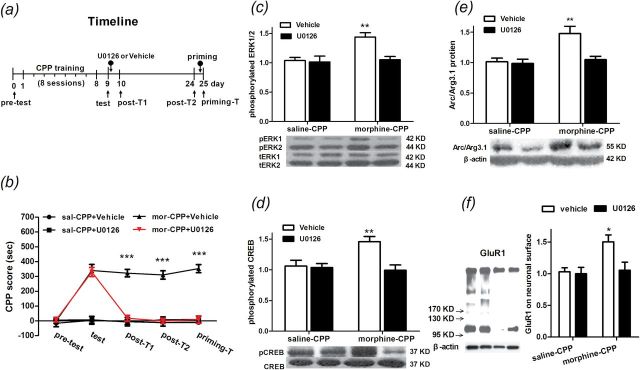
In order to determine whether ERK1/2 coupled with CREB activity is necessary for up-regulation of the Arc/Arg3.1 protein level, we examined AMPA GluR1 cell-surface expression and morphine CPP reconsolidation. Rats were trained for morphine CPP and given bilateral infusions of the MEK1/2 inhibitor U0126 or its vehicle to the NAc shell. Infusions were administered immediately after the CPP test. Then, rats were sacrificed by decapitation 2h after the microinfusion for Western blotting or were subjected to post-CPP tests on day 10, day 24, and day 25 (Figure 4A). Statistical analysis (two-way ANOVA) revealed a significant effect from the interaction between treatments and tests [F(12, 112) = 16.33, p < 0.0001]. Significant differences in the CPP score were found between treatments [F(3, 112) = 163.7, p < 0.0001] and within the tests [F(4, 112) = 25.344, p < 0.001]. The Bonferroni post hoc test demonstrated that, compared to control groups, morphine-conditioned rats microinjected with vehicle (t = 7.98, p < 0.001) or U0126 (t = 7.95, p < 0.001) spent significantly more time in the drug-paired context during the test. Nevertheless, in post-test 1, the CPP score decreased in morphine-conditioned rats microinfused with U0126 compared to vehicle (t = 9.08, p < 0.001), as well as in post-test 2 (t = 9.28, p < 0.001) and the priming test (t = 1.27, p < 0.001; Figure 4B). These results suggested that the NAc shell, microinjected with U0126, can block the reconsolidation of morphine CPP for at least 14 days and cannot be reversed by a 2.5mg/kg morphine priming injection. Two hours after the bilateral infusions of the MEK1/2 inhibitor U0126 or its vehicle to the NAc shell, rats were killed for analysis of ERK1/2 and CREB phosphorylation, Arc/Arg3.1 protein level, and AMPA GluR1 cell-surface expression level in the NAc shell. For ERK1/2 phosphorylation, statistical analysis (two-way ANOVA) revealed a significant effect from the interaction between conditionings and treatments [F(1, 20) = 6.06, p < 0.05]. Significant differences were found between the U0126 and its vehicle microinfusions [F(1, 20) = 7.91, p < 0.05] and within saline and morphine conditions [F(1, 20) = 8.778, p < 0.01]. The Bonferroni post hoc test demonstrated that microinjected U0126 significantly decreased the pERK1/2 level in the NAc shell in morphine-conditioned rats compared to those rats microinjected with the vehicle (t =3.73, p < 0.01; Figure 4C). For CREB phosphorylation, there were significant main effects between treatments [F(1, 20) =8.63, p < 0.01] and within conditions [F(1, 20) = 4.48, p < 0.05], as well as a significant condition and treatment interaction [F(1, 20) = 6.936, p < 0.05]. The Bonferroni post hoc test demonstrated that U0126 can significantly reduce the amount of pCREB (t = 3.94, p < 0.01; Figure 4D). Importantly, as Figure 4E shows, statistical analysis revealed a significant effect from the interaction between the treatments and the conditionings in Arc/Arg3.1 expression [F(1, 20) = 6.13, p < 0.05]. Significant differences were found between U0126 and its vehicle microinfusions [F(1, 20) = 8.03, p < 0.01] and within the saline and morphine conditions [F(1, 20) = 10.72, p < 0.01]. The Bonferroni post hoc test demonstrated that intra-NAc shell infusions of U0126 significantly decreased the Arc/Arg3.1 protein level in morphine-conditioned rats compared to those rats microinjected with the vehicle (t = 3.767, p < 0.01). Finally, we detected the AMPA GluR1 cell-surface expression level in the NAc shell after the U0126 microinfusion. Statistical analysis (two-way ANOVA) revealed significant differences between the U0126 and its vehicle microinfusions [F(1, 16) = 5.50, p < 0.05] and within the saline and morphine conditions [F(1, 16) = 6.69, p < 0.05]. The Bonferroni post hoc test demonstrated that microinjected U0126 into the NAc shell significantly decreased the AMPA GluR1 cell-surface expression level in morphine-conditioned rats compared to those microinjected with the vehicle (t = 3.11, p < 0.05; Figure 4F). These data suggested that the ERK-CREB signaling pathway was the upstream mechanism of Arc/Arg3.1 protein expression and AMPA GluR1 cell-surface expression in the NAc shell during the reconsolidation of morphine CPP.
Figure 4.
Effects of intra-NAc shell injection of U0126 on the reconsolidation of morphine-induced conditioned place preference (CPP) and concomitant protein expression. (A) Diagram outlining the behavioral procedures. (B) NAc shell U0126 injections decreased the CPP score from the post T1 and post T2 to the priming T, n = 8. (C–E) NAc shell U0126 injections decreased extracellular signal-regulated kinase (ERK) and cAMP response element-binding (CREB) phosphorylation (pCREB) and activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) expression induced by retrieval of morphine CPP, n = 6. (F) NAc shell U0126 injections decreased the GluR1 protein on the cell surface that was induced by retrieval of morphine CPP. Data are expressed as the means ± standard error of the mean, n = 5. *p < 0.05, **p < 0.01, ***p < 0.001, compared to the morphine-conditioned group microinfused with vehicle. KD; mor, morphine conditioning; post-T1, post test 1; post-T2, post test 2; priming-T, priming test; sal, saline conditioning; tERK, total of extracellular signal-regulated kinase1/2.
Effect of Arc/Arg3.1 AS on AMPA GluR1 Cell-Surface Expression and the Reconsolidation of Morphine CPP
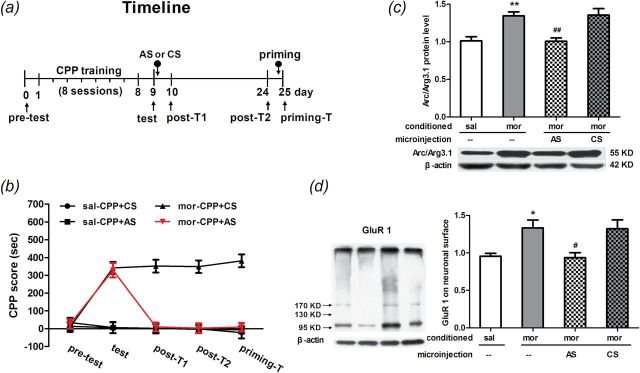
In this experiment, rats were divided into morphine- and saline-treated CPP groups. After 8 days of CPP conditioning, rats received bilateral microinfusions (1 nmol/µl/side) of AS or CS to the NAc shell immediately after the test on day 9. Then, half of rats were sacrificed by decapitation 2h after the microinfusions for Western blotting and the other half received post-CPP tests on day 10, day 24, and day 25 (Figure 5A). Statistical analysis (two-way ANOVA) revealed a significant interaction effect between treatments and times [F(12, 128) = 15.47, p < 0.0001]. Significant differences in the CPP scores were found between AS and CS treatments [F(3, 160) = 50.31, p < 0.0001] and within the different tests [F(4, 128) = 14.54, p < 0.0001]. In the expression test, the post hoc test showed a significant increase in the CPP score in morphine-conditioned rats microinfused with either Arc/Arg3.1 AS (t = 7.60, p < 0.001) or CS (t = 7.94, p < 0.001) when compared to the saline groups. In post-test 1, the CPP score decreased in morphine-conditioned rats microinfused with Arc/Arg3.1 AS compared to CS (t = 8.55, p < 0.001), as well as in post-test 2 (t = 8.66, p < 0.001) and the priming test (t = 9.32, p < 0.001; Figure 5B). These data suggested that Arc/Arg3.1 AS, microinfused into the NAc shell, impaired reconsolidation of morphine CPP at least for 14 days and cannot be reversed by 2.5mg/kg morphine injection.
Figure 5.
Effects of intra-NAc shell injection of activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) AS or CS on the reconsolidation of morphine-induced conditioned place preference (CPP) and GluR1 protein expression on the cell surface. (A) Diagram outlining the behavioral procedures. (B) NAc shell Arc/Arg3.1 AS injections decreased the CPP score from the post T1 and post T2 to the priming T. Data are expressed as the means ± standard error of the mean (SEM), n = 8~10. ***p < 0.001, compared to the morphine-conditioned group microinfused with CS. (C and D) NAc shell Arc/Arg3.1 AS injections decreased the Arc/Arg3.1 protein and GluR1 protein on the cell surface that were induced by retrieval of morphine-induced CPP. Data are expressed as the means ± SEM, n = 7–9. *p < 0.05, **p < 0.01, compared to the saline conditioned group; # p < 0.05, ## p < 0.01, compared to the morphine conditioned group injection with CS. AS, antisense ODN; CS, scrambled ODN; KD; mor, morphine conditioning; post-T1, post test 1; post-T2, post test 2; priming-T, priming test; sal, saline conditioning.
On day 9, half the rats were sacrificed 2 hours after the bilateral infusions of the Arc/Arg3.1 AS or CS to the NAc shell. The other half of the rats were sacrificed by decapitation for analysis of Arc/Arg3.1 protein level, AMPA, and GluR1 cell-surface expression level in the NAc shells. For the Arc/Arg3.1 protein, statistical analysis (one-way ANOVA) revealed significant differences among groups [F(3, 31) = 10.42, p < 0.0001]. Compared to the saline group, the Bonferroni post hoc test showed that the Arc/Arg3.1 protein level was increased in morphine CPP rats (t = 3.70, p < 0.01). In morphine CPP groups, the Arc/Arg3.1 protein level was decreased in rats microinfused with Arc/Arg3.1 AS (t = 4.03, p < 0.01) and or CS (t = 4.18, p < 0.01; Figure 5C) compared to those without microinfusion. For AMPA GluR1 cell-surface expression, statistical analysis (one-way ANOVA) revealed significant differences among groups [F(3, 31) = 6.26, p < 0.01]. The Bonferroni post hoc test showed a significant increase in the AMPA GluR1 cell-surface expression level in morphine CPP rats compared to rats in the saline CPP group (t = 2.91, p < 0.05). In morphine CPP groups, the AMPA GluR1 cell-surface expression level was decreased in rats microinfused with Arc/Arg3.1 AS (t = 3.26, p < 0.05) and or CS (t = 3.18, p < 0.05; Figure 5D) compared to those without microinfusion. These data suggested that Arc/Arg3.1 AS can inhibit Arc/Arg3.1 protein expression and AMPA GluR1 cell-surface expression induced by morphine CPP retrieval in the NAc shell.
Discussion
Previous study in our lab has demonstrated that the Arc/Arg3.1 protein level was increased in the NAc shell, but not the NAc core, after the expression test of morphine CPP (Lv et al., 2011). The present study extended those observations and revealed that morphine context memory reactivation dramatically enhances the Arc/Arg3.1 protein level, accompanied by increases in membranous AMPA GluR1 expression, pERK1/2, and pCREB levels, specifically in the NAc shell. Interestingly, the changes of Arc/Arg3.1-related molecules, including AMPA GluR1, pERK1/2, and pCREB, are selective in the test group of morphine CPP, but not the non-tests of the morphine- or saline-CPP groups, suggesting that the elevated Arc/Arg3.1-related molecules in animals treated with the retrieval cannot be attributed to the earlier training experience but to the exposure to a drug-paired context.
It has been reported that the activation of the ERK signaling pathway plays a critical role in memory reconsolidation. The reconsolidation of recognition memory is associated with an increase in the phosphorylation of ERK in the entorhinal cortex and hippocampal CA1, and inhibition of the ERK pathway with U0126 can block the reconsolidation. This process is shown to be dependent on the reactivation of memory trace by brief reexposure to the objects (Kelly et al., 2003). Additionally, transcription factors CREB is phosphorylated at Ser133 site by upstream kinases result in cellular gene expression, and ERK pathway activation has been coupled to CREB phosphorylation, which is widely implicated in many forms of neuronal plasticity and learning and memory (Hall et al., 2001). Targeted disruption of CREB in the hippocampus, amygdala, and prefrontal cortex could impair the reconsolidation of both auditory fear and contextual fear memories (Kida et al., 2002; Mamiya et al., 2009). A recent report showed that CREB is activated in the striatum of mice following a methamphetamine (METH) CPP expression test (Liu et al., 2014). In brief, the above data suggest that changes in ERK and CREB activity may be the underlying mechanism for the reconsolidation of drug addiction memory, regulating a variety of gene expressions related to addiction behavior; the target genes regulated by CREB need further study.
The present work showed that Arc/Arg3.1 protein induction during morphine CPP reconsolidation was accompanied by ERK-CREB signal pathway activation. Meanwhile, it inhibited ERK1/2 activation with MEK1/2 inhibitor U0126, attenuated CREB activation and Arc/Arg3.1 protein level, and disrupted the reconsolidation of morphine CPP. This behavioral result is similar to those pharmacological studies implying that the inhibition of ERK was almost simultaneous with the reduction of CREB activation and the impairment of contextual memory (Miller and Marshall, 2005; Fricks-Gleason and Marshall, 2011). However, there is some dispute about whether the Arc/Arg3.1 gene expression was regulated by CREB transcription factors. In Waltereit et al.’s (2001) study, they did not detect the cAMP response element (CRE) site within 1737bp of the Arc/Arg3.1 promoter or the entire transcribed region of 3.5kb, which does not exclude the possibility that unidentified CRE sites lie outside of this region. Kawashima et al. (2009) identified a major synaptic activity responsive element of the Arc/Arg3.1 gene, a cis-regulatory element consisting of closely localized binding sites for CREB, myocyte enhancer factor 2, and serum response factor that is located >5kb upstream of the Arc/Arg3.1 transcription initiation site in the mouse genome. This data suggested that the single element is necessary and sufficient for replicating crucial properties of endogenous Arc/Arg3.1’s transcriptional regulation. The present study further recommends that morphine CPP reconsolidation depends on ERK activation, which is required for CREB phosphorylation and induction of Arc/Arg3.1 protein expression.
As we know, Arc/Arg3.1 up-regulation is followed by contextual spatial memory retrieval (Zhang et al., 2005; Kee et al., 2007). These findings are in accord with our present results that a reexposure to the morphine-paired chamber obviously triggered the reconsolidation of morphine CPP and was accompanied by increasing Arc/Arg3.1 expression within the NAc shell in rats. If Arc/Arg3.1 itself plays a key role in the reconsolidation of morphine CPP, disruption of Arc/Arg3.1 expression should prevent these processes. Exactly this result has been obtained in that blockade of Arc/Arg3.1 induced by intra-NAc shell infusion of AS, which impaired the reconsolidation of morphine CPP in a long-term and irreversible manner. These results demonstrate that Arc/Arg3.1 is the key downstream molecule of the ERK-CREB pathway, which plays an essential role in the context-induced morphine CPP reconsolidation.
Studies on the molecular function of Arc/Arg3.1 showed that it is involved in multiple forms of synaptic plasticity (Guzowski et al., 2000; Pandey et al., 2008; Waung et al., 2008). One major mechanism that regulates synaptic strength involves the tightly regulated trafficking of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptors (AMPARs) into and out of synapses (Anggono and Huganir, 2012). In the present study, to explore Arc/Arg3.1’s function, we detected AMPA GluR1, GluR2, and GluR3 on cell surface expression when the Arc/Arg3.1 protein was induced in the NAc shell during morphine CPP reconsolidation. We found that AMPA GluR1 protein expression, but not AMPA GluR2 and GluR3 expression, was significantly increased at the cell-surface following Arc/Arg3.1 protein induction. Disrupting the induction of the Arc/Arg3.1 protein can normalize the GluR1 cell-surface expression level and impair both reconsolidation and reinstatement of morphine CPP. This result suggests that Arc/Arg3.1 in the NAc shell mediated morphine-associated context memory reconsolidation via up-regulating the cell-surface expression of GluR1 in the NAc shell. Significantly, the data is different from the previous finding of Arc/Arg3.1 regulated AMPAR endocytosis (Hearing et al., 2011). Further studies are needed to elucidate the mechanisms of synaptic capture of AMPA GluR1.
Conclusions
In conclusion, our current study suggests that the Arc/Arg3.1 increase in the NAc shell following the reexposure of morphine-associated context relied on the ERK-CREB activation and coupled the elevation of GluR1 membrane surface expression to promote synaptic plasticity. The findings provide new evidence that Arc/Arg3.1 is the key molecule during the reconsolidation of morphine CPP, and local disruption of Arc/Arg3.1 hopefully prevents the reconsolidation of opioid-conditioned memory and further suppresses relapse of drug abuse.
Statement of Interest
The funders had no participation in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no conflicts of interest (financial or otherwise) to declare related to the data presented in this manuscript.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation (grant number 31271163), the National Basic Research Program (grant number 2015CB553500) and Science Fund for Creative Research Groups from the National Natural Science Foundation ( grant number 81221002) of China.
References
- Anggono V, Huganir RL. (2012) Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol 22:461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. (1995) Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev 19:39–51. [DOI] [PubMed] [Google Scholar]
- Chia C, Otto T. (2013) Hippocampal Arc (Arg3.1) expression is induced by memory recall and required for memory reconsolidation in trace fear conditioning. Neurobiol Learn Mem 106:48–55. [DOI] [PubMed] [Google Scholar]
- Fricks-Gleason AN, Marshall JF. (2011) Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuropsychopharmacology 36:434–444. [DOI] [PubMed] [Google Scholar]
- Gusev PA, Gubin AN. (2010) Arc/Arg3.1 mRNA global expression patterns elicited by memory recall in cerebral cortex differ for remote versus recent spatial memories. Front Integr Neurosci 4:15. Retrieved 21 May 2010. 10.3389/fnint.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. (2000) Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci 20:3993–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. (2001) Fear memory retrieval induces CREB phosphorylation and Fos expression within the amygdala. Eur J Neurosci 13:1453–1458. [DOI] [PubMed] [Google Scholar]
- Hearing MC, Schwendt M, McGinty JF. (2011) Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. International Journal of Neuropsychopharmacology 14:784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T, Okuno H, Nonaka M, Adachi-Morishima A, Kyo N, Okamura M, Takemoto-Kimura S, Worley PF, Bito H. (2009) Synaptic activity-responsive element in the Arc/Arg3.1 promoter essential for synapse-to-nucleus signaling in activated neurons. Proc Natl Acad Sci USA 106:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. (2007) Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 10:355–362. [DOI] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S. (2003) Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci 23:5354–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, Pena de Ortiz S, Kogan JH, Chevere I, Masushige S, Silva AJ. (2002) CREB required for the stability of new and reactivated fear memories. Nat Neurosci 5:348–355. [DOI] [PubMed] [Google Scholar]
- Lin J, Liu L, Wen Q, Zheng C, Gao Y, Peng S, Tan Y, Li Y. (2014) Rapamycin prevents drug seeking via disrupting reconsolidation of reward memory in rats. Int J Neuropsychop 17:127–136. [DOI] [PubMed] [Google Scholar]
- Liu W, Peng QX, Lin XL, Luo CH, Jiang MJ, Mo ZX, Yung KK. (2014) Effect of rhynchophylline on the expression of p-CREB and sc-Fos in triatum and hippocampal CA1 area of methamphetamine-induced conditioned place preference rats. Fitoterapia 92:16–22. [DOI] [PubMed] [Google Scholar]
- Lv XF, Xu Y, Han JS, Cui CL. (2011) Expression of activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) in the nucleus accumbens is critical for the acquisition, expression and reinstatement of morphine-induced conditioned place preference. Behav Brain Res 223:182–191. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. (1995) Arc, a Growth-Factor and Activity-Regulated Gene, Encodes a Novel Cytoskeleton-Associated Protein That Is Enriched in Neuronal Dendrites. Neuron 14:433–445. [DOI] [PubMed] [Google Scholar]
- Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, Kida S. (2009) Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci 29:402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. (2006) Persistent disruption of an established morphine conditioned place preference. J Neurosci 26:3010–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. (2005) Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 47:873–884. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. (1968) Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science 160:554–555. [DOI] [PubMed] [Google Scholar]
- Nader K. (2003) Memory traces unbound. Trends Neurosci 26:65–72. [DOI] [PubMed] [Google Scholar]
- Nader K, Einarsson EO. (2010) Memory reconsolidation: an update. Ann NY Acad Sci 1191:27–41. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. (1992) Classical conditioning in drug-dependent humans. Ann NY Acad Sci 654:400–415. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang HB, Ugale R, Prakash A, Xu TJ, Misra K. (2008) Effector immediate-early gene Arc in the amygdala plays a critical role in alcoholism. J Neurosci 28:2589–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1997) The rat brain in stereotaxic coordinates. 3rd edition Amsterdam, Netherlands: Elsevier Academic. [Google Scholar]
- Robinson MJ, Armson M, Franklin KB. (2011) The effect of propranolol and midazolam on the reconsolidation of a morphine place preference in chronically treated rats. Front Behav Neurosci 5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168:3–20. [DOI] [PubMed] [Google Scholar]
- Steward O. (2002) Local synthesis of proteins at synaptic sites on dendrites: role in synaptic plasticity and memory consolidation? Neurobiol Learn Mem 78:508–527. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. (1998) Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron 21:741–751. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. (2007) Molecular mechanisms of memory reconsolidation. Nature reviews Neuroscience 8:262–275. [DOI] [PubMed] [Google Scholar]
- Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, Bundman M, Kuhl D. (2001) Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J Neurosci 21:5484–5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. (2008) Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron 59:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WP, Guzowski JF, Thomas SA. (2005) Mapping neuronal activation and the influence of adrenergic signaling during contextual memory retrieval. Learn Mem 12:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]