Abstract
Background:
Many patients with obsessive-compulsive disorder do not respond adequately to serotonin reuptake inhibitors. Augmentation with antipsychotic drugs can be beneficial in this regard. However, since new relevant randomized controlled trials evaluating new antipsychotics were conducted, a recalculation of the effect sizes appears necessary.
Methods:
We meta-analyzed all double-blind, randomized, placebo-controlled trials comparing augmentation of serotonin reuptake inhibitors with antipsychotics to placebo supplementation in treatment-resistant obsessive-compulsive disorder. The primary outcome was mean change in the Yale-Brown Obsessive–Compulsive Scale total score. Secondary outcomes were obsessions, compulsions, response rates, and attrition rates. The data collection process was conducted independently by 2 authors. Hedges’s g and risks ratios were calculated as effect sizes. In preplanned meta-regressions, subgroup analyses, and sensitivity analyses, we examined the robustness of the results and explored reasons for potential heterogeneity.
Results:
Altogether, 14 double-blind, randomized, placebo-controlled trials (n=491) investigating quetiapine (N=4, n=142), risperidone (N=4, n=132), aripiprazole (N=2, n=79), olanzapine (N=2, n=70), paliperidone (N=1, n=34), and haloperidol (N=1, n=34) were incorporated. Augmentation with antipsychotics was significantly more efficacious than placebo in Yale-Brown Obsessive–Compulsive Scale total reduction (N=14, n=478; Hedges’s g=-0.64, 95% CI: -0.87 to -0.41; P=<.01). Aripiprazole (Hedges’s g=-1.35), haloperidol (Hedges’s g=-0.82), and risperidone (Hedges’s g=-0.59) significantly outperformed placebo. Antipsychotics were superior to placebo in treating obsessions, compulsions, and achieving response. There was no between-group difference concerning all-cause discontinuation. The nonsignificant meta-regressions suggest no influence of the antipsychotic dose or baseline symptom severity on the meta-analytic results.
Conclusions:
According to our findings, antipsychotic augmentation of serotonin reuptake inhibitors can be regarded as an evidence-based measure in treatment-resistant obsessive-compulsive disorder.
Keywords: Obsessive-compulsive disorder, antipsychotics, serotonin reuptake inhibitors, treatment resistance, meta-analysis
Introduction
Cognitive behavioral therapy with exposure exercises and subsequent response prevention can be considered as well-established first-line psychotherapeutic treatment for obsessive-compulsive disorder (OCD) (Bandelow et al., 2008; Bandelow et al., 2012; Koran and Simpson, 2013; Baldwin et al., 2014). In terms of the pharmacological management, there is a large body of evidence for the efficacy of serotonin reuptake inhibitors (SRIs) comprising the selective serotonin reuptake inhibitors and the tricyclic antidepressant clomipramine (Soomro et al., 2008; Fineberg et al., 2013; Pallanti and Hollander, 2014). However, due to their favorable risk profile, preference should be given to the selective serotonin reuptake inhibitors (Bandelow et al., 2008; Bandelow et al., 2012; Koran and Simpson, 2013; Baldwin et al., 2014). Since up to 40%–60% of the OCD patients do not respond satisfactorily to SRI monotherapy (Pallanti and Quercioli, 2006), the question concerning the next therapeutic measures to achieve sufficient treatment response arises. One very frequently applied strategy in this regard contains an augmentation of SRIs with antipsychotic drugs, and recent prescription studies revealed a high and increasing prevalence of the administration of antipsychotics in OCD subjects (Comer et al., 2011; Van Ameringen et al., 2014). Previous reviews could demonstrate significant efficacy for this pharmacological approach, especially for add-on treatment with risperidone that gained the highest effect sizes in meta-analyses (Bloch et al., 2006; Dold et al., 2013). This caused the assumption that risperidone should be preferentially used as augmenting compound and that primarily the antidopaminergic properties of the antipsychotics are responsible for their efficacy in SRI-resistant OCD (Sesia et al., 2013; Ducasse et al., 2014). However, because new, relevant randomized controlled trials (RCTs) with newly introduced second-generation antipsychotic drugs were carried out and published in the meantime, a recalculation of the effect sizes appears necessary to ascertain the value of this augmentation strategy for the clinical routine care. Furthermore, the present meta-analysis is the first that sought to elucidate whether the adjunctive medication with antipsychotics is more beneficial in treating obsessions or compulsions. Thus, we covered and meta-analyzed all double-blind RCTs comparing antipsychotic augmentation of SRIs with placebo augmentation in OCD patients refractory to prior SRI monotherapy.
Methods
Inclusion Criteria: Trial Design
We incorporated all published and unpublished double-blind, parallel-group, placebo-controlled RCTs that enrolled OCD patients with inadequate response to previous pharmacotherapy with SRIs. Continuing the current SRI medication without any dose adjustments, the participants had to be randomized either to augmentation with antipsychotic drugs in the intervention group (SRI + antipsychotic) or adjunctive placebo in the control group (SRI + placebo).
Search Strategy
We used the results of the systematic literature search of a previous meta-analysis of our group (Dold et al., 2013) and updated this search by systematically screening the electronic medical databases ClinicalTrials.gov, Clinicaltrialsregister.eu, Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, PubMed/Medline, and PsycINFO (last search January 2015). Search terms were “obsessive-compulsive disorder” together with “antipsychotics,” “augmentation,” “treatment-resistant,” and the individual names of the single antipsychotics. Additionally, the reference lists of the included trials and relevant reviews/guidelines on this topic were searched manually. Furthermore, the manufacturers of antipsychotics were contacted for unpublished trials.
Outcome Criteria
The primary outcome was mean change (from baseline to endpoint) in the Yale–Brown Obsessive–Compulsive Scale (Y-BOCS) total score (Goodman et al., 1989). Secondary outcomes were mean changes in the Y-BOCS obsession and compulsion subscale, response rates (defined preferably by ≥35% Y-BOCS reduction), and the number of drop-outs due to any reason (all-cause discontinuation), to inefficacy, and to adverse effects.
Study Selection and Data Extraction
Study selection and data extraction were independently conducted by 2 authors (M.D., M.A.). Discrepancies were resolved through discussion and, if necessary, we contacted trial authors for clarification. Intention to treat data were used whenever available. We assumed for dichotomous data that study participants with premature termination of the trial had not achieved response. The workflow was accomplished according to the PRISMA statement to ensure a standardized data collection procedure (Moher et al., 2009).
Statistical Analyses
As effect sizes, we estimated standardized mean differences based on Hedges’s g for continuous outcomes (Y-BOCS changes) and Mantel-Haenszel risks ratios (MH-RRs) for dichotomous outcomes (response and attrition rates). Statistical significance was assumed if the associated 95% CIs did not comprise the numerical value of 0 (Hedges’s g) or 1 (MH-RR) and/or if the P-value of the comparison was <.05. The Mantel–Haenszel random-effects model of Der-Simonian and Laired (1986) was employed to calculate pooled continuous and binary effect sizes. The amount of heterogeneity between the individual studies was explored statistically with I2 statistic and chi2 test of homogeneity (significance level: I2>50%). If present, significant heterogeneity was reported and outlier-studies were excluded in post-hoc sensitivity analyses.
In unrestricted maximum-likelihood meta-regression analyses, we investigated the impact of the continuous moderators: (1) mean administered antipsychotic dose (calculated as olanzapine equivalents according to the International Consensus Study of Antipsychotic Dosing [Gardner et al., 2010]); and (2) baseline symptom severity (measured by the Y-BOCS total score before entering the double-blind supplementation phase) on the effect sizes. In a subgroup analysis, we explored the influence of the categorical variable minimum duration of adequate SRI treatment before randomization into augmentation groups (<8 weeks, ≥8 weeks and <12 weeks, or ≥12 weeks). To ensure that our findings were not biased by the inclusion of participants with comorbid tic disorders, we removed the relevant trials from the meta-analytic statistics within a sensitivity analysis. A potential publication bias was examined by funnel-plot visualization and accomplishment of Egger`s regression intercept test (2-tailed) (Egger et al., 1997). Moreover, we estimated the number of negative unpublished trials (fail-safe N value) that would be necessary to dissolve statistically significant differences (Orwin, 1983). All aforementioned statistical methods were performed for the primary outcome (significance level: P<.05). The software Comprehensive Meta-Analysis version 2.2 (Borenstein et al., 2006) and Review Manager (RevMan) version 5.3.5 (The Cochrane Collaboration, 2014) were used for the meta-analytic calculations.
Evaluation of the Methodological Trial Quality
The methodological quality of every included individual study was rated independently by 2 reviewers (M.D., M.A.) using the risk of bias tool of the Cochrane Collaboration (Higgins and Green, 2011). This measurement instrument comprises judgments concerning sequence generation, allocation concealment, blinding, outcome data presentation, selective reporting, and possible risks for other biases.
Results
Results of the Literature Search and Characteristics of the Included Trials
The updated literature search identified 191 references without duplications, and finally, 3 further trials (Sayyah et al., 2012; Simpson et al., 2013; Storch et al., 2013) could be included. Figure 1 displays the flow diagram of the literature search with a detailed description of the individual steps following the PRISMA statement.
Figure 1.
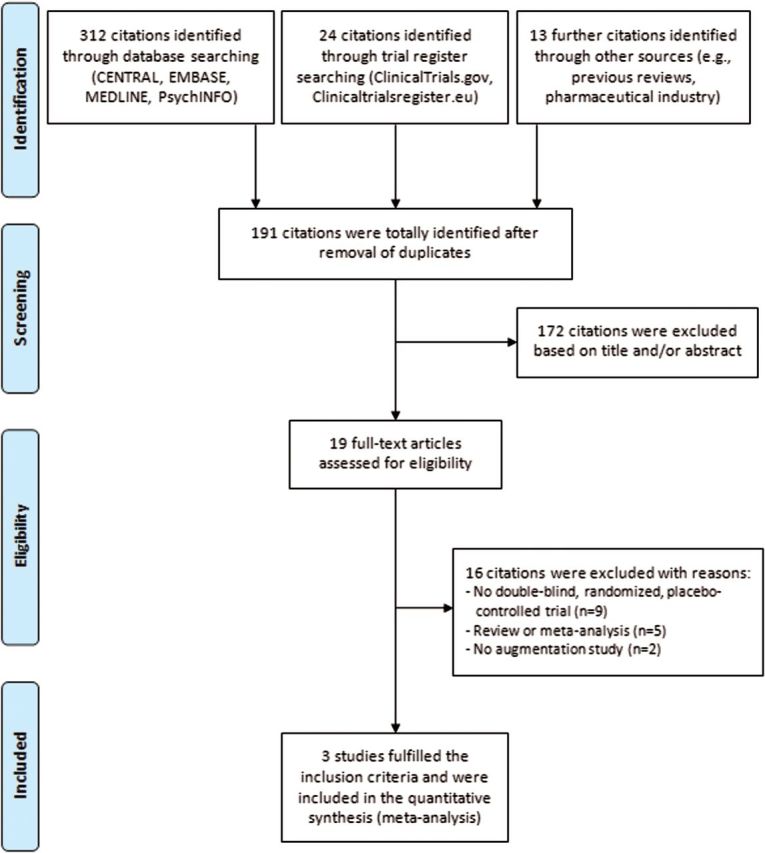
Flowchart of the systematic update literature search according to the PRISMA statement (Moher et al., 2009).
Altogether, this meta-analysis comprises 14 double-blind RCTs representing 491 participants with SRI-resistant OCD. Quetiapine (N=4, n=142) was the most frequently investigated antipsychotic drug followed by risperidone (N=4, n=132), aripiprazole (N=2, n=79), olanzapine (N=2, n=70), paliperidone (N=1, n=34), and haloperidol (N=1, n=34) (Table 1, supplementary Table S1). The duration of the double-blind study phase ranged from 4 (McDougle et al., 1994) to 16 weeks (Fineberg et al., 2005; Muscatello et al., 2011) (mean: 8.71±3.81 weeks), and the number of participants varied between 16 (Hollander et al., 2003) and 60 (Simpson et al., 2013) (mean: 35.14±11.47). The mean age of the participants was 37.15±3.03 years and the mean duration of illness 16.2±5.57 years; 49.3% of all participants were male. Six trials enrolled exclusively outpatients (Hollander et al., 2003; Denys et al., 2004; Fineberg et al., 2005; Muscatello et al., 2011; Sayyah et al., 2012; Simpson et al., 2013) and one study exclusively inpatients (Erzegovesi et al., 2005). The mean antipsychotic dose transferred to olanzapine equivalents ranged from 1.6 (Erzegovesi et al., 2005) to 12.4mg/d (McDougle et al., 1994) (mean: 7.63±3.05mg/d). Fluoxetine (n=≥116) and fluvoxamine (n=≥107) were the most frequently administered SRIs during the double-blind augmentation phase. One-half of the trials were carried out in the United States. Further study characteristics are summarized in supplementary Table S1.
Table 1.
Characteristics of the Double-Blind, Randomized, Placebo-Controlled Trials Included in the Meta-Analysis
| Trial; Country | Study Groups | Duration (wk) | Antipsychotic Dose | Definition of Resistance to Previous SRI Treatment | Sex (% male) |
Age in Years (mean ± SD) |
Y-BOCS Total Score at Baseline (mean ± SD) |
Duration of Illness in Years (mean ± SD) |
|---|---|---|---|---|---|---|---|---|
| Aripiprazole | ||||||||
| Muscatello et al. (2011); Italy | ARI: n=20 PLA: n=20 |
16 | ARI: 15mg/d (fixed dose) | Y-BOCS ≥16 after 1 adequate trial with an SRI for at least 12 wk | ARI: 37.5% PLA: 57.1% |
ARI: 39.4±14.5 PLA: 35.3±5.9 |
ARI: 24.8±5.2 PLA: 24.9±5.2 |
ARI: 5.3±2.7 PLA: 5.0±2.3 |
| Sayyah et al. (2012); Iran | ARI: n=18 OLA: n=21 |
12 | ARI: 10mg/d (fixed dose) | Y-BOCS ≥21 after a 3-mo period of monotherapy with SSRIs at MTD | ARI: 40% PLA: 47.1% |
ARI: 39±3.17 PLA: 37±3.53 |
ARI: 23.2±4.6 PLA: 25.1±4.5 |
Not indicated |
| Haloperidol | ||||||||
| McDougle et al. (1994); USA | HAL: n=17 PLA: n=17 |
4 | HAL: mean 6.2±3.0mg/d, max. 10mg/d |
Y-BOCS ≥16 or Y-BOCS reduction <35%, CGI-I ≥3, and consensus of the clinicians after 8wk fluvoxamine treatment | HAL: 70.6% PLA: 82.4% |
HAL: 35.0±12.0 PLA: 34.7±14.0 |
HAL: 25.4±5.0 PLA: 24.9±4.0 |
HAL: 18.6±13.5 PLA: 20.1±13.8 |
| Olanzapine | ||||||||
| Bystritsky et al. (2004); USA | OLA: n=13 PLA: n=13 |
6 | OLA: mean 11.2±6.5mg/d, max. 20mg/d |
Nonresponse to at least 2 trials of SRIs (at least 12wk) at an adequate dose and at least 1 trial of BT | OLA: 53.8% PLA: 47.2% |
OLA: 44.5±13.7 PLA: 38.3±9.1 |
OLA: 24.2±4.8 PLA: 25.2±4.2 |
Not indicated |
| Shapira et al. (2004); USA | OLA: n=22 PLA: n=22 |
6 | OLA: mean 6.1±2.1mg/d, max. 10mg/d | Nonresponse: Y-BOCS reduction <25%; partial response: Y-BOCS reduction ≥25% but CGI-S ≥4 and Y-BOCS ≥16 after 8wk fluoxetine treatment | 40.9% | 36.9±11.1 | OLA: 19.8±4.7 PLA: 19.9±3.3 |
Not indicated |
| Paliperidone | ||||||||
| Storch et al. (2013); USA | PAL: n=17 PLA: n=17 |
8 | PAL: mean 4.94±2.36mg/d, max. 9mg/d | Y-BOCS ≥19 after at least 2 adequate SRI trials for at least 12wk (at least 8wk in the present dose) | Not indicated | 43.7±11.4 | PAL: 27.1±5.68 PLA: 25.2±4.32 |
Not indicated |
| Quetiapine | ||||||||
| Carey et al. (2005); South Africa, Canada | QUE: n=20 PLA: n=21 |
6 | QUE: mean 168.75±120.82mg/d, max. 300mg/d | Y-BOCS reduction <25% or CGI-I ≥3 after at least 12wk SRI treatment (at least 6wk at adequate dose) | 46.34% | QUE: 33.8±9.7 PLA: 31.8±12.1 |
QUE: 26.4±4.6 PLA: 27.7±3.9 |
Not indicated |
| Denys et al. (2004), the Netherlands | QUE: n=20 PLA: n=20 |
8 | QUE: target dose: 200mg/d, max. 300mg/d | Y-BOCS ≥18 and Y-BOCS reduction <25% after at least 2 SRI trials at MTD and adequate duration (≥8wk) | QUE: 20% PLA: 30% |
QUE: 36±14 PLA: 34±10 |
QUE: 28.2±4.3 PLA: 26.4±6.3 |
QUE: 20±13 PLA: 20±9 |
| Fineberg et al. (2005); United Kingdom | QUE: n=11 PLA: n=10 |
16 | QUE: mean 215±124mg/d, max. 400mg/d | Y-BOCS ≥18 and Y-BOCS reduction <25% after at least 12 wk SSRI treatment at MTD (total ≥6 mo) | QUE: 27.3% PLA: 60% |
QUE: 37.4±11.4 PLA: 37.9±10.7 |
QUE: 24.5±4.6 PLA: 24.1±4.3 |
Not indicated |
| Kordon et al. (2008); Germany | QUE: n=20 PLA: n=20 |
12 | QUE: 400–600mg/d | Y-BOCS ≥18 and Y-BOCS reduction <25% after at least 12wk SRI treatment and at least 20h of CBT | 52.5% | Inclusion criterion: 18 to 65 | QUE: 24.1±5.0 PLA: 25.5±4.1 |
Not indicated |
| Risperidone | ||||||||
| Erzegovesi et al. (2005); Italy | RIS: n=10 PLA: n=10 |
6 | RIS: 0.5mg/d (fixed dose) | Y-BOCS reduction ≤35% and CGI-I ≥3 after 12wk fluvoxamine treatment | 53.3% | RIS: 38±13.4 PLA: 31.8±7.8 |
RIS: 30.9±4.7 PLA: 26.4±4.8 |
RIS: 15.2±10.4 PLA: 11.9±9.9 |
| Hollander et al. (2003); USA | RIS: n=10 PLA: n=6 |
8 | RIS: mean 2.25±0.86mg/d, max. 3mg/d |
CGI-I ≥3 after at least 2 SRI trials of adequate dose and duration (the last for at least 12wk) | RIS: 60% PLA: 50% |
RIS: 36.8±10.4 PLA: 43.2±15.8 |
RIS: 29.2±5.7 PLA: 29.3±2.8 |
RIS: 19.3±12.4 PLA: 26.0±21.1 |
| McDougle et al. (2000); USA | RIS: n=20 PLA: n=16 |
6 | RIS: mean 2.2±0.7mg/d, max. 6mg/d | Y-BOCS reduction <35% or Y-BOCS >16, CGI-I ≥3, and consensus of the therapists after 12wk of SRI treatment (at least 8wk at MTD) | RIS: 70% PLA: 43.8% |
RIS: 39.8±10.2 PLA: 34.6±10.3 |
RIS: 27.5±5.4 PLA: 27.7±3.6 |
RIS: 19.5±10.9 PLA: 14.9±11.6 |
| Simpson et al. (2013); USA | RIS: n=40 PLA: n=20 |
8 | RIS: mean 1.9±1.1mg/d, max. 4mg/d | Y-BOCS ≥16 after at least 12 wk SRI treatment at MTD | RIS: 47.5% PLA: 70% |
RIS: 33.8±10.8 PLA: 33.4±10.4 |
RIS: 26.1±4.3 PLA: 25.9±4.6 |
RIS: 16.2±11.4 PLA: 15.8±9.9 |
Abbreviations: ARI, aripiprazole; BT, behavior therapy; CBT, cognitive behavioral therapy; CGI-I, Clinical Global Impression Improvement Scale; CGI-S, Clinical Global Impression Severity Scale; HAL, haloperidol; max., maximum; MTD, maximum tolerated dose; OCD, obsessive-compulsive disorder; OLA, olanzapine; PAL, paliperidone; PLA, placebo; QUE, quetiapine; RIS, risperidone; SRI, serotonin reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; Y-BOCS, Yale–Brown Obsessive-Compulsive Scale.
The trial duration refers to the double-blind augmentation phase with antipsychotic or placebo. Further trial characteristics are provided in supplementary Table S1.
Methodological Quality of the Included Trials
Supplementary Figures S1 and S2 illustrate the single ratings for each item of the risk of bias tool. Briefly, 8 studies described an appropriate (mainly computer-based) randomization procedure (McDougle et al., 2000; Bystritsky et al., 2004; Carey et al., 2005; Erzegovesi et al., 2005; Muscatello et al., 2011; Sayyah et al., 2012; Simpson et al., 2013; Storch et al., 2013) and 3 adequate concealment of allocation (Bystritsky et al., 2004; Carey et al., 2005; Erzegovesi et al., 2005). The blinding was insufficiently indicated in 4 trials (Bystritsky et al., 2004; Shapira et al., 2004; Fineberg et al., 2005; Kordon et al., 2008) for both participants/personnel (performance bias) and outcome assessment (detection bias). Overall attrition (outcome data reporting) was low (<10%) in 6 studies (McDougle et al., 1994, 2000; Denys et al., 2004; Carey et al., 2005; Erzegovesi et al., 2005; Fineberg et al., 2005), moderate (10%-25%) in 4 (Hollander et al., 2003; Shapira et al., 2004; Sayyah et al., 2012; Simpson et al., 2013), and high (>25%) in 4 trials (Bystritsky et al., 2004; Kordon et al., 2008; Muscatello et al., 2011; Storch et al., 2013). Outcome data necessary to accomplish this meta-analysis have not been sufficiently reported (missing standard deviations) in 2 studies (McDougle et al., 1994; Erzegovesi et al., 2005). Regarding other potential sources of bias, we noticed that one trial was characterized by significant baseline imbalance in terms of sex distribution (Denys et al., 2004), and in Carey et al. (2005), the minimum duration of the previous SRI treatment at adequate doses was shorter (6 weeks) than in the other incorporated trials.
Primary Outcome: Mean Y-BOCS Total Score Change
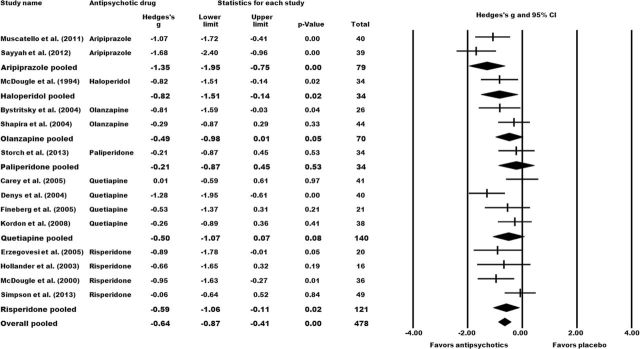
Augmentation of SRIs with antipsychotic drugs was significantly efficacious in the management of treatment-resistant OCD. The mean Y-BOCS total reduction was significantly higher in the pooled antipsychotic augmentation group than in the placebo group (N=14, n=478; Hedges’s g=-0.64, 95% CI: -0.87 to -0.41; P=<.01). Stratification according to the individual antipsychotics revealed significant superiority over placebo for aripiprazole (N=2, n=79; Hedges’s g=-1.35, 95% CI: -1.95 to -0.75; P=<.01), haloperidol (N=1, n=34; Hedges’s g=-0.82, 95% CI: -1.51 to -0.14; P=.02), and risperidone (N=4, n=121; Hedges’s g=-0.59, 95% CI: -1.06 to -0.11; P=.02). Olanzapine (N=2, n=70), paliperidone (N=1, n=34), and quetiapine (N=5, n=140) failed to differentiate significantly from placebo (Figure 2).
Figure 2.
Effect sizes for the primary outcome mean change in Y-BOCS total scores from baseline to study endpoint. Comparison: antipsychotic augmentation vs placebo augmentation. The forest plot illustrates the standardized mean differences based on Hedges’s g with the corresponding 95% CIs. Numerical values <0 indicate a higher Yale–Brown Obsessive–Compulsive Scale (Y-BOCS) reduction in the antipsychotic group compared to the control group. Statistical significance can be assumed if the 95% CI does not comprise the numerical value of 0 and/or if the P-value of the comparison is <.05.
Secondary Outcome: Obsessions and Compulsions
Antipsychotic drugs were significantly more efficacious than placebo in the management of both obsessions (N=6, n=199; Hedges’s g=-0.58, 95% CI: -0.89 to -0.27; P=<.01) and compulsions (N=6, n=199; Hedges’s g=-0.72, 95% CI: -1.06 to -0.38; P=<.01). Individually, we found a significant superiority of aripiprazole in treating obsessions (N=1, n=40; Hedges’s g=-0.77, 95% CI: -1.40 to -0.14; P=<.01) and compulsions (N=1, n=40; Hedges’s g=-1.20, 95% CI: -1.87 to -0.54; P=<.01), for quetiapine in treating obsessions (N=3, n=99; Hedges’s g=-0.66, 95% CI: -1.18 to -0.14; P=<.01), and for olanzapine in treating compulsions (N=1, n=26; Hedges’s g=-1.16, 95% CI: -1.97 to -0.35; P=<.01) (supplementary Figures S3 and S4).
Secondary Outcome: Response Rates
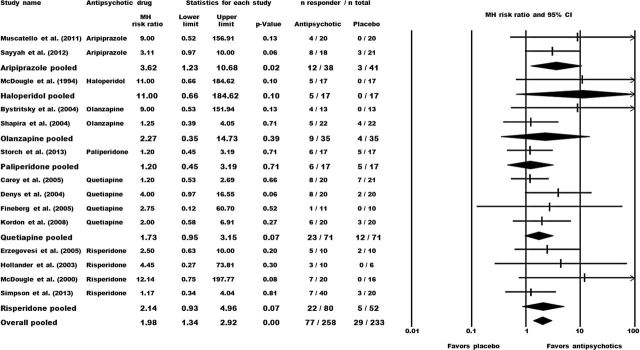
Altogether, significantly more patients allocated to antipsychotics exhibited treatment response (Y-BOCS reduction ≥35%) compared to placebo (response rates: 29.8% vs. 12.5%; N=14, n=491; MH-RR=1.98, 95% CI: 1.34 to 2.92; P=<.01). With regard to the individual drugs, only aripiprazole could significantly outperform placebo (N=2, n=79; MH-RR=3.62, 95% CI: 1.23 to 10.68; P=.02). Patients receiving haloperidol (N=1, n=34), olanzapine (N=2, n=70), paliperidone (N=1, n=34), quetiapine (N=4, n=142), and risperidone (N=4, n=132) did not significantly differ from placebo (Figure 3).
Figure 3.
Effect sizes for the response rates (preferably ≥35% Y-BOCS total improvement during the double-blind augmentation phase). Comparison: antipsychotic augmentation vs placebo augmentation. The forest plot illustrates the Mantel-Haenszel (MH) risk ratios with the corresponding 95% CIs. Numerical values >1 indicate a higher proportion of responders in the antipsychotic group compared to the control group. Statistical significance is present if the 95% CI does not include the numerical value of 1 and/or if the P-value of the comparison is <.05.
Secondary Outcome: Drop-Out Rates
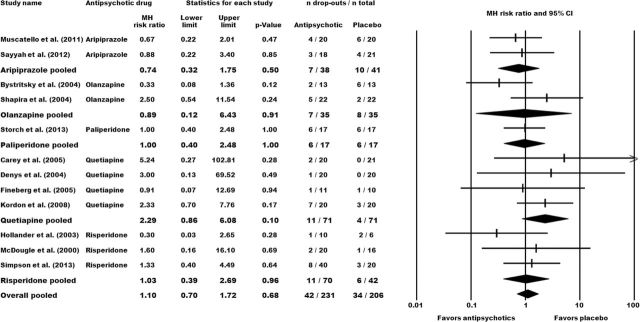
We did not identify any significant between-group differences for the number of drop-outs due to any reason (all-cause discontinuation) (N=12, n=437) and due to inefficacy (N=5, n=137); neither for the combined antipsychotic group nor for the individual antipsychotics (Figure 4, supplementary Figure S5). Attrition rates caused by the occurrence of adverse effects were significantly higher in the pooled antipsychotic group (N=8, n=302; MH-RR=2.38, 95% CI: 1.04 to 5.43; P=.04) (supplementary Figure S6).
Figure 4.
Effect sizes for the drop-out rate due to any reason (all-cause discontinuation). Comparison: antipsychotic augmentation vs placebo augmentation. The forest plot illustrates the Mantel-Haenszel (MH) risk ratios with the corresponding 95% CIs. Numerical values >1 indicate a higher attrition rate in the antipsychotic group compared with the control group.
Meta-Regressions and Subgroup-Analysis
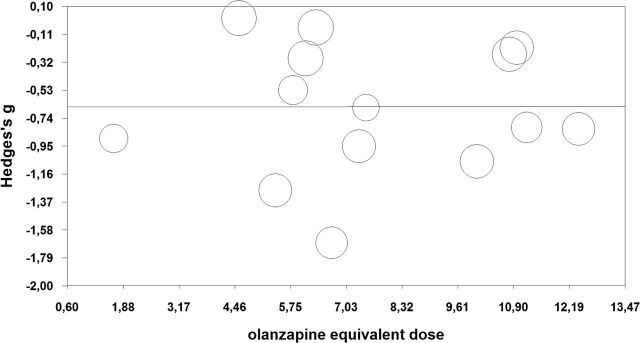
The preplanned, unrestricted, maximum-likelihood meta-regression analyses detected no significant relationship between the effect sizes and both the mean administered antipsychotic doses (P=.996) (Figure 5) and the Y-BOCS total score at baseline (P=.70) (supplementary Figure S7). The subgroup analysis investigating the minimal duration of adequate SRI treatment before randomization to the augmentation groups revealed significant differences in disfavor of the subgroup with the shortest time frame (<8 weeks). Both other subgroups (≥8 weeks to <12 weeks and ≥12 weeks) were statistically significantly more efficacious than the <8 weeks group (for both comparisons: P=<.01) (supplementary Figure S8).
Figure 5.
Unrestricted maximum-likelihood meta-regression with mean olanzapine equivalents as continuous moderator variable. Hedges’s g refers to the effect sizes of the primary outcome (mean Yale–Brown Obsessive–Compulsive Scale [Y-BOCS] total score change). The olanzapine dose equivalents were calculated following the International Consensus Study of Antipsychotic Dosing (Gardner et al., 2010). The circle size reflects the weight a study obtained in this meta-regression. Slope=0.0002, 95% CI: -0.09 to 0.09; P=.996.
Sensitivity Analysis: Exclusion of Trials Including Patients with Comorbid Tic Disorder
Removing the trials that included OCD patients with any comorbid tic disorder (McDougle et al., 1994, 2000; Shapira et al., 2004; Carey et al., 2005; Erzegovesi et al., 2005; Fineberg et al., 2005; Kordon et al., 2008; Simpson et al., 2013) did not alter the combined overall effect sizes in terms of statistical significance (N=6, n=195; Hedges’s g=-0.91, 95% CI: -1.23 to -0.60; P=<.01) (supplementary Figure S9). The comparison for haloperidol was no longer available.
Publication Bias
The funnel-plot visualization (supplementary Figure S10) and the nonsignificant Egger`s regression intercept test (P=.14) did not provide any evidence of the presence of publication bias. The fail-safe N value estimates that 160 negative unpublished trials are necessary to dissolve statistically significant differences regarding the primary outcome.
Discussion
Meta-analyzing 14 double-blind RCTs with a total of 491 participants, we found a significant efficacy for adding antipsychotic drugs to SRIs in treatment-resistant OCD. Aripiprazole, haloperidol, and risperidone significantly outperformed the control groups, whereas olanzapine, paliperidone, and quetiapine failed to demonstrate efficacy compared with placebo. The pooled effect size of -0.64 based on nearly 500 participants suggests that augmentation treatment with antipsychotics can be regarded as evidence-based treatment strategy in OCD patients refractory to monotherapy with SRIs. This obtained effect size is even higher than the mean continuous effect size (0.49) that was determined in a review of 33 psychopharmacological medications across all psychiatric disorders (Leucht et al., 2012). When translating our effect size to differences in means, the difference between the Y-BOCS total reduction in the pooled antipsychotic group and the pooled placebo group was 4.02 points. The significant results in favor of the adjunctive treatment with antipsychotics in terms of the response rates (antipsychotic: 29.8%; placebo: 12.5%) corroborate the positive findings of the primary outcome analysis. All in all, almost one-third of the study participants achieved treatment response (≥35% Y-BOCS improvement). In conclusion, the body of evidence for antipsychotic augmentation strategies in the management of therapy-refractory OCD is currently considerably larger than for any other pharmacological options. However, it should be taken into account that no antipsychotic compound is officially approved to treat OCD, and its use presents an off-label treatment. If tried, the antipsychotic medication should be closely monitored and quickly discontinued in case of inefficacy.
In this meta-analysis, we aimed to elucidate for the first time whether there is a different response to the add-on antipsychotic medication between prevailing obsessions or compulsions. As the effect sizes for both OCD subtypes significantly outperformed placebo (-0.58 for obsessions and -0.72 for compulsions), we see no justification to assume that one of these OCD subtypes can serve as compelling predictor for treatment response.
Discussion of the Results in the Context of Guidelines and Previous Reviews
Our meta-analytic findings are in agreement with the guidelines for the pharmacological treatment of OCD, which consistently advise antipsychotic augmentation in SRI-resistant conditions (Bandelow, 2008; Koran and Simpson, 2013; Baldwin et al., 2014). We could corroborate these recommendations with high-quality meta-analytic statistics. Furthermore, we could confirm previous meta-analytic findings demonstrating an efficacy of the adjunctive antipsychotic pharmacotherapy (Bloch et al., 2006; Skapinakis et al., 2007). These systematic reviews comprised a fewer number of individual trials and were mainly based on the analyses of dichotomous response rates. In our previous meta-analysis on this topic (Dold et al., 2013), we concluded that risperidone can be considered as the augmenting drug of first choice and should be preferred to olanzapine and quetiapine. However, in the present analysis, incorporating new relevant drugs and trials (Sayyah et al., 2012; Simpson et al., 2013; Storch et al., 2013), we found the highest effect size for aripiprazole (-1.35) followed by haloperidol (-0.82), risperidone (-0.59), quetiapine (-0.50), olanzapine (-0.49), and paliperidone (-0.21). Although not all effect sizes reached statistical significance in comparison to placebo, the differences of the Hedges’s g values are rather low. Moreover, the significant superiority of haloperidol and risperidone over placebo in terms of Y-BOCS improvement was not accompanied by significant superiority over placebo in achieving dichotomous treatment response. To appraise the efficacy of the individual antipsychotic agents, some trials that were excluded from our meta-analysis should also be taken into account. In a double-blind RCT investigating drug-naïve OCD patients, quetiapine addition to citalopram was significantly more efficacious than placebo supplementation (Vulink et al., 2009). In a single-blind, randomized head-to-head trial, there was no significant difference in efficacy between olanzapine and risperidone (Maina et al., 2008), and in another single-blind, randomized study, adjunctive risperidone was significantly superior to aripiprazole (Selvi et al., 2011).
Differently from our previous meta-analysis (Dold et al., 2013), we adjusted the inclusion criteria and required a stable dose of the ongoing SRI medication. We therefore excluded studies that performed dose adjustments in the maintaining SRI treatment in order to avoid meaningful clinical heterogeneity between the included RCTs. Hence, we excluded the study of Diniz et al. (2011) in which the fluoxetine dose was decreased from 80mg/d to 40mg/d in the quetiapine group, whereas it remained unchanged in the placebo group.
Tolerability
We did not find any statistically significant differences in terms of all-cause discontinuation. This outcome is more and more frequently employed in large effectiveness trials investigating antipsychotics (Lieberman et al., 2005; Kahn et al., 2008) because it combines efficacy and safety aspects of the pharmacotherapy. It should be taken into account with regard to the individual drug choice that any gains in efficacy are not necessarily accompanied by similar effects in such a global measure. We determined a significantly higher attrition rate due to adverse effects in the pooled antipsychotic group compared to the placebo group. This could probably be caused by the high amount of comorbidities in the trials that accounted mainly for this finding. Even though we did not systematically examine the occurrence of the specific adverse effects, we noticed in a descriptive way that the observed adverse effects in the antipsychotic augmentation groups are comparable with those that are covered in schizophrenia trials for which many systematic evaluations are available. The most frequently reported adverse effects were sedation, dryness of the mouth, headache, and increased appetite. Moreover, in OCD, the risk of induced psychosis by second-generation antipsychotic drugs, especially by clozapine, must be considered (Schirmbeck and Zink, 2012), although this did not emerge within the included trials. However, the potential advantages of the adjunctive antipsychotic medication should be carefully balanced against the risk of undesired effects.
Antipsychotic Dose
The antipsychotic doses applied within the individual trials were mainly moderate, and the nonsignificant meta-regression with the administered mean dose as moderator revealed no association between dose and treatment response. This suggests that high antipsychotic doses are not associated with high efficacy. Nevertheless, as no trials used high doses of antipsychotic medication, it cannot definitely be ruled out that potentially antipsychotic high-dose pharmacotherapy can improve the proportion of responders. However, we presume analogies to the use of second-generation antipsychotics in unipolar depression. In this indication, the officially approved dose ranges of the licensed antipsychotics are lower than those that are recommended to treat acute schizophrenic symptoms effectively.
Prior SRI Treatment
The subgroup analysis investigating the duration of SRI treatment before entering the double-blind augmentation phase failed to demonstrate significance for the studies with fewer than 8 weeks SRI medication. Although this finding is based on only one single trial (Carey et al., 2005), it should be considered that the significant Y-BOCS improvement in the placebo group of this trial prevented the identification of a significant superiority for quetiapine. Apparently, the symptom improvement in the control group reflects the response to the ongoing SRI medication during the placebo supplementation. This finding underlines the need for a sufficient long duration of the initial serotonergic medication to verify SRI nonresponse before a change of the pharmacological strategy should be considered.
Comorbid Tic Disorders
To consider a potential bias in favor of the antipsychotics caused by the inclusion of participants with comorbid tic disorders, we decided a priori to exclude individual trials that enrolled participants suffering from any tic disorder in a sensitivity analysis. Removing these trials did not cause diminished effect sizes, suggesting robustness of our findings. Nevertheless, it should be noted that some trials enrolled only a very small number of participants with tic disorders. Even in this case, we had to exclude the whole study from the meta-analytic calculations within the sensitivity analysis. Therefore, the findings in this regard can be interpreted only with reservation and the conclusions should be drawn very carefully.
Limitations and Strengths of the Meta-Analysis
Several clinical and methodological limitations potentially confining the conclusions of this meta-analysis should be considered. First of all, some antipsychotics drugs have not been examined in double-blind studies in SRI-resistant OCD. Therefore, their efficacy in this condition remains unknown. For example, it would be clinically meaningful to evaluate with an appropriate high-quality study design the efficacy of adjunctive amisulpride, a drug for which very promising results based on open studies and case series exist (Metin et al., 2003; Miodownik et al., 2015). Thus, it should be taken into account that future studies could probably change the overall findings. Moreover, we are currently not aware of double-blind direct (head-to-head) comparisons of antipsychotic drugs in SRI-resistant OCD.
A further limitation arises from the mostly small sample sizes in the individual studies (mean: 35 participants). Furthermore, the included RCTs differ in terms of the investigated participants’ collective (eg, degree of treatment-resistance), therapeutic modalities (eg, outpatient or inpatient treatment), trial duration, comorbidities, and the administered antipsychotic doses. However, neither the preplanned meta-regressions, subgroup analyses, and sensitivity analyses nor the statistical tests for detecting significant heterogeneity indicate the presence of possible methodological or clinical limitations hampering the conclusions of our statistical findings. With regard to the meta-regression investigating the baseline symptom severity as moderator variable, it should be considered that with one exception, all baseline Y-BOCS total scores are in the range between 24 and 30. Therefore, it does not appear justified to exclude on this basis any influence of the baseline symptom severity on the effect sizes, although the meta-regression analysis was not significant. The symmetrical funnel plot, the nonsignificant Egger’s regression intercept test, and the high fail-safe N value of 160 did not provide any evidence for the existence of a publication bias. However, we cannot definitely rule out that some study results, especially with negative findings, were not published and subsequently not covered by our systematic literature search. Therefore, the possibility of publication bias needs to be considered.
Despite these above-mentioned limitations, the strengths of our analyses should be highlighted. The methodological quality of the included trials was high. The outcomes of interest for this meta-analysis were substantially completely reported throughout all included individual studies, enhancing the validity of our statistical findings.
Even though we could verify that antipsychotic augmentation of SRIs significantly improved OCD symptoms refractory to SRI monotherapy, further research is still needed and should focus, for example, on the evaluation of the optimum antipsychotic dose, the optimal duration of the adjunctive treatment, the long-term tolerability, the identification of response predictors, and the elucidation of subgroups that could take an advantage of this treatment option with high probability. Our results do not suggest that predominant obsessions or compulsions are factors associated with response. From a clinical point of view, antipsychotics should be preferentially used if there are comorbidities for which antipsychotic drugs are indicated; for example, psychosis that represents a frequent comorbidity in OCD (Schirmbeck et al., 2013; Zink et al., 2014). Generally, it should be critically considered that in many trials, OCD subjects with clinically relevant comorbidities were excluded even if especially the presence of comorbidities is highly associated with treatment resistance in OCD (Pallanti and Quercioli, 2006).
Conclusions
Based on the results of 14 double-blind RCTs representing 491 resistant OCD patients, augmentation of SRIs with antipsychotic drugs can be considered as evidence-based treatment option in this condition. Aripiprazole, haloperidol (finding based on only one trial), and risperidone were significantly superior to placebo, whereas olanzapine, paliperidone, and quetiapine could not differentiate from placebo as measured by mean Y-BOCS total improvement.
Interest Statement
Dr. Dold has received a travel grant from Janssen-Cilag. Dr. Aigner has served as a consultant for CSC and has received travel grants and speakers fees from CSC, Eli Lilly, Germania, Janssen-Cilag, and Pfizer. Dr. Lanzenberger has received travel grants and conference speaker honoraria from AstraZeneca, Lundbeck A/S, Dr. Willmar Schwabe GmbH & Co. KG, and Roche Austria GmbH. Dr. Kasper has received grant/research support from Bristol Myers-Squibb, Eli Lilly, GlaxoSmithKline, Lundbeck, Organon, Pfizer, Sepracor, and Servier; he has served as a consultant or on advisory boards for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Lundbeck, Merck Sharp and Dome (MSD), Novartis, Organon, Pfizer, Schwabe, Sepracor, and Servier; and he has served on speakers’ bureaus for Angelini, AOP-Pharma, AstraZeneca, Bristol Myers-Squibb, Eli Lilly, Janssen, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, Sepracor, Servier, and Wyeth.
Supplementary Material
Acknowledgments
We thank Professors Diniz, Fineberg, McDougle, Muscatello, and Simpson for providing further information on their trials.
References
- Baldwin DS, Anderson IM, Nutt DJ, Allgulander C, Bandelow B, den Boer JA, Christmas DM, Davies S, Fineberg N, Lidbetter N, Malizia A, McCrone P, Nabarro D, O’Neill C, Scott J, van der Wee N, Wittchen HU. (2014) Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Clin Psychopharmacol 28:403–439. [DOI] [PubMed] [Google Scholar]
- Bandelow B. (2008) The medical treatment of obsessive-compulsive disorder and anxiety. CNS Spectr 13:37–46. [DOI] [PubMed] [Google Scholar]
- Bandelow B, et al. (2008) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders - first revision. World J Biol Psychiatry 9:248–312. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Sher L, Bunevicius R, Hollander E, Kasper S, Zohar J, Moller HJ, WFSBP Task Force on Anxiety Disorders, OCD, and PTSD (2012) Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract 16:77–84. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Landeros-Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF. (2006) A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry 11:622–632. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein H. (2006) Comprehensive meta-analysis, version 2. Englewood, New York: Biostat. [Google Scholar]
- Bystritsky A, Ackerman DL, Rosen RM, Vapnik T, Gorbis E, Maidment KM, Saxena S. (2004) Augmentation of serotonin reuptake inhibitors in refractory obsessive-compulsive disorder using adjunctive olanzapine: a placebo-controlled trial. J Clin Psychiatry 65:565–568. [DOI] [PubMed] [Google Scholar]
- Carey PD, Vythilingum B, Seedat S, Muller JE, van Ameringen M, Stein DJ. (2005) Quetiapine augmentation of SRIs in treatment refractory obsessive-compulsive disorder: a double-blind, randomised, placebo-controlled study. BMC Psychiatry 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer JS, Mojtabai R, Olfson M. (2011) National trends in the antipsychotic treatment of psychiatric outpatients with anxiety disorders. Am J Psychiatr 168:1057–1065. [DOI] [PubMed] [Google Scholar]
- Denys D, de Geus F, van Megen HJ, Westenberg HG. (2004) A double-blind, randomized, placebo-controlled trial of quetiapine addition in patients with obsessive-compulsive disorder refractory to serotonin reuptake inhibitors. J Clin Psychiatry 65:1040–1048. [DOI] [PubMed] [Google Scholar]
- Der-Simonian R, Laird N. (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. [DOI] [PubMed] [Google Scholar]
- Diniz JB, Shavitt RG, Fossaluza V, Koran L, Pereira CA, Miguel EC. (2011) A double-blind, randomized, controlled trial of fluoxetine plus quetiapine or clomipramine versus fluoxetine plus placebo for obsessive-compulsive disorder. J Clin Psychopharmacology 31:763–768. [DOI] [PubMed] [Google Scholar]
- Dold M, Aigner M, Lanzenberger R, Kasper S. (2013) Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol 16:557–574. [DOI] [PubMed] [Google Scholar]
- Ducasse D, Boyer L, Michel P, Loundou A, Macgregor A, Micoulaud-Franchi JA, Courtet P, Abbar M, Leboyer M, Fond G. (2014) D2 and D3 dopamine receptor affinity predicts effectiveness of antipsychotic drugs in obsessive-compulsive disorders: a metaregression analysis. Psychopharmacol 231:3765–3770. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzegovesi S, Guglielmo E, Siliprandi F, Bellodi L. (2005) Low-dose risperidone augmentation of fluvoxamine treatment in obsessive-compulsive disorder: a double-blind, placebo-controlled study. Eur Neuropsychopharmacol 15:69–74. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Sivakumaran T, Roberts A, Gale T. (2005) Adding quetiapine to SRI in treatment-resistant obsessive-compulsive disorder: a randomized controlled treatment study. Int Clin Psychopharmacol 20:223–226. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Reghunandanan S, Brown A, Pampaloni I. (2013) Pharmacotherapy of obsessive-compulsive disorder: evidence-based treatment and beyond. Aust N Z J Psychiatry 47:121–141. [DOI] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. (2010) International consensus study of antipsychotic dosing. Am J Psychiatr 167:686–693. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. (1989) Yale-Brown Obsessive-Compulsive Scale I. Development, use, and reliability. Arch Gen Psychiatry 46:1006–1011. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. (2011) Cochrane handbook for systematic reviews of interventions, version 5.1.0 [updated March 2011] The Cochrane Collaboration, available from www.cochrane-handbook.org.
- Hollander E, Baldini Rossi N, Sood E, Pallanti S. (2003) Risperidone augmentation in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. Int J Neuropsychopharmacol 6:397–401. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, Gheorghe MD, Rybakowski JK, Galderisi S, Libiger J, Hummer M, Dollfus S, Lopez-Ibor JJ, Hranov LG, Gaebel W, Peuskens J, Lindefors N, Riecher-Rossler A, Grobbee DE, EUFEST study group (2008) Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 371:1085–1097. [DOI] [PubMed] [Google Scholar]
- Koran LM, Simpson HB. (2013) Guideline watch (March 2013): practice guideline for the treatment of patients with obsessive-compulsive disorder. Arlington, VA: American Psychiatric Association. [PubMed] [Google Scholar]
- Kordon A, Wahl K, Koch N, Zurowski B, Anlauf M, Vielhaber K, Kahl KG, Broocks A, Voderholzer U, Hohagen F. (2008) Quetiapine addition to serotonin reuptake inhibitors in patients with severe obsessive-compulsive disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol 28:550–554. [DOI] [PubMed] [Google Scholar]
- Leucht S, Hierl S, Kissling W, Dold M, Davis JM. (2012) Putting the efficacy of psychiatric and general medicine medication into perspective: review of meta-analyses. Br J Psychiatry 200:97–106. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK, Clinical Antipsychotic Trials of Intervention Effectiveness Investigators (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223. [DOI] [PubMed] [Google Scholar]
- Maina G, Pessina E, Albert U, Bogetto F. (2008) 8-week, single-blind, randomized trial comparing risperidone versus olanzapine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder. Eur Neuropsychopharmacol 18:364–372. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Goodman WK, Leckman JF, Lee NC, Heninger GR, Price LH. (1994) Haloperidol addition in fluvoxamine-refractory obsessive-compulsive disorder. A double-blind, placebo-controlled study in patients with and without tics. Arch Gen Psychiatry 51:302–308. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Epperson CN, Pelton GH, Wasylink S, Price LH. (2000) A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 57:794–801. [DOI] [PubMed] [Google Scholar]
- Metin O, Yazici K, Tot S, Yazici AE. (2003) Amisulpiride augmentation in treatment resistant obsessive-compulsive disorder: an open trial. Hum Psychopharmacol 18:463–467. [DOI] [PubMed] [Google Scholar]
- Miodownik C, Bergman J, Lerner PP, Kreinin A, Lerner V. (2015) Amisulpride as add-on treatment for resistant obsessive-compulsive disorder: retrospective case series. Clin Neuropharmacol 38:26–29. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatello MR, Bruno A, Pandolfo G, Mico U, Scimeca G, Romeo VM, Santoro V, Settineri S, Spina E, Zoccali RA. (2011) Effect of aripiprazole augmentation of serotonin reuptake inhibitors or clomipramine in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. J Clin Psychopharmacol 31:174–179. [DOI] [PubMed] [Google Scholar]
- Orwin R. (1983) A fail-safe N for effect-size in meta-analysis. J Stat Educ 8:157–159. [Google Scholar]
- Pallanti S, Quercioli L. (2006) Treatment-refractory obsessive-compulsive disorder: methodological issues, operational definitions and therapeutic lines. Prog Neuropsychopharmacol Biol Psychiatry 30:400–412. [DOI] [PubMed] [Google Scholar]
- Pallanti S, Hollander E. (2014) Pharmacological, experimental therapeutic, and transcranial magnetic stimulation treatments for compulsivity and impulsivity. CNS Spectr 19:50–61. [DOI] [PubMed] [Google Scholar]
- Sayyah M, Sayyah M, Boostani H, Ghaffari SM, Hoseini A. (2012) Effects of aripiprazole augmentation in treatment-resistant obsessive-compulsive disorder (a double blind clinical trial). Depress Anxiety 29:850–854. [DOI] [PubMed] [Google Scholar]
- Schirmbeck F, Zink M. (2012) Clozapine-induced obsessive-compulsive symptoms in schizophrenia: a critical review. Curr Neuropharmacol 10:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmbeck F, Rausch F, Englisch S, Eifler S, Esslinger C, Meyer-Lindenberg A, Zink M. (2013) Stable cognitive deficits in schizophrenia patients with comorbid obsessive-compulsive symptoms: a 12-month longitudinal study. Schizophr Bull 39:1261–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvi Y, Atli A, Aydin A, Besiroglu L, Ozdemir P, Ozdemir O. (2011) The comparison of aripiprazole and risperidone augmentation in selective serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a single-blind, randomised study. Hum Psychopharmacol 26:51–57. [DOI] [PubMed] [Google Scholar]
- Sesia T, Bizup B, Grace AA. (2013) Evaluation of animal models of obsessive-compulsive disorder: correlation with phasic dopamine neuron activity. Int J Neuropsychopharmacol 16:1295–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira NA, Ward HE, Mandoki M, Murphy TK, Yang MC, Blier P, Goodman WK. (2004) A double-blind, placebo-controlled trial of olanzapine addition in fluoxetine-refractory obsessive-compulsive disorder. Biol Psychiatry 55: 553–555. [DOI] [PubMed] [Google Scholar]
- Simpson HB, Foa EB, Liebowitz MR, Huppert JD, Cahill S, Maher MJ, McLean CP, Bender J, Jr, Marcus SM, Williams MT, Weaver J, Vermes D, Van Meter PE, Rodriguez CI, Powers M, Pinto A, Imms P, Hahn CG, Campeas R. (2013) Cognitive-behavioral therapy vs risperidone for augmenting serotonin reuptake inhibitors in obsessive-compulsive disorder: a randomized clinical trial. JAMA Psychiatry 70:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapinakis P, Papatheodorou T, Mavreas V. (2007) Antipsychotic augmentation of serotonergic antidepressants in treatment-resistant obsessive-compulsive disorder: a meta-analysis of the randomized controlled trials. Eur Neuropsychopharmacol 17:79–93. [DOI] [PubMed] [Google Scholar]
- Soomro GM, Altman D, Rajagopal S, Oakley-Browne M. (2008) Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev:CD001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Goddard AW, Grant JE, De Nadai AS, Goodman WK, Mutch PJ, Medlock C, Odlaug B, McDougle CJ, Murphy TK. (2013) Double-blind, placebo-controlled, pilot trial of paliperidone augmentation in serotonin reuptake inhibitor-resistant obsessive-compulsive disorder. J Clin Psychiatry 74:e527–532. [DOI] [PubMed] [Google Scholar]
- The Cochrane Collaboration (2014) Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre. [Google Scholar]
- Van Ameringen M, Simpson W, Patterson B, Dell’Osso B, Fineberg N, Hollander E, Hranov L, Hranov G, Lochner C, Karamustafalioglu O, Marazziti D, Menchon JM, Nicolini H, Pallanti S, Stein DJ, Zohar J. (2014) Pharmacological treatment strategies in obsessive compulsive disorder: a cross-sectional view in nine international OCD centers. J Psychopharmacol 28:596–602. [DOI] [PubMed] [Google Scholar]
- Vulink NC, Denys D, Fluitman SB, Meinardi JC, Westenberg HG. (2009) Quetiapine augments the effect of citalopram in non-refractory obsessive-compulsive disorder: a randomized, double-blind, placebo-controlled study of 76 patients. J Clin Psychiatry 70:1001–1008. [DOI] [PubMed] [Google Scholar]
- Zink M, Schirmbeck F, Rausch F, Eifler S, Elkin H, Solojenkina X, Englisch S, Wagner M, Maier W, Lautenschlager M, Heinz A, Gudlowski Y, Janssen B, Gaebel W, Michel TM, Schneider F, Lambert M, Naber D, Juckel G, Krueger-Oezguerdal S, et al. (2014) Obsessive-compulsive symptoms in at-risk mental states for psychosis: associations with clinical impairment and cognitive function. Acta Psychiatr Scand 130:214–226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.