Abstract
Background:
Schizophrenia has been associated with disturbances of thalamic functioning. In light of recent evidence suggesting a significant impact of the glutamatergic system on key symptoms of schizophrenia, we assessed whether modulation of the glutamatergic system via blockage of the N-methyl-d-aspartate (NMDA)-receptor might lead to changes of thalamic functional connectivity.
Methods:
Based on the ketamine model of psychosis, we investigated changes in cortico-thalamic functional connectivity by intravenous ketamine challenge during a 55-minute resting-state scan. Thirty healthy volunteers were measured with pharmacological functional magnetic resonance imaging using a double-blind, randomized, placebo-controlled, crossover design.
Results:
Functional connectivity analysis revealed significant ketamine-specific changes within the thalamus hub network, more precisely, an increase of cortico-thalamic connectivity of the somatosensory and temporal cortex.
Conclusions:
Our results indicate that changes of thalamic functioning as described for schizophrenia can be partly mimicked by NMDA-receptor blockage. This adds substantial knowledge about the neurobiological mechanisms underlying the profound changes of perception and behavior during the application of NMDA-receptor antagonists.
Keywords: ketamine, functional MRI, thalamus, glutamate, schizophrenia
Introduction
In the last years, compelling evidence has been raised that the glutamatergic system plays a major role in the neurobiology of schizophrenia. This has been supported by the observation that dissociative anesthetics, which block the N-methyl-d-aspartate (NMDA) receptor, such as phencyclidine and ketamine, can elicit transient effects in healthy volunteers, which mimic positive, negative, and cognitive symptoms seen in patients with schizophrenia. Furthermore, previous studies using ketamine in healthy volunteers have reported a significant impact of NMDA blockage on neuronal activation and functional connectivity (Anticevic et al., 2012; Driesen et al., 2013). Ketamine-induced changes of neuronal activation have been shown during a number of MRI tasks with major relevance in the context of schizophrenia, such as self-monitoring, attentional functions, and affective processing (Abel et al., 2003; Daumann et al., 2010; Stone et al., 2011) as well as during resting state (Scheidegger et al., 2012; Driesen et al., 2013). Furthermore, a recent multimodal imaging study using simultaneous acquisition of functional MRI (fMRI) and EEG during a visual oddball task could show a significant impact of a subanesthetic dose of ketamine both on the blood oxygenation level dependent (BOLD) signal and the P300 amplitude. These effects were evident in regions involved in sensory processing and selective attention (Musso et al., 2011). The implication of glutamatergic dysfunction in schizophrenia has been further substantiated by studies in patients with schizophrenia showing abnormalities of glutamatergic pathways compared with healthy volunteers. Postmortem studies in patients with schizophrenia showed significant alterations of glutamate metabolism, with abnormalities of phosphate-activated glutaminase and glutamic acid decarboxylase activities and NMDA receptor binding (Kornhuber et al., 1989; Gluck et al., 2002). More recently, a number of functional neuroimaging studies showed changes of glutamine levels or the glutamine/glutamate ratio in the anterior cingulate cortex and the thalamus in patients with schizophrenia in vivo as measured with magnetic resonance spectroscopy (MRS) (Theberge et al., 2002; Bustillo et al., 2010; Kegeles et al., 2012), indicating higher turnover rates of glutamate. It was also shown that clinical response to antipsychotic treatment is associated with the degree of glutamatergic dysfunction as assessed with MRS (Szulc et al., 2013; Egerton et al., 2012).
Clinical symptoms seen in patients with schizophrenia may partly arise from dysfunction in sensory processing. Therefore, the thalamus as a major modulator of integration of sensory, cognitive, and emotional information has gained particular attention. In this context, several studies have investigated the involvement of the glutamatergic system in thalamic dysfunction in schizophrenia (Meador-Woodruff et al., 2003; Watis et al., 2008). It was thus shown that glutamatergic transmission of the thalamus is disturbed in patients with schizophrenia, with histological, structural, and functional findings supporting this concept (Clinton and Meador-Woodruff, 2004; Smith et al., 2011).
A number of task-independent functional networks, so-called resting-state networks, have been defined and investigated in healthy individuals, patients suffering from neuropsychiatric disorders, and during pharmacological interventions (Whitfield-Gabrieli and Ford, 2012). Subsequently, these networks were shown to be associated with a number of functions, which are highly relevant in the context of schizophrenia. Tomasi and Volkow (2011) were able to detect the thalamus hub network involving the thalamus, motor, premotor, visual, auditory, and limbic regions and the cerebellum in a large cohort of healthy volunteers. This network was shown to represent a functional correlate of the sensory gating function of the thalamus based on the extensive functional connections to primary sensory cortical areas and limbic regions such as the cingulate gyrus. A number of neuroimaging studies in animals and humans have shown differential involvement of thalamic nuclei and their functional projections in distinct behavioral processes as recently reviewed by Metzger et al. (2013).
Several fMRI investigations in patients with schizophrenia have demonstrated significant disruption of thalamo-cortical connectivity compared with healthy volunteers. Using various analysis techniques for fMRI data, these studies showed connectivity dysfunctions, in particular dysfunction of thalamo-prefrontal connections (Szulc et al., 2013; Meador-Woodruff et al., 2003; Watis et al., 2008). Importantly, alterations of cortico-thalamic connectivity with reduced prefrontal-thalamic connectivity and increased motor/somatosensory-thalamic connectivity in patients with schizophrenia were recently reported (Zhang et al., 2010; Woodward et al., 2012).
Based on this vast scientific evidence relating the glutamatergic system in general and the NMDA receptor more specifically to the neurobiology of schizophrenia, the aim of the present study was to further investigate the involvement of NMDA blockage on thalamic functioning. Using the ketamine model of psychosis, we assessed time-dependent changes of functional connectivity before, during, and after intravenous application of a subanaesthetic dose of ketamine in healthy volunteers using long-term resting-state fMRI lasting for 55 minutes. Two functional systems were analyzed using 2 independent seed-voxel correlation analyses. First, we analyzed the thalamus hub network described by Tomasi and Volkow (2011) for assessment of overall thalamic functional connectivity. Second, we analyzed cortico-thalamic functional connections using the analysis approach recently published by Woodward et al. (2012) to assign network changes more specifically to different connections of thalamic nuclei. Based on the already existing literature, the goal of this study was thus to further explore the involvement of specific functional connections of the thalamus in the schizophrenia-like state seen in healthy volunteers caused by the administration of ketamine.
Methods
Participants
Thirty-five healthy volunteers (mean age±SD: 25±4.58, 18 males) without any history of psychiatric, neurological, or somatic disorders were included in the study. Recruitment was performed by advertisement on dedicated message boards of the General Hospital of Vienna. Prior to inclusion, potential participants underwent a medical examination and a psychiatric interview performed by experienced psychiatrists. To ensure psychiatric health, the Structured Clinical Interview for DSM-IV was performed as well as routine medical check-up, including physical examination, electrocardiogram, and blood and urine analyses. Exclusion criteria were the history of any psychiatric, neurological, or relevant somatic illness; current or former substance abuse; current use of any prescribed or nonprescribed medication; treatment with psychotropic agents within the last 6 months; lifetime use of antipsychotic drugs; the existence of first-degree relatives with a history of psychiatric illness or substance abuse; pregnancy; or any contraindication for the performance of MRI. All procedures were approved by the Ethics committee of the Medical University of Vienna, and the study was registered at the European Union Drug Regulating Authorities Clinical Trials (EudraCT Nr. 2010-022772-31) and approved by the Austrian Federal Office for Safety in Health Care. The study was further registered on clinicaltrials.gov (NCT01394757).
Study Design and Medication
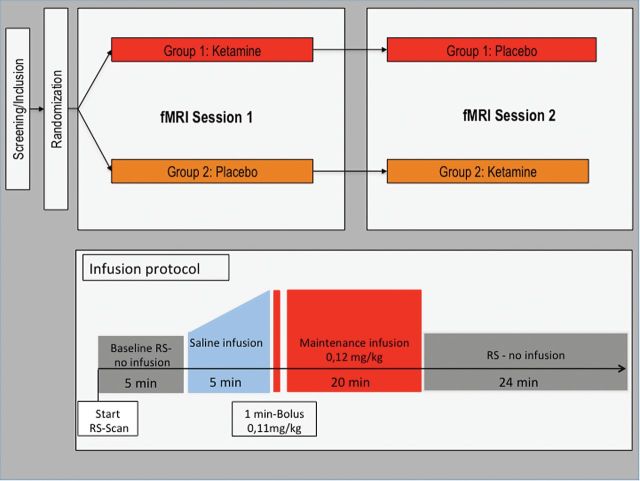
Thirty of 35 participants (25±4.58 years, 18 males) included in the study completed 2 fMRI sessions in a double-blind, placebo-controlled, randomized, crossover study design. Patients were randomly assigned to 1 of 2 experimental arms, one receiving ketamine at first measurement and placebo at the second measurement, the other vice versa (for a graphical depiction of the study design, see Figure 3).
Figure 3.
Graphical representation of study design, resting-state fMRI protocol, and the application of the study drug.
The study drug was provided in standard syringes of 55-mL volume containing either 5mL S-ketamine (Ketanest S 5mg/mL ampoules, Actavis Italy S.P.A./Pfizer) diluted in 50mL 0.90% NaCl or placebo (55mL of 0.90% NaCl). The administration of the study drug was performed using an MR-compatible fully-automated infusion system (65/115 Injector System, MEDRAD) in the MRI scanner. The MR-compatible infusion system consisted of 2 separate syringes, one of which was filled with the study drug and the other with a standard volume of 100mL of 0.90% NaCl. The ketamine dosage was applied using a 1-minute bolus of 0.11mg/kg body weight followed by a maintenance infusion of 0.12mg/kg for 19 minutes. The dosage was based on previous studies using the S(+)-enantiomer of ketamine, which was shown to have a 3 to 4 times higher affinity or potency at specific receptors than the R(-)enantiomer, usually leading to a 50% reduction of the dosage compared with studies using the racemate of ketamine (Sinner and Graf, 2008). Furthermore, the dosage was chosen to lie within a range that provokes reliable psychoactive effects and shows tolerability of an application in the MRI scanner and the following MRI measurement of about 1 hour, which was validated in a pilot study prior to the main investigation (n=10). Equal dosage per kilogram body weight as well as constant bolus and maintenance times across subjects were ensured by adjusting the flow rate of the infusion. The infusion protocol was timed to begin 5 minutes after the start of the resting state scan. First, a standardized infusion of 0.90% NaCl for 5 minutes with increasing injection speed was applied to ensure that subjects did not know from the injection itself when ketamine was administered. On few occasions, long fMRI scanning times caused technical problems and/or subjects to preemptively terminate the scan towards the end of the measurement. Thus, data for the time period between 22.5 and 55 minutes after the start of the resting-state scan refer to 25 participants, whereas results for the preceding time periods refer to all 30 participants. After the MRI measurement, participants were observed in the clinical setting for a minimum of 2 hours to ensure the lack of side effects. This period further comprised a debriefing done with the experimenter and collection of blood samples for the analysis of plasma ketamine levels (see below). Furthermore, participants were asked if they fell asleep during the resting-state scan; this information was recorded in addition to neurophysiological data, which gave indirect information about the status of the participants during the scan.
Psychometric Measures
PSychoactive effects of ketamine were assessed using the German versions of Positive and Negative Syndrome Scale (PANSS), the Brief Psychiatric Rating Scale (BPRS) and the Altered State of Consciousness Scale (5D-ASC) (Dittrich, 1998). PANSS and BPRS were tested both before and after the MRI measurement to have a baseline value for every participant.
Resting-State fMRI Acquisition and Analysis
During the resting-state scan, subjects were instructed to relax with eyes open, let their mind wander, and not think of anything specific, as previously described (Weissenbacher et al., 2009; Hahn et al., 2011). Resting-state measurements were performed at 3 Tesla (Siemens Trio, Erlangen, Germany) using single-shot gradient-recalled echo planar imaging (EPI) with repetition time/echo time (TR/TE)=1800/38ms, a matrix size of 128×128 voxel, and a field-of-view of 190×190mm. Hence, voxel size was 1.48×1.48×3.0mm with 23 axial slices (slice gap =1.8mm).
Functional Connectivity Analysis
Preprocessing was performed as previously described (Hahn et al., 2013) using SPM8 (www.fil.ion.ucl.ac.uk/spm) with default parameters unless specified. Preprocessing included correction for slice-timing differences (reference=middle slice) and head motion (reference=mean image), normalization to Montreal Neurological Institute space using a scanner-specific EPI template, spatial smoothing with an isotropic Gaussian kernel of 9mm FWHM. In addition to standard preprocessing, resting-state data were corrected as proposed in a previous study (Weissenbacher et al., 2009) and successfully used in other studies of our research laboratory (eg, Hahn et al., 2011). In short, linear regression was used to correct for changes in movement-related, ventricular, white matter, and global signals as well as physiological confounds (heart rate; blood pressure). Data were band-pass filtered with a 12-term finite impulse response filter (0.007<f<0.08 Hz). Head motion was quantified from realignment parameters as framewise displacement (Power et al., 2012). Functional connectivity analysis was carried out by applying a seed-based approach (Biswal et al., 1995).
Analysis 1: Functional Connectivity of the Thalamus Hub Network
To investigate overall connectivity changes of the thalamus during ketamine infusion, the thalamus hub network as defined by Tomasi and Volkow (2011) was used as seed region. To avoid seed selection bias (Cole et al., 2010) for the thalamus hub network, we used the averaged time courses of 4 seeds, namely the thalamus bilaterally (x/y/z=10,-10/-18/8mm MNI space), the cingulate cortex (x/y/z=0/12/38mm), and the lingual gyrus (x/y/z =0/-80/-1mm).
To evaluate the time course of the effect of ketamine on functional connectivity, the total time-period of ketamine infusion (20 minutes) plus the following 35 minutes were split in periods of 2.5 minutes, as previously proposed in pharmaco-fMRI (McKie et al., 2005). Spontaneous fluctuations in BOLD signal within these periods in the respective seed region were correlated voxel-wise with the entire brain. Here, the average time course across all 4 seeds (cubes of 3×3×3 voxel at each coordinate) was correlated with the entire brain. Correlation maps were converted to z-values using Fisher’s r-to-z transformation to enable statistic comparisons across subjects.
Analysis 2: Cortico-Thalamic Functional Connectivity
For the calculation of thalamo-cortical connectivity, the cortex was divided in nonoverlapping regions of interest, as previously proposed (Behrens et al., 2003; Zhou et al., 2007; Woodward et al., 2012). The prefrontal cortex, motor cortex/supplementary motor area, somatosensory cortex, temporal lobe, posterior parietal cortex, and occipital lobe were defined with the automated anatomical labeling atlas (Tzourio-Mazoyer et al., 2002) included in the MRIcro software package (http://www.sph.sc.edu/comd/rorden/mricro.html). Similar to the assessment of the thalamus hub network, BOLD signal fluctuations of 2.5-minute blocks were correlated between respective seeds and the entire brain. Correlation maps were converted to z-values, whereas statistical evaluation was focused on the thalamus.
Statistical Analysis
Using repeated-measures ANOVA in SPM8, the interaction effect of time (ie, 2.5-minute periods of infusion vs baseline) and drug (ie, ketamine vs placebo) was evaluated for both the cortico-thalamic connectivity and the thalamus hub network analysis. Following an overall F-test, posthoc t tests were computed. Hence, for each 2.5-minute time period, the change from baseline during the ketamine condition was compared with the corresponding change from baseline in the placebo condition. Again, the baseline in each condition was given by a 5-minute resting-state period before the infusion. Statistical inference was drawn at P<.05 corrected using the family wise error rate (FWE) at voxel level. Since for the cortico-thalamic connectivity we were only interested in subregions of the thalamus, small volume correction was applied using the thalamus of the anatomical labeling atlas as a mask (P<.05 FWE-corrected voxel level).
Blood Samples
As the collection of blood samples during MRI measurements may disturb participants as well as introduce changes in the magnetic field and thus signal fluctuations, ketamine levels were measured via HPLC and triple-quadrupol mass spectrometry on blood samples taken after the resting-state MRI scan, that is, 60, 75, 90, and 120 minutes after the end of the drug application on the descending part of the pharmacokinetic curve.
Results
Descriptive data are summarized in Table 1. The mean dosage of intravenous ketamine was 15.46±3.13mg, with mean ketamine plasma level of 17.77±4.49ng/mL (10–27ng/mL) 60 minutes after the end of the administration of ketamine. Compared with placebo, the infusion of ketamine led to a significant increase on the positive and negative syndrome scale (PANSS) and the Altered State of Consciousness Scale. There was no significant difference between the ketamine and placebo condition regarding movement during the MRI scan (ketamine mean±SD=0.12±0.06mm; placebo mean±SD =0.13±0.06mm; P=.57 paired t test).
Table 1.
Clinical Effects of Ketamine on Neuropsychological Parameters
| Test | Placebo Scan | Ketamine Scan | P |
|---|---|---|---|
| PANSS | |||
| Positive scale | 7.2±0.4 | 14.5±4.7 | <.001 |
| Negative scale | 7.3±1.0 | 9.2±2.9 | .002 |
| General scale | 16.6±1.5 | 30.4±8.9 | <.001 |
| Overall score | 31.1±2.4 | 54.1±14.6 | <.001 |
| 5D-ASC | |||
| Oceanic boundlessness | 1.45±3.3 | 84.26±57.9 | <.001 |
| Anxious ego-dissolution | 3.08±5.6 | 40±34.83 | <.001 |
| Visual reconstruction | 0.6±2.5 | 44.9±33.9 | <.001 |
| Auditory alterations | 1.63±4.3 | 21.72±18,8 | <.001 |
| Vigilance reduction | 8.75±12.8 | 40.33±25.15 | <.001 |
Abbreviations: 5D-ASC, Altered State of Consciousness Scale, PANSS, Positive and Negative Syndrome Scale.
P-values derived from a paired t test; mean values are indicated±SD; n=30.
Analysis 1: Ketamine Effects on the Thalamus Hub Network
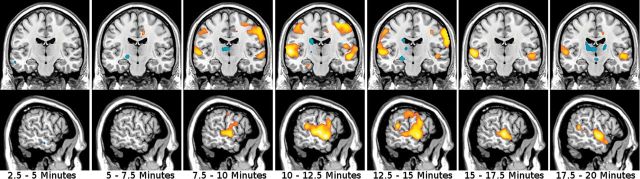
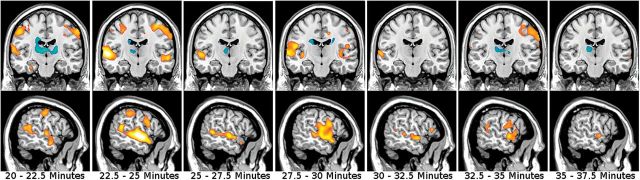
The investigation of the thalamus hub network showed significantly higher functional connectivity within the network in the ketamine condition compared with placebo. The overall F-test of the interaction (levels: drug+placebo; 22 time points of 2.5 minutes) showed significant results with a maximum P [41,984]=.001 (FWE-corrected, voxel-level) for bilateral temporo-parietal areas. Posthoc t tests of the interaction drug*time revealed a significant increase of connectivity 2.5 minutes after the start of the ketamine infusion in a bilateral cluster extending from the superior parietal lobule toward the temporal cortex, including the post- and precentral gyri. This cluster proved to be largely stable over the total time period of ketamine infusion as shown in Figure 1 and Table 2 (peak t=6.51). After the infusion, significant differences in temporal regions (peak t=5.48, P<.001, FWE-corrected) remained evident until 17.5 minutes after the end of the ketamine application with no significant results thereafter.
Figure 1.
Ketamine effects on functional connectivity of the thalamus hub network (analysis 1). Each period of 2.5 minutes is depicted. Interaction effects drug*time of posthoc t tests are displayed and data overlaid on a standard-MNI brain. Warm colors stand for increase of connectivity and cold colors for decreased connectivity, while color intensity refers to t-values (range t=3.09…6). A significant increase is shown in temporo-parietal regions throughout the ketamine application. x=-58mm, y=-16mm.
Table 2.
Differences of Functional Connectivity of the Thalamus Hub Network (Analysis 1) during and after Ketamine Infusion
| Time | Anatomical Region (Anatomical Labeling Atlas) | MNI Coordinates | Statistic | ||
|---|---|---|---|---|---|
| x | y | z | T-value | ||
| 2.5–5 min | Calcarine L | -16 | -80 | 4 | -5.19 |
| 5–7.5 min | Anterior cingulate cortex L | -12 | 30 | 26 | -5.16 |
| Gyrus postcentralis L | -48 | -24 | 44 | 4.61 | |
| Gyrus postcentralis R | 64 | -14 | 30 | 5.04 | |
| Middle temporal gyrus L | -58 | -14 | 0 | 5.36 | |
| 7.5–10 min | Inferior occipital gyrus R | 40 | -86 | -8 | 4.74 |
| Gyrus precentralis R | 36 | -14 | 60 | 5.09 | |
| Superior temporal gyrus L | -58 | -4 | -6 | 5.55 | |
| 10–12.5 min | Gyrus precentralis R | 62 | 8 | 32 | 4.91 |
| Supramarginal gyrus R | 64 | -18 | 34 | 4.82 | |
| Rolandic operculum R | 48 | 4 | 14 | 4.74 | |
| Superior temporal gyrus L | -58 | -4 | -4 | 5.71 | |
| 12.5–15 min | Middle Temporal Gyrus L | -58 | -14 | 0 | 4.87 |
| Middle Temporal Gyrus L | -56 | -8 | -8 | 5.79 | |
| 15–17.5 min | Superior Temporal Pole L | -54 | 8 | -18 | 4.68 |
| Superior Temporal Gyrus R | 56 | -8 | 0 | 4.75 | |
| 17.5–20 min | Thalamus R | 18 | -16 | 8 | -4.60 |
| Middle temporal pole R | 56 | 14 | -22 | 4.85 | |
| Gyrus precentralis R | 62 | 4 | 32 | 4.64 | |
| 20–22.5 min | Superior temporal gyrus L | -58 | -6 | 6 | 6.51 |
| Middle temporal pole R | 54 | 10 | -18 | 4.74 | |
| 22.5–25 min | Middle temporal gyrus L | -56 | -14 | -2 | 4.61 |
| Superior temporal pole R | 52 | 8 | -12 | 5.19 | |
| 25–27.5 min | Superior temporal gyrus L | -62 | -2 | -6 | 5.00 |
| Middle temporal gyrus L | -64 | -28 | 4 | 4.96 | |
| Rolandic operculum R | 52 | 6 | 12 | 4.91 | |
| Insula L | -40 | -2 | 2 | 4.87 | |
| Insula R | 38 | 2 | 6 | 4.85 | |
| Rolandic operculum L | -52 | -16 | 16 | 4.74 | |
| Medial temporal pole R | 58 | 10 | -20 | 4.76 | |
| 27.5–30 min | Middle temporal gyrus R | 46 | -40 | 4 | 5.11 |
| 30–32.5 min | Supplementary motor area L | -8 | 0 | 56 | 4.92 |
| Middle temporal gyrus L | -58 | -6 | -6 | 5.48 | |
| 35–37.5 min | Thalamus L | -10 | -20 | 8 | -4.84 |
Time points refer to the start of the ketamine application. All values are family wise error rate (FWE)-corrected at P<.05 voxel-level.
Analysis 2: Ketamine Effects on Cortico-Thalamic Connectivity
Using the method recently published by Zhang et al. (2010) and Woodward et al. (2012), the dynamics of cortico-thalamic connectivity during and after ketamine infusion were investigated. First, baseline resting-state data before the application of ketamine were used to identify specific subregions of the thalamus; the results of our analysis turned out to largely overlap with findings reported previously using the same methodology (Zhang et al., 2008, 2010; Woodward et al., 2012). Functional connections of the somatosensory and motor seed regions were found to ventral-lateral and ventral posterior-lateral thalamic regions; the prefrontal cortex was functionally connected to anterior and dorsomedial regions of the thalamus. The temporal lobe and occipital cortex seed region were connected to anterolateral and dorsal medial thalamic subregions. The posterior parietal cortex was found to be connected to the lateral posterior nucleus and the pulvinar.
Comparing ketamine with placebo infusion showed significant differences in cortico-thalamic connectivity for 2 seed regions: the somatosensory and temporal cortices. A detailed description of the results is presented in Table 3 and Figure 2. No significant effect of ketamine compared with placebo was observed in the connectivity of the prefrontal, motor, posterior parietal, and occipital cortex.
Table 3.
Ketamine-Induced Difference of Cortico-Thalamic Connections (Analysis 2)
| Cortico-Thalamic Connectivity | |||||
|---|---|---|---|---|---|
| Time, min | Anatomical Region (Oxford thalamic connectivity atlas) | MNI coordinates | Statistics | ||
| x | y | z | T | ||
| Somatosensory cortex seed | |||||
| 2.5–5 | Ventral lateral nucleus | -10 | -12 | 4 | 4.00 |
| 5–7.5 | Ventral anterior nucleus | 10 | -4 | 4 | 4.11 |
| Pulvinar | 18 | -24 | 12 | 4.06 | |
| Medial dorsal nucleus | 4 | -16 | 10 | 3.58 | |
| 7.5–10 | Ventral anterior nucleus | -12 | -4 | 6 | 4.58 |
| Pulvinar | 20 | -26 | 14 | 4.21 | |
| Ventral lateral nucleus | 16 | -8 | 8 | 3.78 | |
| 10–12.5 | Ventral lateral nucleus | -12 | -8 | 10 | 3.99 |
| 12.5–15 | Ventral anterior nucleus | 12 | -4 | 6 | 3.94 |
| 15–17.5 | Ventral anterior nucleus | -12 | -6 | 10 | 3.75 |
| Ventral lateral nucleus | 14 | -10 | 0 | 3.50 | |
| 17.5–20 | Ventral anterior nucleus | -14 | -6 | 8 | 3.84 |
| 20–22.5 | Ventral lateral nucleus | 10 | -6 | 2 | 3.66 |
| 22.5–25 | Ventral anterior nucleus | -10 | -6 | 8 | 4.57 |
| Ventral lateral nucleus | -16 | -16 | 18 | 3.72 | |
| 25–27.5 | --- | -- | -- | -- | -- |
| 30–32.5 | Medial dorsal nucleus | -10 | -20 | 6 | 4.69 |
| Ventral anterior nucleus | -10 | -6 | 6 | 4.04 | |
| Ventral lateral nucleus | -14 | -10 | 14 | 3.79 | |
| 32.5–35 | Pulvinar | -8 | -30 | 4 | 3.61 |
| 35–37.5 | Ventral anterior nucleus | -10 | -6 | 10 | 3.89 |
| Medial dorsal nucleus | 6 | -14 | 10 | 3.52 | |
| 37.5–40 | Ventral anterior nucleus | -10 | -6 | 8 | 3.95 |
| Medial dorsal nucleus | -10 | -20 | 6 | 3.77 | |
| Temporal gyrus seed | |||||
| 2.5–5 | Medial dorsal nucleus | 2 | -18 | 8 | 3.64 |
| Ventral anterior nucleus | 8 | -8 | 12 | 4.37 | |
| Ventral lateral nucleus | -14 | -14 | 14 | 4.36 | |
| 5–7.5 | Ventral lateral nucleus | 16 | -14 | 14 | 4.16 |
| 7.5–10 | Ventral anterior nucleus | -10 | -6 | 8 | 3.86 |
| Medial dorsal nucleus | 8 | -18 | 4 | 3.75 | |
| 10–12.5 | Ventral lateral nucleus | -14 | -14 | 6 | 4.20 |
| 12.5–15 | Medial dorsal nucleus | 10 | -14 | 14 | 3.82 |
| Ventral anterior nucleus | 10 | -4 | 6 | 3.83 | |
| Ventral lateral nucleus | 8 | -10 | 4 | 3.45 | |
| 15–17.5 | Medial dorsal nucleus | -8 | -14 | 8 | 4.14 |
| Ventral lateral nucleus | 12 | -16 | 2 | 3.76 | |
| 17.5–20 | Medial dorsal nucleus | -8 | -12 | 10 | 3.63 |
| Ventral lateral nucleus | 20 | -16 | 12 | 4.46 | |
| 20–22.5 | Medial dorsal nucleus | -10 | -18 | 6 | 3.98 |
| 22.5–25 | Medial dorsal nucleus | 10 | -18 | 6 | 3.72 |
| Ventral lateral nucleus | -10 | -10 | 4 | 4.58 | |
| 25–27.5 | Ventral lateral nucleus | 16 | -8 | 8 | 3.90 |
| 27.5–30 | Ventral lateral nucleus | -12 | -8 | 6 | 4.16 |
| 30–32.5 | Medial dorsal nucleus | 6 | -18 | 8 | 4.34 |
Only the 2 cortical seed regions (somatosensory and temporal) are shown. Time points refer to the start of ketamine infusion. All values are family wise error rate (FWE)-corrected at P<.05 voxel-level after small volume correction for the thalamus.
Figure 2.
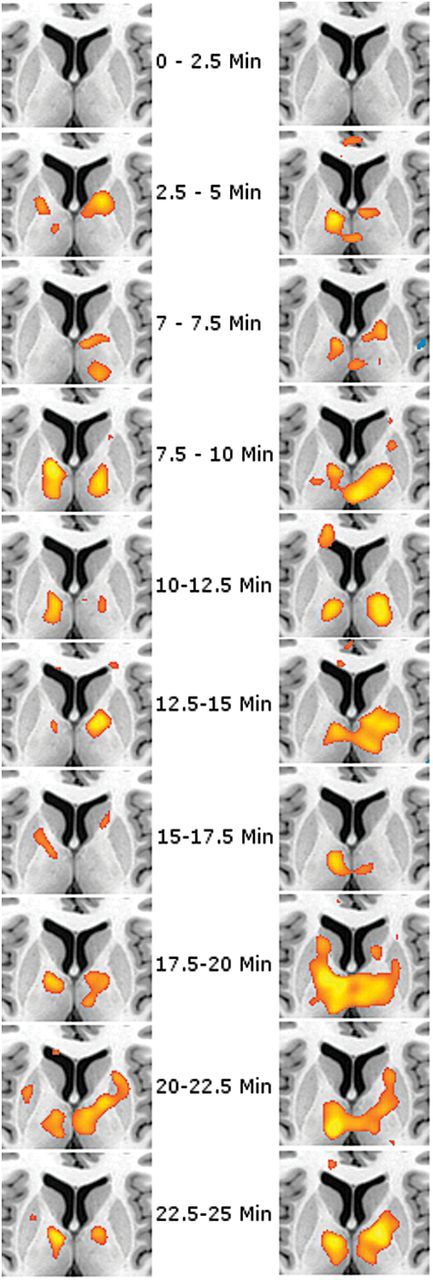
Ketamine-induced alterations of cortic-thalamic functional connectivity (analysis 2). Posthoc t tests of the interaction drug*time show a significant increase of functional connectivity for the somatosensory (left row) and temporal cortex (right row). Other regions without significant results are not shown. Results of seed-to-voxel correlation analysis are overlaid onto a single-subject standard brain (range of t-values=3.09…6). Results are shown for each period of 2.5 minutes. z=7mm.
For the somatosensory cortex, a significant increase in functional connectivity of the postcentral gyrus with the ventrolateral region of the thalamus was observed. The overall F-test showed significant results with a maximum P [41,984]=<.001 (FWE-corrected, voxel-level) for the thalamus. Posthoc t-values ranged between 3.50 and 4.69, all P<.05, FWE-corrected for the volume of the thalamus. According to the Oxford thalamic connectivity atlas, the increase was allocated mainly in the ventral anterior nucleus and ventral lateral nucleus.
The temporo-thalamic functional connectivity revealed a maximum P [41,984]=<.001 (FWE-corrected, voxel-level) for the thalamus. The posthoc analysis showed a ketamine-associated increase of the temporal seed region with the medial dorsal nucleus, ventral lateral, and ventral anterior nucleus. Again, differences between the ketamine and placebo scan were present shortly after start of the infusion, with t-values ranging from 3.45 to 4.58, all P<.05, FWE-corrected for the volume of the thalamus.
Discussion
Here, we show that the application of ketamine has a substantial impact on thalamic functioning in healthy volunteers, with 2 main findings. First, we demonstrate that the administration of a subanesthetic dose of ketamine leads to a significantly higher functional connectivity in the thalamus hub network consisting of motor, premotor, visual, auditory, and limbic regions and the cerebellum compared with placebo (analysis 1). Second, the investigation of specific cortico-thalamic connections revealed significant increases of the connectivity of the somatosensory cortex to ventrolateral and ventral anterior thalamic areas and the temporal cortex to mediodorsal and antero-ventral and -lateral thalamic areas (analysis 2).
The results of this study fit well into the context of theoretical concepts that propagate a significant impact of the glutamatergic system on key symptoms of schizophrenia, such as perturbation of perception. Accordingly, our study provides a more comprehensive understanding of the connection between the glutamtergic system and thalamic functioning. More specifically, we could show that the blockage of the NMDA receptor can cause functional alterations of thalamic connectivity in healthy volunteers similar to those reported for patients with schizophrenia.
A number of previous studies have investigated thalamic alterations in schizophrenia. These include differences in morphology such as significant changes of thalamic volume as well as disruption of functioning using neuroimaging techniques such as PET and fMRI (for review, see Sim et al. 2006). Importantly, nucleus-specific volume reductions, particularly in the mediodorsal and anterior nucleus and the pulvinar, have been described, pointing toward a differential involvement of specific thalamic nuclei in schizophrenia. In accordance with these results, we found a specific strengthening of functional cortico-thalamic connectivity for the somatosensory and temporal seed regions but not for prefrontal, occipital, and parietal regions.
Significant effects of NMDA antagonist on thalamic nuclei and thalamo-cortical networks have also been reported in animal studies. Importantly, phencyclidine and acute ketamine administration were shown to provoke a significant alteration of the discharge rate of the centromedial and mediodorsal thalamic nucleus, which project to the medial prefrontal cortex and within the nucleus reuniens of the thalamus, respectively (Santana et al., 2011; Zhang et al., 2012) (for review, see Celada et al. 2013). Furthermore, another recently published animal study could show that phencyclidine leads to a significant reduction of the discharge rate of GABAergic interneurons in the reticular nucleus of the thalamus, leading to a disinhibition of thalamo-cortical activity (Troyano-Rodriguez et al., 2014). In accordance with the results of the present study, an [18F] FDG-PET investigation in unmedicated patients with schizophrenia revealed metabolic disconnectivity of the mediodorsal nucleus of the thalamus and medial temporal areas compared with healthy controls (Adolphs, 2003).
Recently, a general hyperconnectivity was described after intravenous application of ketamine in a group of healthy volunteers, which persisted for more than 45 minutes (Driesen et al., 2013). In line with the findings of this study, our study indicates that ketamine induces hyperconnectivity rather than reduction of connectivity both on a larger network level and on thalamus-specific connectivity.
Recently, Woodward et al. (2012) described differences of cortico-thalamic connectivity between patients with schizophrenia and healthy volunteers using resting-state fMRI. In this study, a significant increase of motor/somatosensory-thalamic connectivity along with a reduction of prefrontal-thalamic connectivity was shown (Woodward et al., 2012). Similarly, Klingner et al. (2013) reported a significant increase in thalamo-cortical connectivity in patients with schizophrenia compared with healthy controls, which was caused both by increased connectivity of the thalamus to prefrontal and superior temporal correlations and a relative lack of negative correlation of the thalamus to secondary sensory areas, somatosensory, visual, and auditory cortices.
Notably, results found with the ketamine model of psychosis in healthy volunteers seem to only partly overlap with results found in patients with schizophrenia. The finding of increased somatosensory-thalamic connectivity was evident both in studies in schizophrenia and under ketamine application. This result is also compatible with findings in patients with schizophrenia showing deficits in sensory and higher-order processing regions as measured with EEG (Leitman et al., 2010). In contrast, we could not replicate the finding of decreased prefrontal-thalamic connectivity. As this finding may be a highly relevant one, given the vast number of evidence reporting deficits of top-down functional control of prefrontal areas in schizophrenia, this issue might be worth highlighting (Lesh et al., 2011; Tu et al., 2013). The discrepancy might be based on the fact that disruption of functional connections in patients with schizophrenia is influenced by a number of endogenous disease-specific and treatment-associated molecular changes, whereas the present study included solely the modulation of the glutamatergic system. This difference could be further based on the smaller sample size of our study, as in the investigation by Woodward et al. (2012). Eighty patients were included, showing effect sizes of prefrontal-thalamic connectivity, which were clearly lower than for somatosensory regions.
The changes of connectivity within the thalamus network are also complementary to findings in patients with schizophrenia. In accordance with our results of a significant increase of connectivity to temporo-parietal regions, changes of the functional connectivity of the temporal lobe during resting state have been shown for patients suffering from a first episode of psychosis (Alonso-Solís et al., 2012) and patients with schizophrenia with chronic auditory verbal hallucinations (Sommer et al., 2012). Moreover, structural deviations of the superior, middle, and inferior temporal gyrus have been found in patients with first-episode psychosis (Kasai et al., 2003). These alterations might partly account for psychosis-like symptoms, as the areas of significant changes found in our analysis cover both somatosensory cortex and temporal regions, including the primary and secondary auditory cortex. Furthermore, the superior temporal gyrus has been shown to be involved in perception of emotional facial stimuli and social cognition processes (Adolphs, 2003; Radua et al., 2010), and the middle and inferior temporal gyrus are thought to subserve functions such as language and semantic memory processing, visual perception, and multimodal sensory integration. Concerning the calcarine, which covers the primary visual cortex (V1), an absence of contextual modulation within V1 was detected in patients with schizophrenia. This disruption was hypothesized to affect local inhibitory mechanisms within V1 or feedback connections from higher areas as described by Seymour et al (2013).
The neurochemical mechanisms that link glutamatergic modulation via NMDA blockage and large-scale changes in distinct cortico-thalamic networks are not fully understood. Our study reveals that the intravenous application of ketamine leads to an acute alteration of the thalamic network and cortico-thalamic connections within a few minutes, which is paralleled by profound clinical symptoms. Current models describing the modulation of the glutamatergic system propose that the blockage of NMDA receptors on GABAergic interneurons leads to a disinhibition of the activity of pyramidal cells, resulting in an increase of glutamate release within afferent and connected regions (Lorrain et al., 2003; Lopez-Gil et al., 2007). MRS studies directly investigating the impact of acute ketamine on glutamate in the brain have shown a significant increase of these glutamine and glutamate levels in the ACC (Rowland et al., 2005; Stone et al., 2012), although not all studies have shown positive results (Taylor et al., 2012). This increase of the glutamate level is thought to have a significant impact on the BOLD signal, as it is known that glutamate is decisively involved in neuronal activity in the cerebral cortex. In fact, modulation of the glutamatergic system, more specifically the NMDA and AMPA receptor, has been shown to have a significant impact on the BOLD signal in response to somatosensory modulation in an animal model (Gsell et al., 2006). However, imaging studies using MRS combined with fMRI paint a more complex picture of the connection between excitatory and inhibitory neurotransmitter systems with regard to the influence of the BOLD signal. It was thus hypothesized by Falkenberg et al. (2012) that glutamate might have a global effect on BOLD response, whereas GABA might have a more local impact (Falkenberg et al., 2012).
Aside from its role as a model for schizophrenia, ketamine has gained attention as a treatment for treatment-resistant depression. Although research in this scientific field has focused particularly on neuroplastic properties of ketamine (Li et al., 2010, 2011), the fact that depression significantly alters thalamic functioning as revealed by fMRI studies in depressed patients (Fu et al., 2004; Dichter et al., 2009; for review, see Hoflich et al., 2012) might point toward the relevance of the results gained in this study also in the context of depression.
A limitation of this study is the lack of direct comparison of results in patients with schizophrenia and changes seen in healthy volunteers under ketamine infusion. Therefore, differences in scanning protocols and analysis techniques, which might influence the retrieved results, cannot be excluded and the comparison must rely on indirect information with regard to patients with schizophrenia. Further studies might overcome this limitation by using data gained within the same research infrastructure or by including both groups within one study.
Furthermore, it should be mentioned that ketamine has a relevant impact on a number of physiological parameters such as heart rate, respiratory rate, and blood pressure. As parameters such as heart rate variability were shown to have a significant effect on resting state functional connectivity (Chang et al., 2013), this data might have had an influence on the results retrieved in our analysis, although we tried to account for this fact by applying state-of-the-art preprocessing steps, as described earlier (see Methods).
Another issue is the fact that fMRI does not allow for a very accurate discrimination of thalamic nuclei. To relate the peak coordinates gained in the analysis with specific nuclei, MNI coordinates derived from a standardized atlas implemented in SPM were used. However, this approach does not account for potential individual differences in the thalamic area. To overcome this issue, future studies might additionally employ DTI to gain information about individual connections that can be included in the analysis.
In summary, the present study shows that findings of thalamic dysfunction can be partly mimicked by blockage of the NMDA receptor in healthy volunteers. These findings provide additional information on how the modulation of the glutamatergic system can lead to profound changes of perception and behavior, creating a psychosis-like state in healthy volunteers. According to its gating function for the integration of sensory, cognitive, and emotional information, the finding that thalamic functioning is disrupted gives an intuitive explanation for the loss of sensory filtering observed in these individuals.
Statement of Interest
Without any relevance to this work, S. Kasper declares that he has received grant/research support from Eli Lilly, Lundbeck A/S, Bristol-Myers Squibb, Servier, Sepracor, GlaxoSmithKline, Organon, and Dr. Willmar Schwabe GmbH & Co. KG and has served as a consultant or on advisory boards for AstraZeneca, Austrian Sick Found, Bristol-Myers Squibb, German Research Foundation (DFG), GlaxoSmithKline, Eli Lily, Lundbeck A/S, Pfizer, Organon, Sepracor, Janssen, and Novartis, and has served on speakers’ bureaus for AstraZeneca, Eli Lilly, Lundbeck A/S, Servier, Sepracor, and Janssen. R. Lanzenberger received travel grants and conference speaker honoraria from AstraZeneca, Lundbeck A/S, and Roche Austria GmbH. D. Winkler has received lecture fees from Bristol-Myers Squibb, CSC Pharmaceuticals, Novartis, Pfizer, and Servier.
Acknowledgments
This research was funded by Austrian National Bank Grant P 14193. The authors are especially grateful to staff at the MRI center and the Department of Psychiatry and Psychotherapy for their technical and medical support, to Sebastian Ganger for assistance in the analysis, and to Marie Spies and Gregor Gryglewski for linguistic support.
References
- Abel KM, Allin MP, Kucharska-Pietura K, David A, Andrew C, Williams S, Brammer MJ, Phillips ML. (2003) Ketamine alters neural processing of facial emotion recognition in healthy men: an fMRI study. Neuroreport 14:387–391. [DOI] [PubMed] [Google Scholar]
- Adolphs R. (2003) Investigating the cognitive neuroscience of social behavior. Neuropsychologia 41:119–126. [DOI] [PubMed] [Google Scholar]
- Alonso-Solís A, Corripio I, de Castro-Manglano P, Duran-Sindreu S, Garcia-Garcia M, Proal E, Nuñez-Marín F, Soutullo C, Alvarez E, Gómez-Ansón B, Kelly C, Castellanos FX. (2012) Altered default network resting state functional connectivity in patients with a first episode of psychosis. Schizophr Res 139:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, Niciu MJ, Morgan PT, Surti TS, Bloch MH, Ramani R, Smith MA, Wang XJ, Krystal JH, Corlett PR. (2012) NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A 109:16720–16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. (2003) Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Mullins P, Jung R, Chen H, Qualls C, Hammond R, Brooks WM, Lauriello J. (2010) 1H-MRS at 4 tesla in minimally treated early schizophrenia. Mol Psychiatry 15:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Llado-Pelfort L, Santana N, Kargieman L, Troyano-Rodriguez E, Riga MS, Artigas F. (2013) Disruption of thalamocortical activity in schizophrenia models: relevance to antipsychotic drug action. Int J Neuropsychopharmacol:1–19. [DOI] [PubMed] [Google Scholar]
- Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. (2013) Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage 68:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Meador-Woodruff JH. (2004) Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophr Res 69:237–253. [DOI] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. (2010) Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumann J, Wagner D, Heekeren K, Neukirch A, Thiel CM, Gouzoulis-Mayfrank E. (2010) Neuronal correlates of visual and auditory alertness in the DMT and ketamine model of psychosis. J Psychopharmacol 24:1515–1524. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Smoski MJ. (2009) Affective context interferes with cognitive control in unipolar depression: an fMRI investigation. J Affect Disord 114:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich A (1998) The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 31 Suppl 2:80–84. [DOI] [PubMed] [Google Scholar]
- Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, Gueorguieva R, He G, Ramachandran R, Suckow RF, Anticevic A, Morgan PT, Krystal JH. (2013) Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry 18:1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Brugger S, Raffin M, Barker GJ, Lythgoe DJ, McGuire PK, Stone JM. (2012) Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology 37:2515–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg LE, Westerhausen R, Specht K, Hugdahl K. (2012) Resting-state glutamate level in the anterior cingulate predicts blood-oxygen level-dependent response to cognitive control. Proc Natl Acad Sci U S A 109:5069–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. (2004) Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry 61:877–889. [DOI] [PubMed] [Google Scholar]
- Gluck MR, Thomas RG, Davis KL, Haroutunian V. (2002) Implications for altered glutamate and GABA metabolism in the dorsolateral prefrontal cortex of aged schizophrenic patients. Am J Psychiatry 159:1165–1173. [DOI] [PubMed] [Google Scholar]
- Gsell W, Burke M, Wiedermann D, Bonvento G, Silva AC, Dauphin F, Buhrle C, Hoehn M, Schwindt W. (2006) Differential effects of NMDA and AMPA glutamate receptors on functional magnetic resonance imaging signals and evoked neuronal activity during forepaw stimulation of the rat. J Neurosci 26:8409–8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Kasper S, Lanzenberger R. (2011) Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage 56:881–889. [DOI] [PubMed] [Google Scholar]
- Hahn A, Kranz GS, Seidel EM, Sladky R, Kraus C, Kublbock M, Pfabigan DM, Hummer A, Grahl A, Ganger S, Windischberger C, Lamm C, Lanzenberger R. (2013) Comparing neural response to painful electrical stimulation with functional MRI at 3 and 7T. Neuroimage 82C:336–343. [DOI] [PubMed] [Google Scholar]
- Hoflich A, Baldinger P, Savli M, Lanzenberger R, Kasper S. (2012) Imaging treatment effects in depression. Rev Neurosci 23:227–252. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee CU, Ciszewski AA, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. (2003) Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry 160:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, Gil R, Slifstein M, Abi-Dargham A, Lisanby SH, Shungu DC (2012) Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry 69:449–459. [DOI] [PubMed] [Google Scholar]
- Klingner CM, Langbein K, Dietzek M, Smesny S, Witte OW, Sauer H, Nenadic I. (2013) Thalamocortical connectivity during resting state in schizophrenia. Eur Arch Psychiatry Clin Neurosci 264:111–119. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Mack-Burkhardt F, Riederer P, Hebenstreit GF, Reynolds GP, Andrews HB, Beckmann H. (1989) [3H]MK-801 binding sites in postmortem brain regions of schizophrenic patients. J Neural Transm 77:231–236. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC. (2010) Sensory deficits and distributed hierarchical dysfunction in schizophrenia. Am J Psychiatry 167:818–827. [DOI] [PubMed] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. (2011) Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology 36:316–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gil X, Babot Z, Amargos-Bosch M, Sunol C, Artigas F, Adell A. (2007) Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology 32:2087–2097. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. (2003) Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 117:697–706. [DOI] [PubMed] [Google Scholar]
- McKie S, Del-Ben C, Elliott R, Williams S, del Vai N, Anderson I, Deakin JF. (2005) Neuronal effects of acute citalopram detected by pharmacoMRI. Psychopharmacology (Berl) 180:680–686. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Clinton SM, Beneyto M, McCullumsmith RE. (2003) Molecular abnormalities of the glutamate synapse in the thalamus in schizophrenia. Ann NY Acad Sci 1003:75–93. [DOI] [PubMed] [Google Scholar]
- Metzger CD, van der Werf YD, Walter M. (2013) Functional mapping of thalamic nuclei and their integration into cortico-striatal-thalamo-cortical loops via ultra-high resolution imaging-from animal anatomy to in vivo imaging in humans. Front Neurosci 7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso F, Brinkmeyer J, Ecker D, London MK, Thieme G, Warbrick T, Wittsack HJ, Saleh A, Greb W, de Boer P, Winterer G. (2011) Ketamine effects on brain function: simultaneous fMRI/EEG during a visual oddball task. Neuroimage 58:508–525. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J, Phillips ML, Russell T, Lawrence N, Marshall N, Kalidindi S, El-Hage W, McDonald C, Giampietro V, Brammer MJ, David AS, Surguladze SA. (2010) Neural response to specific components of fearful faces in healthy and schizophrenic adults. Neuroimage 49:939–946. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, Barrow R, Yeo R, Lauriello J, Brooks WM. (2005) Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry 162:394–396. [DOI] [PubMed] [Google Scholar]
- Santana N, Troyano-Rodriguez E, Mengod G, Celada P, Artigas F. (2011) Activation of thalamocortical networks by the N-methyl-D-aspartate receptor antagonist phencyclidine: reversal by clozapine. Biol Psychiatry 69:918–927. [DOI] [PubMed] [Google Scholar]
- Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, Boesiger P, Henning A, Seifritz E. (2012) Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One 7:e44799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour K, Stein T, Sanders LL, Guggenmos M, Theophil I, Sterzer P. (2013) Altered contextual modulation of primary visual cortex responses in schizophrenia. Neuropsychopharmacology 38:2607–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim K, Cullen T, Ongur D, Heckers S. (2006) Testing models of thalamic dysfunction in schizophrenia using neuroimaging. J Neural Transm 113:907–928. [DOI] [PubMed] [Google Scholar]
- Sinner B, Graf B. (2008) Ketamine. In: Modern anesthetics. Handbook of experimental pharmacology (Schüttler J, Schwilden H, eds), pp 313–333. Berlin, Heidelberg: Springer. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Wang L, Cronenwett W, Mamah D, Barch DM, Csernansky JG. (2011) Thalamic morphology in schizophrenia and schizoaffective disorder. J Psychiatr Res 45:378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, Clos M, Meijering AL, Diederen KM, Eickhoff SB. (2012) Resting state functional connectivity in patients with chronic hallucinations. PLoS One 7:e43516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Abel KM, Allin MP, van Haren N, Matsumoto K, McGuire PK, Fu CH. (2011) Ketamine-induced disruption of verbal self-monitoring linked to superior temporal activation. Pharmacopsychiatry 44:33–48. [DOI] [PubMed] [Google Scholar]
- Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, Krystal JH, Nutt D, Barker GJ. (2012) Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry 17:664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulc A, Konarzewska B, Galinska-Skok B, Lazarczyk J, Waszkiewicz N, Tarasow E, Milewski R, Walecki J. (2013) Proton magnetic resonance spectroscopy measures related to short-term symptomatic outcome in chronic schizophrenia. Neurosci Lett 547:37–41. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Tiangga ER, Mhuircheartaigh RN, Cowen PJ. (2012) Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J Psychopharmacol 26:733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, Neufeld RW, Rogers J, Pavlosky W, Schaefer B, Densmore M, Al-Semaan Y, Williamson PC. (2002) Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry 159:1944–1946. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. (2011) Association between functional connectivity hubs and brain networks. Cereb Cortex 21:2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyano-Rodriguez E, Llado-Pelfort L, Santana N, Teruel-Marti V, Celada P, Artigas F. (2014) Phencyclidine inhibits the activity of thalamic reticular gamma-aminobutyric acidergic neurons in rat brain. Biol Psychiatry 76:937–945. [DOI] [PubMed] [Google Scholar]
- Tu P-C, Lee Y-C, Chen Y-S, Li C-T, Su T-P. (2013) Schizophrenia and the brain’s control network: aberrant within- and between-network connectivity of the frontoparietal network in schizophrenia. Schizophr Res 147:339–347. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Watis L, Chen SH, Chua HC, Chong SA, Sim K. (2008) Glutamatergic abnormalities of the thalamus in schizophrenia: a systematic review. J Neural Transm 115:493–511. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. (2009) Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage 47:1408–1416. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. (2012) Default mode network activity and connectivity in psychopathology. Ann Rev Clin Psychol 8:49–76. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. (2012) Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry 169:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. (2010) Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex 20:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. (2008) Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol 100:1740–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yoshida T, Katz DB, Lisman JE. (2012) NMDAR antagonist action in thalamus imposes delta oscillations on the hippocampus. J Neurophysiol 107:3181–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. (2007) Functional disintegration in paranoid schizophrenia using resting–state fMRI. Schizophr Res 97:194–205. [DOI] [PubMed] [Google Scholar]