Abstract
P-glycoprotein (P-gp) has been associated with a number of neurodegenerative diseases, including Parkinson’s disease, although the mechanisms remain unclear. Altered transport of neurotoxic pesticides has been proposed in Parkinson’s disease, but it is unknown whether these pesticides are P-gp substrates. We used three in vitro transport models, stimulation of ATPase activity, xenobiotic-induced cytotoxicity, and inhibition of rhodamine-123 efflux, to evaluate P-gp transport of diazinon, dieldrin, endosulfan, ivermectin, maneb, 1-methyl-4-phenyl-4-phenylpyridinium ion (MPP+), and rotenone. Diazinon and rotenone stimulated ATPase activity in P-gp–expressing membranes, with Vmax values of 22.4 ± 2.1 and 16.8 ± 1.0 nmol inorganic phosphate/min per mg protein, respectively, and Km values of 9.72 ± 3.91 and 1.62 ± 0.51 µM, respectively, compared with the P-gp substrate verapamil, with a Vmax of 20.8 ± 0.7 nmol inorganic phosphate/min per mg protein and Km of 0.871 ± 0.172 μM. None of the other pesticides stimulated ATPase activity. We observed an increased resistance to MPP+ and rotenone in LLC-MDR1-WT cells compared with LLC-vector cells, with 15.4- and 2.2-fold increases in EC50 values, respectively. The resistance was reversed in the presence of the P-gp inhibitor verapamil. None of the other pesticides displayed differential cytotoxicity. Ivermectin was the only pesticide to inhibit P-gp transport of rhodamine-123, with an IC50 of 0.249 ± 0.048 μM. Our data demonstrate that dieldrin, endosulfan, and maneb are not P-gp substrates or inhibitors. We identified diazinon, MPP+, and rotenone as P-gp substrates, although further investigation is needed to understand the role of P-gp transport in their disposition in vivo and associations with Parkinson’s disease.
Introduction
P-glycoprotein (P-gp), encoded by the multidrug resistance gene ABCB1 or MDR1, is a member of the ATP-binding cassette superfamily of transporters. P-gp is a transporter that effluxes a diverse array of xenobiotics in healthy tissues throughout the body, which is particularly important in limiting the distribution of xenobiotics across blood-tissue barriers, such as the blood-brain barrier (BBB) (Giacomini, 1997; Lin and Yamazaki, 2003a,b; Giacomini et al., 2010; Sharom, 2011). P-gp expression at the BBB protects the brain from toxic substances circulating in the blood. Numerous studies have suggested a role for P-gp in the development of neurodegenerative diseases, including Parkinson’s disease. Potential mechanisms of these associations include changes in P-gp expression or activity with age, disease progression, and ABCB1 pharmacogenomics (Le Couteur et al., 2001; Furuno et al., 2002; Drozdzik et al., 2003; Lee and Bendayan, 2004; Lee et al., 2004; Tan et al., 2004, 2005; Kortekaas et al., 2005; Bartels et al., 2008, 2009; Westerlund et al., 2008, 2009; Rapposelli et al., 2009; Vautier and Fernandez, 2009; Dutheil et al., 2010; Bartels, 2011; Li et al., 2014). Since P-gp is an efflux transporter, changes in P-gp function at the BBB may result in increased brain accumulation of neurotoxicants that are normally excluded from the brain. Although it has been suggested that the modulation of P-gp at the BBB in neurodegenerative diseases may not have a clinically significant effect on exposures of therapeutic compounds (<25%) (Kalvass et al., 2013), this decrease in function could be significant for neurotoxic pesticides where exposures occur chronically over decades and compounds typically have long half-lives and bioaccumulate (Hatcher et al., 2008).
We evaluated P-gp transport of the major neurotoxic pesticides that have been associated with Parkinson’s disease to elucidate the role of P-gp in the development of disease. Environmental exposures to neurotoxicants are risk factors for Parkinson’s disease (Langston et al., 1984; Semchuk et al., 1992; Bonnet and Houeto, 1999; Steece-Collier et al., 2002; Gatto et al., 2009; Wirdefeldt et al., 2011; Kamel, 2013; Mostafalou and Abdollahi, 2013; Pezzoli and Cereda, 2013). One of the first discoveries of an environmental risk factor was 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine is converted to 1-methyl-4-phenyl-4-phenylpyridinium ion (MPP+) (the pesticide cyperquat) in the brain, resulting in parkinsonism (Langston et al., 1983, 1984). Several pesticides, including paraquat, diazinon, dieldrin, endosulfan, maneb, MPP+, and rotenone, have been associated with an increased risk of Parkinson’s disease in epidemiologic studies in humans, toxicity models in animals, and in vitro studies (Langston et al., 1984; Bradbury et al., 1986; Fleming et al., 1994; Liou et al., 1997; Thiffault et al., 2000; Uversky et al., 2001; Gao et al., 2002; Uversky, 2004; Firestone et al., 2005; Li et al., 2005; Richardson et al., 2006; Wang et al., 2006; Hatcher et al., 2007; Jia and Misra, 2007a,b; Dhillon et al., 2008; Kanthasamy et al., 2008; Sonsalla et al., 2008; Costello et al., 2009; Gatto et al., 2009; Sharma et al., 2010; Weisskopf et al., 2010; Slotkin and Seidler, 2011; Tanner et al., 2011). Pesticides are a diverse class of compounds with different mechanisms of action, pharmacokinetic properties, and uses, i.e., fungicides, herbicides, and insecticides (Hatcher et al., 2008; Goldman, 2014; Chin-Chan et al., 2015). We reported previously that P-gp does not transport paraquat (Lacher et al., 2014). Our current study is not an exhaustive screen of P-gp transport of pesticides, but rather a focused evaluation of pesticides most commonly associated with Parkinson’s disease: diazinon, dieldrin, endosulfan, maneb, MPP+, and rotenone (Hatcher et al., 2008; Goldman, 2014; Chin-Chan et al., 2015).
Currently, there are sparse data to evaluate the P-gp transport of pesticides associated with Parkinson’s disease (Bain and LeBlanc, 1996; Bleasby et al., 2000; Martel et al., 2001; Lecoeur et al., 2006; Pivčević and Zaja, 2006; Sreeramulu et al., 2007; Bircsak et al., 2013). Previous reports are often contradictory and many lack appropriate controls, making interpretation of the data difficult. Additionally, previous studies have often relied on a single in vitro transport model; however, it is clear in evaluating xenobiotic transporters that multiple models are necessary (Polli et al., 2001; Feng et al., 2008; Giacomini et al., 2010; Brouwer et al., 2013; Hillgren et al., 2013; Zamek-Gliszczynski et al., 2013). Therefore, classifying pesticides as P-gp substrates or inhibitors is challenging based on previous work.
To overcome some of the challenges of previous studies, we used a combination of in vitro P-gp transport models to systematically screen the major pesticides that have been linked to Parkinson’s disease: diazinon, dieldrin, endosulfan, maneb, MPP+, and rotenone. We also evaluated P-gp transport of the pesticide ivermectin. Although ivermectin has not been associated with Parkinson’s, it is a pesticide that has been reported to be a P-gp substrate and was used as a comparator. We screened each compound using three models: 1) xenobiotic-induced stimulation of ATPase activity in P-gp–expressing membranes, 2) xenobiotic-induced cytotoxicity in recombinant cell lines, and 3) inhibition of intracellular rhodamine-123 (R123) efflux in recombinant cell lines. This study is the first comprehensive investigation of P-gp–mediated transport of the major pesticides associated with Parkinson’s disease.
Materials and Methods
Chemicals.
Diazinon, dieldrin, endosulfan, maneb, MPP+, rotenone, doxorubicin, colchicine, ivermectin, verapamil, R123, and cyclosporine 4′,6-diamidino-2-phenylindole were purchased from the Sigma-Aldrich Chemical Company (St. Louis, MO). N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918) was provided by GlaxoSmithKline (Research Triangle Park, NC).
ATPase Activity.
Stimulation of ATPase activity by xenobiotics was measured using the SB MDR1/P-gp PREDEASY ATPase Kit (SOLVO Biotechnology, Budaörs, Hungary) according to the manufacturer’s instructions. The assay utilizes the known P-gp substrate verapamil as a positive control. P-gp membranes were preincubated at 37°C for 10 minutes with diazinon, dieldrin, endosulfan, maneb, MPP+, rotenone, ivermectin, or verapamil over a concentration range of 0.14–300 μM. The concentration range was selected based on the manufacturer’s instructions. ATPase activity was measured as the rate of inorganic phosphate (Pi) liberation in the presence and absence of 1.2 mM sodium orthovanadate, a nonspecific ATPase inhibitor. The reaction was started with the addition of 2 mM MgATP and incubated at 37°C for an additional 10 minutes. The developer solution was added and incubated for 30 minutes at 37°C. Absorbance was read at 620 nm using a SpectraMax Gemini XS microplate reader (Molecular Devices, Sunnyvale, CA). The amount of Pi was estimated from a phosphate standard curve, and orthovanadate-sensitive activity was subtracted from each value to account for background ATPase activity. Xenobiotic-stimulated ATPase activity was reported as nmol Pi liberated per minute incubation time per milligram total protein (nmol Pi/min per mg protein). Each xenobiotic was evaluated in at least triplicate and repeated once. Nonlinear regression least-squares model fit on Prism 5.0 software (GraphPad, San Diego, CA) was used to estimate Michaelis-Menten parameters (Vmax and Km).
Cell Culture.
LLC-PK1 vector cells (LLC-vector) and recombinant ABCB1 cells (LLC-MDR1-WT), provided by Michael M. Gottesman in the Laboratory of Cell Biology at the National Cancer Institute (Bethesda, MD), were cultured in complete Media 199 (Mediatech, Manassas, VA) supplemented with 3% (v/v) fetal bovine serum (Mediatech), 1% (v/v) L-glutamine (Mediatech), 1% (v/v) penicillin/streptomycin (Mediatech), and 1% (v/v) geneticin (Life Technologies, Carlsbad, CA) and grown at 37°C in the presence of 5% CO2.
Xenobiotic-Induced Cytotoxicity.
Sensitivity to cytotoxic agents was evaluated in LLC-vector and LLC-MDR1-WT cells as previously described (Lacher et al., 2014) using the CellTiter-Glo cell viability assay (Promega, Fitchburg, WI). Cells were plated overnight at a density of 1000 cells/well in 96-well plates and grown in the presence of test compounds for 72 hours at 37°C (Lacher et al., 2014). Cells were treated with the following pesticides over a wide concentration range: diazinon (11–1000 µM), dieldrin (4–1000 µM), endosulfan (0.4–100 µM), maneb (0.16–200 µM), MPP+ (0.46–3000 µM), rotenone (0.76–5000 nM), and ivermectin (0.4–35 µM). The cytotoxic P-gp substrates doxorubicin (0.02–200,000 nM) and colchicine (0.01–50,000 nM) were used as positive controls. Concentration ranges were experimentally determined to capture the spectrum of the sigmoidal cell viability dose-response curve for each individual test compound. Inhibition of transport was performed with the known P-gp inhibitors GF120918 (0.5 µM) and verapamil (10 µM). Compounds were evaluated in triplicate. The final concentration of dimethylsulfoxide did not exceed 2% in cell culture experiments. A nonlinear regression log (agonist) versus response variable slope least-squares model was used to estimate cell sensitivity as the effective concentration necessary for 50% cell death (EC50) with Prism 5.0 software. The fold change in cellular resistance was calculated as the ratio of EC50 values in LLC-MDR1-WT cells divided by the values in LLC-vector cells.
Inhibition of Intracellular Accumulation.
Inhibition of R123 efflux in LLC-vector and LLC-MDR1-WT cells was performed based on a previously developed assay (Woodahl et al., 2004; Lacher et al., 2014). Concentration ranges of the following pesticides were experimentally determined based on solubility and to minimize cell death: diazinon (7–500 µM), dieldrin (3–200 µM), endosulfan (3–200 µM), maneb (1–250 µM), MPP+ (1–1000 µM), rotenone (1–100 µM), and ivermectin (0.05–50 µM). The following known P-gp inhibitors were used as positive controls: GF120918 (1.56–1000 nM), cyclosporine (0.1–100 µM), and verapamil (0.1–500 µM). The final concentration of dimethylsulfoxide did not exceed 2% in cell culture experiments. 4′,6-Diamidino-2-phenylindole was added to the cells as a measure of cell viability immediately prior to flow cytometry, and dead cells were excluded from the analysis. A nonlinear regression log (inhibitor) versus normalized response variable slope least-squares model was used to estimate the inhibitor concentration necessary for 50% inhibition (IC50) with Prism 5.0 software.
Statistical Analysis.
EC50 estimates were compared between cell types and within a cell type using an extra sum of squares F test for the cytotoxic sensitivity assay. P values < 0.05 were considered statistically significant.
Results
Membrane-Based Analysis of P-gp Transport of Neurotoxicants.
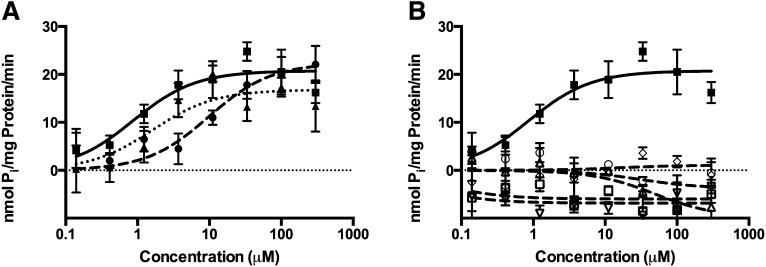
Diazinon and rotenone stimulated ATPase activity in P-gp–expressing membranes (Fig. 1A; Table 1) and were compared with the positive control verapamil. Estimates of Vmax were similar among diazinon, rotenone, and verapamil. The Km of diazinon is more than 10-fold higher than that of verapamil, and the Km of rotenone is almost 2-fold higher than that of verapamil. Dieldrin, endosulfan, maneb, MPP+, and ivermectin failed to stimulate ATPase activity (Fig. 1B; Table 1).
Fig. 1.
Pesticide stimulation of ATPase activity in P-gp–expressing membranes. Compounds that stimulated ATPase activity: diazinon (closed circle, dashed line), rotenone (closed triangle, dotted line), or P-gp substrate verapamil (closed square, solid line) (A). Compounds that did not stimulate ATPase activity: dieldrin (open circle, dashed line), endosulfan (open triangle, dashed line), maneb (open diamond, dashed line), MPP+ (open square, dashed line), ivermectin (open upside-down triangle, dashed line), or P-gp substrate verapamil (closed square, solid line) (B).
TABLE 1.
ATPase activity in P-gp–expressing membranes
| Mean ± S.E. |
||
|---|---|---|
|
Vmax |
Km |
|
| nmol Pi/min per mg protein | µM | |
| Diazinon | 22.4 ± 2.1 | 9.72 ± 3.91 |
| Dieldrin | —a | —a |
| Endosulfan | —a | —a |
| Maneb | —a | —a |
| MPP+ | —a | —a |
| Rotenone | 16.8 ± 1.0 | 1.62 ± 0.51 |
| Ivermectin | —a | —a |
| Verapamil | 20.8 ± 0.7 | 0.871 ± 0.172 |
No ATPase activity above orthovanadate control.
Cell-Based Analysis of P-gp Transport of Neurotoxicants.
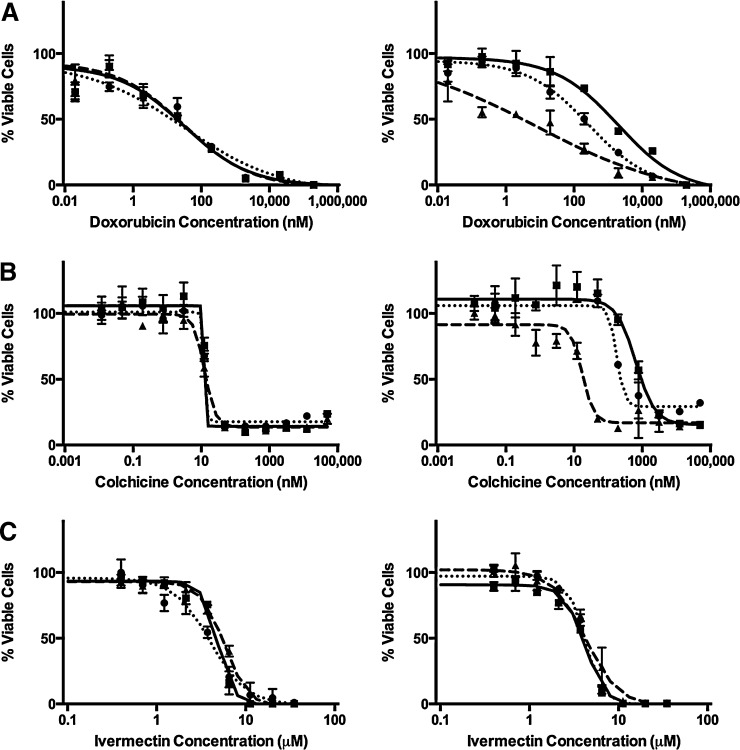
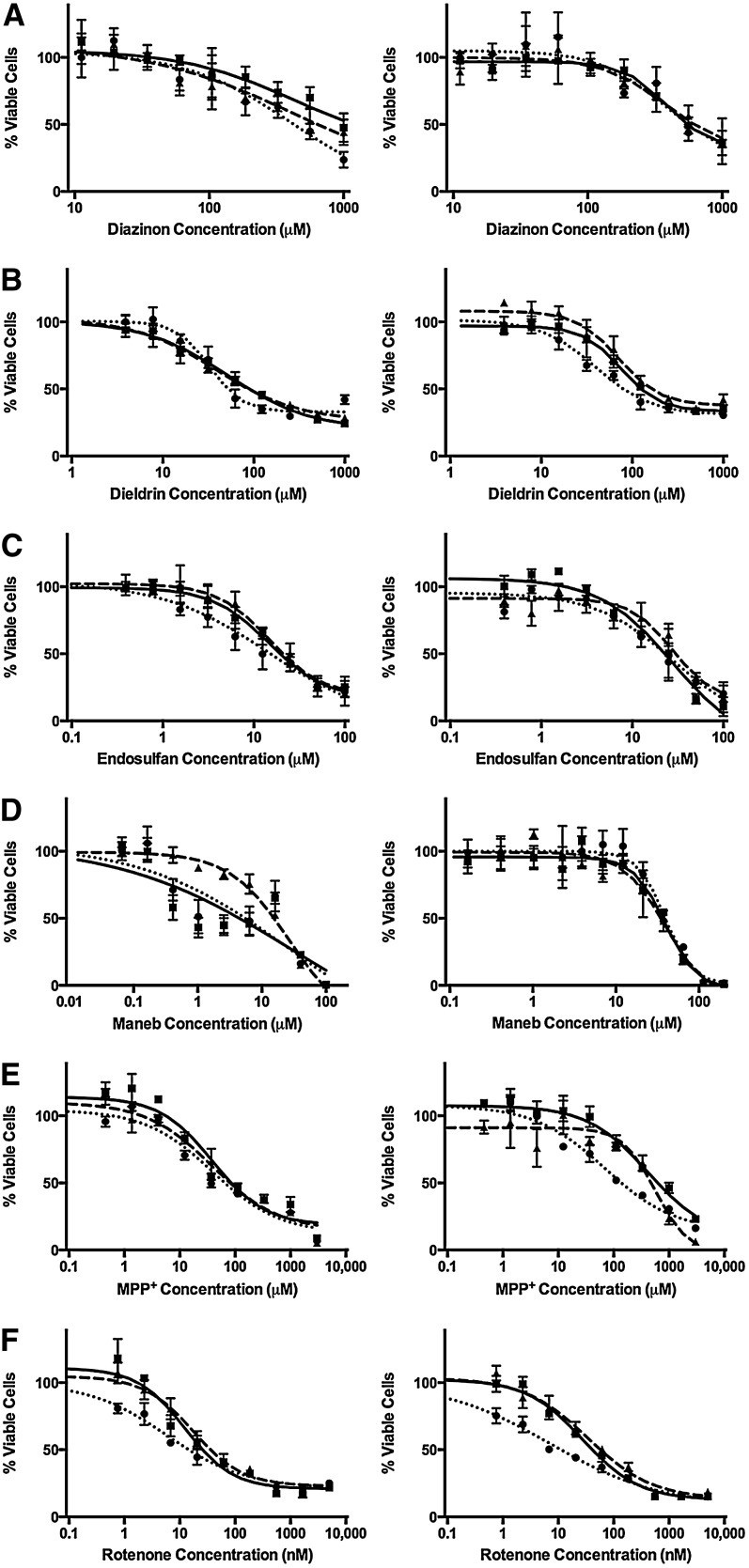
Cytotoxicity was measured in LLC-vector and LLC-MDR1-WT cells in the presence of neurotoxic pesticides and compared with known cytotoxic P-gp substrates as positive controls (doxorubicin and colchicine). EC50 values were estimated (Table 2) and dose-response curves were generated following exposure to P-gp substrates (Fig. 2) (doxorubicin, colchicine, and ivermectin) and the pesticides associated with Parkinson’s disease (Fig. 3) (diazinon, dieldrin, endosulfan, maneb, MPP+, and rotenone). Doxorubicin and colchicine data have been previously reported (Lacher et al., 2014). To confirm that differences in sensitivities between LLC-vector and LLC-MDR1-WT cells were due to P-gp, we used the P-gp inhibitors GF120918 and verapamil. Linearity in the assay was assured by optimizing the number of cells and the time course of neurotoxicant exposure. The two P-gp inhibitors did not cause cytotoxicity at the inhibitor concentrations used (data not shown).
TABLE 2.
Xenobiotic-induced cytotoxicity in LLC-vector and LLC-MDR1-WT cells
| LLC-PK1 Cellsa | EC50 Values (95% Confidence Interval) |
|
|---|---|---|
| LLC-Vector Cellsb | LLC-MDR1-WT Cellsb | |
| Doxorubicin (nM)c | 32.2 (13.7–85.6) | 1978 (1015–3850)*** |
| + GF120918c | 24.8 (12.0–51.3) | 8.06 (1.32–49.2)††† |
| + Verapamilc | 43.4 (12.0–155.9) | 268 (90.7–98.6)††† |
| Colchicine (nM)c | 13.3 (N.D.)d | 645 (467–892) |
| + GF120918c | 12.6 (10.8–14.5) | 17.5 (12.0–25.4)††† |
| + Verapamilc | 12.8 (N.D.)d | 179 (117–273)††† |
| Ivermectin (µM) | 4.86 (4.51–5.23) | 4.04 (3.76–4.35)*** |
| + GF120918 | 5.76 (5.33–6.23)†† | 4.59 (3.99–5.28) |
| + Verapamil | 3.99 (3.31–4.81)† | 4.25 (4.02–4.50) |
| Diazinon (µM) | 1789 (N.D.)d | 384 (279–528) |
| + GF120918 | 132 (68.1–255) | 414 (165–1040) |
| + Verapamil | 592 (50.6–6920) | 373 (159–877) |
| Dieldrin (µM) | 51.4 (41.1–64.4) | 75.4 (62.9–90.4)* |
| + GF120918 | 38.2 (28.0–52.0) | 70.7 (57.0–87.6) |
| + Verapamil | 33.0 (27.3–39.8)† | 40.0 (32.4–49.4)††† |
| Endosulfan (µM) | 14.0 (10.4–18.8) | 19.4 (12.5–30.2) |
| + GF120918 | 17.4 (11.6–26.0) | 40.7 (16.9–98.0) |
| + Verapamil | 8.40 (5.01–14.1) | 16.8 (10.7–26.4) |
| Maneb (µM) | N.D.d | 40.3 (32.7–49.6) |
| + GF120918 | 24.6 (13.9 – 43.7) | 37.7 (30.0–47.3) |
| + Verapamil | N.D.d | 39.2 (32.9–46.7) |
| MPP+ (µM) | 27.1 (16.7–43.9) | 418 (170–1030)*** |
| + GF120918 | 84.9 (37.1–194) | 566 (299–1070) |
| + Verapamil | 31.9 (18.1–56.2) | 69.0 (39.4–121)†† |
| Rotenone (nM) | 12.5 (7.9–19.8) | 27.7 (21.7–35.3)** |
| + GF120918 | 17.4 (12.7–23.8) | 35.0 (23.5–52.1) |
| + Verapamil | 5.89 (3.97–8.73) | 7.24 (4.50–11.6)††† |
N.D., not determined.
Significant differences between cell types in the xenobiotic treatment alone group; *P < 0.05, **P < 0.01, and ***P < 0.001.
Significant differences within a cell type between the xenobiotic treatment alone group and in the presence of a P-gp inhibitor; †P < 0.05, ††P < 0.01, and †††P < 0.001.
Doxorubicin and colchicine have been reported previously (Lacher et al., 2014).
Nonlinear regression was unable to estimate confidence intervals.
Fig. 2.
P-gp substrate cytotoxicity in LLC-vector and LLC-MDR1-WT cells. Cell viability was tested in the presence of doxorubicin (A), colchicine (B), and ivermectin (C). LLC-vector (left column) and LLC-MDR1-WT cells (right column) treated over a range of substrate concentrations alone (square, solid line) or in the presence of the P-gp inhibitors GF120918 (triangle, dashed line) or verapamil (circle, dotted line). Compounds were tested in triplicate (n = 3) at each concentration point; data are represented as mean ± S.D.
Fig. 3.
Parkinson’s disease–associated pesticide cytotoxicity in LLC-vector and LLC-MDR1-WT cells. Cell viability was tested in the presence of diazinon (A), dieldrin (B), endosulfan (C), maneb (D), MPP+ (E), and rotenone (F). LLC-vector (left column) and LLC-MDR1-WT cells (right column) treated over a range of pesticide concentrations alone (square, solid line) or in the presence of the P-gp inhibitors GF120918 (triangle, dashed line) or verapamil (circle, dotted line). Compounds were tested in triplicate (n = 3) at each concentration point; data are represented as mean ± S.D.
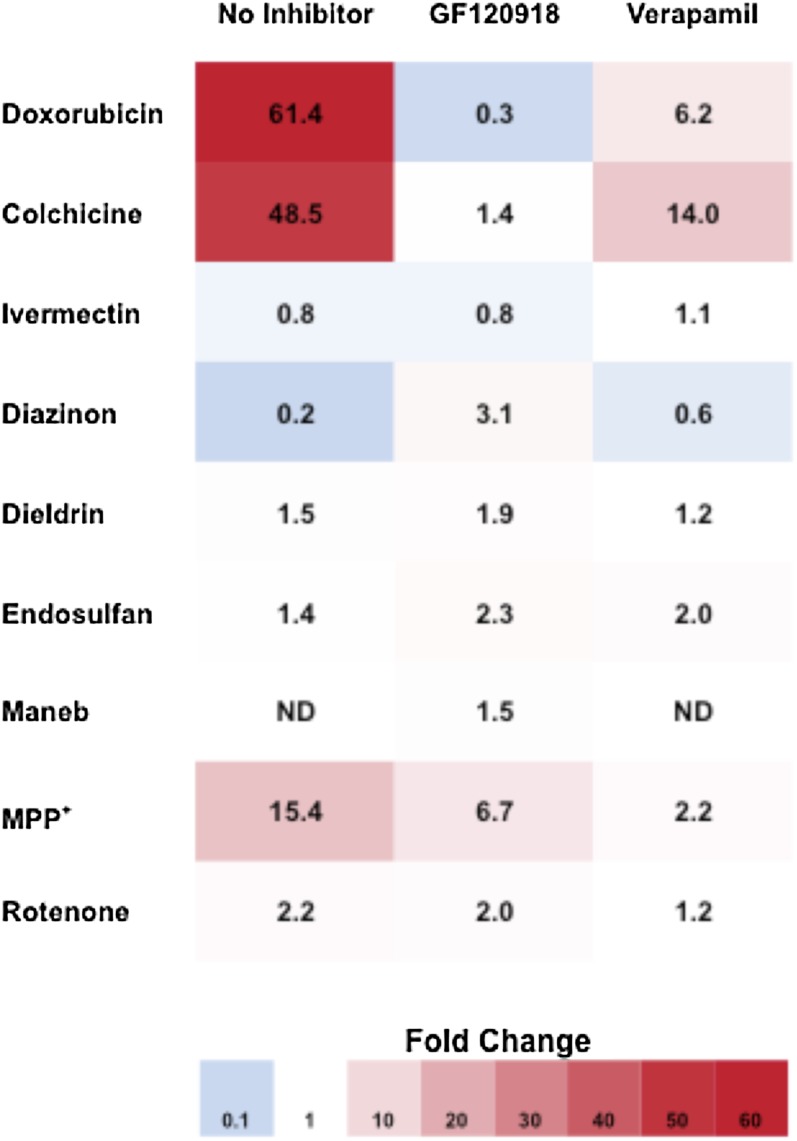
For each compound tested, we estimated the fold change in resistance between LLC-MDR1-WT and LLC-vector control cells (Fig. 4). A fold change greater than one indicates P-gp–mediated resistance (red), and a fold change of one (white) indicates no difference in cellular resistance. To confirm that an increase in resistance was due to P-gp transport, compounds were cotreated with P-gp inhibitors, leading to a decrease in the fold change. LLC-MDR1-WT cells were significantly more resistant to doxorubicin and colchicine than LLC-vector control cells with 61.4- and 48.5-fold increases in EC50 values, respectively. Importantly, the P-gp inhibitors GF120918 and verapamil reversed the resistance to doxorubicin and colchicine, verifying resistance was mediated by P-gp. We observed more modest increases in resistance to MPP+ and rotenone in LLC-MDR1-WT cells compared with LLC-vector cells (15.4- and 2.2-fold, respectively), and this resistance to MPP+ and rotenone was reversed in the presence of verapamil, but not by GF120918, displaying an inhibitor-specific reversal of resistance. LLC-MDR1-WT cells were slightly more resistant to dieldrin than LLC-vector cells (1.5-fold); however, although verapamil had a small effect on increasing sensitivity to dieldrin, this was observed in both LLC-MDR1-WT and LLC-vector cells, indicating that the effect was not mediated by P-gp. We observed no differential resistance to diazinon, endosulfan, maneb, and ivermectin between LLC-MDR1-WT and LLC-vector cells, and GF120918 and verapamil had no effect on cytotoxicity. Together, these data indicate that P-gp mediates cellular resistance to MPP+ and rotenone.
Fig. 4.
Visual representation of the fold change in cellular resistance to cytotoxic agents in LLC-MDR1-WT cells compared with LLC-vector cells. LLC-MDR1-WT cells display increased cellular resistance to P-gp substrates compared with LLC-vector cells, leading to fold changes greater than one (red). No difference between cellular sensitivities results in a fold change of one (white). A fold change of less than one (blue) indicates a result that is not P-gp mediated. Compounds were tested alone or in the presence of P-gp inhibitors (GF120918 and verapamil).
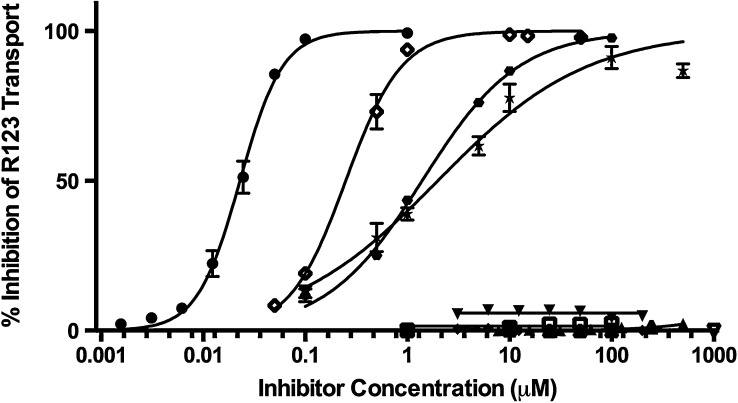
To evaluate if the pesticides were P-gp inhibitors, we measured their ability to inhibit R123 efflux in LLC-vector and LLC-MDR1-WT cells and compared them to the known P-gp inhibitors GF120918, verapamil, and cyclosporine (Fig. 5). The test compounds had no effect on R123 intracellular accumulation in LLC-vector cells (data not shown). Verapamil, cyclosporine, and GF120928 produced 100% inhibition of R123 transport, with IC50 values of 1.98 ± 0.12 μM, 1.39 ± 0.07 μM, and 22.9 ± 1.9 nM, respectively [data reported previously (Lacher et al., 2014)]. Of the pesticides tested, only ivermectin, which is not associated with Parkinson’s disease, inhibited R123 efflux, with an IC50 value of 0.249 ± 0.048 μM. Diazinon, dieldrin, endosulfan, maneb, MPP+, and rotenone failed to inhibit R123 accumulation in P-gp–expressing cells and are not P-gp inhibitors.
Fig. 5.
Inhibition of rhodamine-123 transport by pesticides. Percentage inhibition of rhodamine-123 transport was evaluated in LLC-MDR1-WT cells in the presence of diazinon (closed triangle), dieldrin (closed upside down triangle), endosulfan (closed diamond), maneb (open triangle), MPP+ (open upside down triangle), rotenone (open square), ivermectin (open diamond), GF120918 (closed circle), verapamil (closed star), and cyclosporine (closed hexagon). GF120918, verapamil, and cyclosporine have been previously reported (Lacher et al., 2014). Compounds were tested in triplicate (n = 3) at each concentration point; data are presented as mean ± S.D.
Discussion
P-gp transport may be a protective mechanism in the development of neurodegenerative diseases by effluxing neurotoxic compounds from the brain. We screened a panel of pesticides associated with Parkinson’s disease, diazinon, dieldrin, endosulfan, maneb, MPP+, and rotenone using three different P-gp transport models (summarized in Table 3). We identified diazinon, MPP+, and rotenone as P-gp substrates. None of the pesticides were P-gp inhibitors. These findings are interesting in light of our previous work to demonstrate that paraquat, also a risk factor in Parkinson’s disease, is not a P-gp substrate or inhibitor (Lacher et al., 2014).
TABLE 3.
Summary of membrane-based and cell-based assays to evaluate Parkinson’s disease–associated pesticides as P-gp substrates and inhibitors
| Membrane-Based Assay |
Cell-Based Assay |
||
|---|---|---|---|
| ATPase | Cytotoxicity | R123 Inhibition | |
| Diazinon | ✔ | — | — |
| Dieldrin | — | — | — |
| Endosulfan | — | — | — |
| Maneb | — | — | — |
| MPP+ | — | ✔ | — |
| Rotenone | ✔ | ✔ | — |
Pesticide stimulation of P-gp ATPase activity was measured in a membrane-based system. Diazinon and rotenone both stimulated ATPase activity, indicating that they are P-gp substrates. Dieldrin, endosulfan, maneb, and MPP+ did not stimulate ATPase activity. Although the estimates of Vmax were similar between diazinon and rotenone, the Km values differed significantly. Diazinon has a Km more than 10-fold higher verapamil, indicating a weak binding affinity for P-gp. The Km for rotenone was comparable to verapamil, suggesting that rotenone binds P-gp with a similar affinity to a known substrate.
One limitation of ATPase assays is that slowly transported substrates may not stimulate ATPase activity (Giacomini et al., 2010; Bircsak et al., 2013); therefore, we also used a cell-based model to screen pesticides as P-gp substrates. We evaluated P-gp transport of pesticides using recombinant LLC-vector and LLC-MDR1-WT cells. LLC-PK1 cells are commonly used to study P-gp transport (Giacomini et al., 2010; Brouwer et al., 2013; Hillgren et al., 2013; Zamek-Gliszczynski et al., 2013). We used known P-gp substrates and inhibitors as positive controls to validate the cellular models. As expected, a dramatic increase in resistance was observed in LLC-MDR1-WT cells compared with LLC-vector cells for the classic P-gp cytotoxic substrates doxorubicin and colchicine (61.4- and 48.5-fold, respectively), and resistance was reversed in the presence of the P-gp inhibitors GF120918 and verapamil. Increased resistance was also observed in LLC-MDR1-WT cells to the pesticides MPP+ and rotenone (15.4- and 2.2-fold, respectively). Interestingly, there was an inhibitor-specific ability to reverse the resistance. GF120918 did not have any effect on the resistance, whereas verapamil increased the sensitivity to both pesticides in LLC-MDR1-WT cells. Therefore, MPP+ and rotenone were classified as P-gp substrates from the cytotoxicity data. Although diazinon stimulated ATPase activity, we did not observe an increased resistance in LLC-MDR1-WT cells or alterations in cytotoxicity by P-gp inhibitors. In fact, the LLC-vector cells appear to be slightly more resistant to diazinon than LLC-MDR1-WT cells, which could be due to compensatory alterations in the LLC-MDR1-WT cells, such as upregulation of an uptake transporter for diazinon. There was a slight increase in resistance to dieldrin (1.5-fold); however, while verapamil modestly increased sensitivity in LLC-MDR1-WT cells, it also increased the sensitivity in LLC-vector cells, indicating that dieldrin is not a P-gp substrate. We observed no significant differences in cellular sensitivities to endosulfan, and neither P-gp inhibitor had an effect on cytotoxicity in the LLC-MDR1-WT cells. Although LLC-MDR1-WT cells appear slightly more resistant to maneb, the P-gp inhibitors had no effect on cytotoxicity. Together with the results of the ATPase assay, these data confirm endosulfan and maneb are not P-gp substrates. We also screened the pesticides associated with Parkinson’s disease as potential inhibitors of P-gp efflux of R123. None of the pesticides were able to inhibit R123 efflux in LLC-MDR1-WT cells, even at high concentrations.
We identified rotenone as a P-gp substrate in both membrane- and cell-based models, which strongly suggests that rotenone is a P-gp substrate and may provide a mechanistic link between P-gp transport at the BBB and Parkinson’s disease risk. Rotenone stimulated ATPase activity with a Km similar to that of the known P-gp substrate verapamil. We also observed differential cytotoxicity to rotenone in LLC-MDR1-WT cells compared with LLC-vector cells, and this resistance was reversed in the presence of verapamil, further suggesting that rotenone is a P-gp substrate. MPP+ did not stimulate ATPase activity, but we did observe differential cytotoxicity to MPP+ between LLC-MDR1-WT and LLC-vector cells that was reversed by verapamil, suggesting that it is a P-gp substrate. ATPase methods can give false negatives (Giacomini et al., 2010; Bircsak et al., 2013), especially with slowly transported substrates, which may explain why we did not detect ATPase stimulation by MPP+. Diazinon, on the other hand, stimulated ATPase activity, but did not lead to altered cytotoxicity. The observed Km for diazinon in the ATPase assay was approximately 40-fold lower than the diazinon concentrations used in the cytotoxicity assay. This suggests that diazinon concentrations necessary to induce cytotoxicity in LLC-PK1 cells may be in the nonlinear range of P-gp transport and further cell-based studies may be needed.
Our study is the first to evaluate the P-gp transport of rotenone and maneb. Although there have been sparse reports about P-gp transport of the other pesticides under study, results have been mixed and often contradictory (Bain and LeBlanc, 1996; Bleasby et al., 2000; Martel et al., 2001; Lecoeur et al., 2006; Pivčević and Zaja, 2006; Sreeramulu et al., 2007; Bircsak et al., 2013). Therefore, our study is important to clarify the role of P-gp in the transport of these pesticides. An example of a lack of consistency in the literature includes the results for endosulfan (Bain and LeBlanc, 1996; Sreeramulu et al., 2007; Bircsak et al., 2013). Another issue in interpreting previous studies is the lack of appropriate controls. For example, previous reports have identified pesticides as modest substrates in P-gp–expressing cells lines but did not use P-gp inhibitors to confirm the effects were P-gp mediated (Bain and LeBlanc, 1996; Lecoeur et al., 2006). Our study clearly demonstrates the importance of using P-gp inhibitors to interpret results in recombinant cell lines. We showed that LLC-MDR1-WT cells are more resistant to maneb; however, since P-gp inhibitors do not reverse the resistance, we can disregard maneb as a P-gp substrate. Additionally, the use of a vector control cell line is critical. For example, verapamil appears to slightly reverse resistance to dieldrin in LLC-MDR1-WT cells, but we observed the same phenomenon in LLC-vector cells, which also eliminates dieldrin as a P-gp substrate. Our approach of screening pesticides with three in vitro models and proper controls provides confidence in our observations regarding the P-gp transport of pesticides.
Although not linked to Parkinson’s disease, we also screened the neurotoxic pesticide ivermectin since P-gp is thought to play a role in its in vivo brain disposition. Studies that support ivermectin as a P-gp substrate are in mdr1a knockout mice (Schinkel et al., 1994) and collie dogs with a natural deletion of the mdr1 gene (Mealey et al., 2001, 2003; Edwards, 2003; Nelson et al., 2003; Roulet et al., 2003) that were more sensitive to ivermectin. Interestingly, there has been little direct in vitro investigation of ivermectin as a P-gp substrate other than a previous study that identified ivermectin as an inhibitor, not a substrate, of P-gp (Bain and LeBlanc, 1996). Our results support these findings. We found that ivermectin is a good inhibitor of P-gp, but that it did not stimulate ATPase activity or contribute to differential cytotoxicity in P-gp–expressing cells, indicating that it was not transported by P-gp. Therefore, the role of P-gp in ivermectin neurotoxicity is likely more complicated than what has been previously predicted and may merit further study.
Our goal was to comprehensively study the P-gp transport of the key pesticides associated with Parkinson’s disease to provide a potential mechanism for the role of P-gp in neurodegenerative diseases. An important aspect of our work was to use a combination of in vitro P-gp transport models, which is important in studying xenobiotic interactions with membrane transporters (Polli et al., 2001; Feng et al., 2008; Giacomini et al., 2010; Brouwer et al., 2013; Hillgren et al., 2013; Zamek-Gliszczynski et al., 2013). Our strategy also allowed us to prioritize which pesticides require further study to understand the role of P-gp in their brain accumulation. Further investigation of diazinon, MPP+, and rotenone using in vitro transepithelial transport models and in vivo P-gp–deficient mice models, similar to our work with paraquat (Lacher et al., 2014), will be valuable. One of the limitations of in vitro transport models using recombinant cells is the possibility that endogenous uptake transporters may complicate data interpretation. Little is known about the uptake transporters of these pesticides. Organic cation transporters are thought to mediate MPP+ uptake in humans and rodents (Ahmadimoghaddam et al., 2012; Belzer et al., 2013; Ingoglia et al., 2015; Wu et al., 2015), and a organic anion transporting polypeptide may be involved in diazinon uptake in zebrafish (Popovic et al., 2014). However, it is likely that other uptake transporters of pesticides will be identified in the future.
In summary, we have screened for P-gp transport of the key neurotoxic pesticides linked to Parkinson’s disease using three different in vitro transport models and identified diazinon, MPP+, and rotenone as P-gp substrates. Our data also highlight the value in using multiple models to assess P-gp transport. Further work is needed to determine if P-gp limits the brain distribution of diazinon, MPP+, and rotenone in vivo. Although we selected these pesticides because of their link to Parkinson’s disease, the relevance of P-gp in pesticide-associated toxicity may have a much broader impact because of the wide distribution of P-gp in tissues important in xenobiotic disposition. Therefore, it is important to characterize the P-gp transport of pesticides associated with a spectrum of human diseases.
Acknowledgments
The authors thank Pam Shaw at the Fluorescence Cytometry Core at the University of Montana for technical support, Fernando Cardozo-Pelaez at the University of Montana for meaningful discussions, and Joslynn S. Lee at the University of Minnesota Duluth for help with visually presenting the cellular cytotoxicity data.
Abbreviations
- BBB
blood-brain barrier
- GF120918
N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide
- MPP+
1-methyl-4-phenyl-4-phenylpyridinium ion
- P-gp
P-glycoprotein
- Pi
inorganic phosphate
- R123
rhodamine-123
Authorship Contributions
Participated in research design: Lacher, Woodahl.
Conducted experiments: Lacher, Skagen, Veit, Dalton.
Performed data analysis: Lacher, Skagen, Veit, Dalton, Woodahl.
Wrote or contributed to the writing of the manuscript: Lacher, Skagen, Veit, Dalton, Woodahl.
Footnotes
This work was supported by the National Institutes of Health Center of Biomedical Research Excellence grants that support the Center for Biomolecular Structure and Dynamics [Grant P20GM103546], the Center for Environmental Health Sciences [Grant P20RR017670], and the Center for Structural and Functional Neuroscience [Grant P20RR015583]; the American Foundation for Pharmaceutical Education, American Association of College of Pharmacy, New Investigators Program (E.L.W.); the Institute of Translational Health Sciences [Grant UL1TR000423 to E.L.W.]; and the American Foundation for Pharmacy Education Pre-Doctoral Fellowship (S.E.L.).
References
- Ahmadimoghaddam D, Hofman J, Zemankova L, Nachtigal P, Dolezelova E, Cerveny L, Ceckova M, Micuda S, Staud F. (2012) Synchronized activity of organic cation transporter 3 (Oct3/Slc22a3) and multidrug and toxin extrusion 1 (Mate1/Slc47a1) transporter in transplacental passage of MPP+ in rat. Toxicol Sci 128:471–481. [DOI] [PubMed] [Google Scholar]
- Bain LJ, LeBlanc GA. (1996) Interaction of structurally diverse pesticides with the human MDR1 gene product P-glycoprotein. Toxicol Appl Pharmacol 141:288–298. [DOI] [PubMed] [Google Scholar]
- Bartels AL. (2011) Blood-brain barrier P-glycoprotein function in neurodegenerative disease. Curr Pharm Des 17:2771–2777. [DOI] [PubMed] [Google Scholar]
- Bartels AL, Kortekaas R, Bart J, Willemsen AT, de Klerk OL, de Vries JJ, van Oostrom JC, Leenders KL. (2009) Blood-brain barrier P-glycoprotein function decreases in specific brain regions with aging: a possible role in progressive neurodegeneration. Neurobiol Aging 30:1818–1824. [DOI] [PubMed] [Google Scholar]
- Bartels AL, Willemsen AT, Kortekaas R, de Jong BM, de Vries R, de Klerk O, van Oostrom JC, Portman A, Leenders KL. (2008) Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J Neural Transm 115:1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzer M, Morales M, Jagadish B, Mash EA, Wright SH. (2013) Substrate-dependent ligand inhibition of the human organic cation transporter OCT2. J Pharmacol Exp Ther 346:300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bircsak KM, Richardson JR, Aleksunes LM. (2013) Inhibition of human MDR1 and BCRP transporter ATPase activity by organochlorine and pyrethroid insecticides. J Biochem Mol Toxicol 27:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasby K, Chauhan S, Brown CD. (2000) Characterization of MPP+ secretion across human intestinal Caco-2 cell monolayers: role of P-glycoprotein and a novel Na(+)-dependent organic cation transport mechanism. Br J Pharmacol 129:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet AM, Houeto JL. (1999) Pathophysiology of Parkinson’s disease. Biomed Pharmacother 53:117–121. [DOI] [PubMed] [Google Scholar]
- Bradbury AJ, Costall B, Domeney AM, Jenner P, Kelly ME, Marsden CD, Naylor RJ. (1986) 1-methyl-4-phenylpyridine is neurotoxic to the nigrostriatal dopamine pathway. Nature 319:56–57. [DOI] [PubMed] [Google Scholar]
- Brouwer KL, Keppler D, Hoffmaster KA, Bow DA, Cheng Y, Lai Y, Palm JE, Stieger B, Evers R, International Transporter Consortium (2013) In vitro methods to support transporter evaluation in drug discovery and development. Clin Pharmacol Ther 94:95–112. [DOI] [PubMed] [Google Scholar]
- Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. (2015) Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci 9:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. (2009) Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol 169:919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon AS, Tarbutton GL, Levin JL, Plotkin GM, Lowry LK, Nalbone JT, Shepherd S. (2008) Pesticide/environmental exposures and Parkinson’s disease in East Texas. J Agromed 13:37–48. [DOI] [PubMed] [Google Scholar]
- Droździk M, Białecka M, Myśliwiec K, Honczarenko K, Stankiewicz J, Sych Z. (2003) Polymorphism in the P-glycoprotein drug transporter MDR1 gene: a possible link between environmental and genetic factors in Parkinson’s disease. Pharmacogenetics 13:259–263. [DOI] [PubMed] [Google Scholar]
- Dutheil F, Beaune P, Tzourio C, Loriot MA, Elbaz A. (2010) Interaction between ABCB1 and professional exposure to organochlorine insecticides in Parkinson disease. Arch Neurol 67:739–745. [DOI] [PubMed] [Google Scholar]
- Edwards G. (2003) Ivermectin: does P-glycoprotein play a role in neurotoxicity? Filaria J 2 (Suppl 1):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Mills JB, Davidson RE, Mireles RJ, Janiszewski JS, Troutman MD, de Morais SM. (2008) In vitro P-glycoprotein assays to predict the in vivo interactions of P-glycoprotein with drugs in the central nervous system. Drug Metab Dispos 36:268–275. [DOI] [PubMed] [Google Scholar]
- Firestone JA, Smith-Weller T, Franklin G, Swanson P, Longstreth WT, Jr, Checkoway H. (2005) Pesticides and risk of Parkinson disease: a population-based case-control study. Arch Neurol 62:91–95. [DOI] [PubMed] [Google Scholar]
- Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. (1994) Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol 36:100–103. [DOI] [PubMed] [Google Scholar]
- Furuno T, Landi MT, Ceroni M, Caporaso N, Bernucci I, Nappi G, Martignoni E, Schaeffeler E, Eichelbaum M, Schwab M, et al. (2002) Expression polymorphism of the blood-brain barrier component P-glycoprotein (MDR1) in relation to Parkinson’s disease. Pharmacogenetics 12:529–534. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. (2002) Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto NM, Cockburn M, Bronstein J, Manthripragada AD, Ritz B. (2009) Well-water consumption and Parkinson’s disease in rural California. Environ Health Perspect 117:1912–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini KM. (1997) Membrane transporters in drug disposition. J Pharmacokinet Biopharm 25:731–741. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, et al. International Transporter Consortium (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SM. (2014) Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol 54:141–164. [DOI] [PubMed] [Google Scholar]
- Hatcher JM, Pennell KD, Miller GW. (2008) Parkinson’s disease and pesticides: a toxicological perspective. Trends Pharmacol Sci 29:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher JM, Richardson JR, Guillot TS, McCormack AL, Di Monte DA, Jones DP, Pennell KD, Miller GW. (2007) Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol 204:619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillgren KM, Keppler D, Zur AA, Giacomini KM, Stieger B, Cass CE, Zhang L, International Transporter Consortium (2013) Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clin Pharmacol Ther 94:52–63. [DOI] [PubMed] [Google Scholar]
- Ingoglia F, Visigalli R, Rotoli BM, Barilli A, Riccardi B, Puccini P, Dall’Asta V. (2015) Functional characterization of the organic cation transporters (OCTs) in human airway pulmonary epithelial cells. Biochim Biophys Acta 1848:1563–1572. [DOI] [PubMed] [Google Scholar]
- Jia Z, Misra HP. (2007a) Developmental exposure to pesticides zineb and/or endosulfan renders the nigrostriatal dopamine system more susceptible to these environmental chemicals later in life. Neurotoxicology 28:727–735. [DOI] [PubMed] [Google Scholar]
- Jia Z, Misra HP. (2007b) Exposure to mixtures of endosulfan and zineb induces apoptotic and necrotic cell death in SH-SY5Y neuroblastoma cells, in vitro. J Appl Toxicol 27:434–446. [DOI] [PubMed] [Google Scholar]
- Kalvass JC, Polli JW, Bourdet DL, Feng B, Huang SM, Liu X, Smith QR, Zhang LK, Zamek-Gliszczynski MJ, International Transporter Consortium (2013) Why clinical modulation of efflux transport at the human blood-brain barrier is unlikely: the ITC evidence-based position. Clin Pharmacol Ther 94:80–94. [DOI] [PubMed] [Google Scholar]
- Kamel F. (2013) Epidemiology. Paths from pesticides to Parkinson’s. Science 341:722–723. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Yang Y, Anantharam V, Kanthasamy A. (2008) Environmental neurotoxin dieldrin induces apoptosis via caspase-3-dependent proteolytic activation of protein kinase C delta (PKCdelta): implications for neurodegeneration in Parkinson’s disease. Mol Brain 1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, Hendrikse NH. (2005) Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 57:176–179. [DOI] [PubMed] [Google Scholar]
- Lacher SE, Gremaud JN, Skagen K, Steed E, Dalton R, Sugden KD, Cardozo-Pelaez F, Sherwin CM, Woodahl EL. (2014) Absence of P-glycoprotein transport in the pharmacokinetics and toxicity of the herbicide paraquat. J Pharmacol Exp Ther 348:336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. (1983) Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219:979–980. [DOI] [PubMed] [Google Scholar]
- Langston JW, Irwin I, Langston EB, Forno LS. (1984) 1-Methyl-4-phenylpyridinium ion (MPP+): identification of a metabolite of MPTP, a toxin selective to the substantia nigra. Neurosci Lett 48:87–92. [DOI] [PubMed] [Google Scholar]
- Le Couteur DG, Davis MW, Webb M, Board PG. (2001) P-glycoprotein, multidrug-resistance-associated protein and Parkinson’s disease. Eur Neurol 45:289–290. [DOI] [PubMed] [Google Scholar]
- Lecoeur S, Videmann B, Mazallon M. (2006) Effect of organophosphate pesticide diazinon on expression and activity of intestinal P-glycoprotein. Toxicol Lett 161:200–209. [DOI] [PubMed] [Google Scholar]
- Lee CG, Tang K, Cheung YB, Wong LP, Tan C, Shen H, Zhao Y, Pavanni R, Lee EJ, Wong MC, et al. (2004) MDR1, the blood-brain barrier transporter, is associated with Parkinson’s disease in ethnic Chinese. J Med Genet 41:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Bendayan R. (2004) Functional expression and localization of P-glycoprotein in the central nervous system: relevance to the pathogenesis and treatment of neurological disorders. Pharm Res 21:1313–1330. [DOI] [PubMed] [Google Scholar]
- Li AA, Mink PJ, McIntosh LJ, Teta MJ, Finley B. (2005) Evaluation of epidemiologic and animal data associating pesticides with Parkinson’s disease. J Occup Environ Med 47:1059–1087. [DOI] [PubMed] [Google Scholar]
- Li Y, Li Y, Pang S, Huang W, Zhang A, Hawley RG, Yan B. (2014) Novel and functional ABCB1 gene variant in sporadic Parkinson’s disease. Neurosci Lett 566:61–66. [DOI] [PubMed] [Google Scholar]
- Lin JH, Yamazaki M. (2003a) Clinical relevance of P-glycoprotein in drug therapy. Drug Metab Rev 35:417–454. [DOI] [PubMed] [Google Scholar]
- Lin JH, Yamazaki M. (2003b) Role of P-glycoprotein in pharmacokinetics: clinical implications. Clin Pharmacokinet 42:59–98. [DOI] [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. (1997) Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology 48:1583–1588. [DOI] [PubMed] [Google Scholar]
- Martel F, Keating E, Azevedo I. (2001) Effect of P-glycoprotein modulators on the human extraneuronal monoamine transporter. Eur J Pharmacol 422:31–37. [DOI] [PubMed] [Google Scholar]
- Mealey KL, Bentjen SA, Gay JM, Cantor GH. (2001) Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics 11:727–733. [DOI] [PubMed] [Google Scholar]
- Mealey KL, Northrup NC, Bentjen SA. (2003) Increased toxicity of P-glycoprotein-substrate chemotherapeutic agents in a dog with the MDR1 deletion mutation associated with ivermectin sensitivity. J Am Vet Med Assoc 223:1453–1455, 1434. [DOI] [PubMed] [Google Scholar]
- Mostafalou S, Abdollahi M. (2013) Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol 268:157–177. [DOI] [PubMed] [Google Scholar]
- Nelson OL, Carsten E, Bentjen SA, Mealey KL. (2003) Ivermectin toxicity in an Australian Shepherd dog with the MDR1 mutation associated with ivermectin sensitivity in Collies. J Vet Intern Med 17:354–356. [PubMed] [Google Scholar]
- Pezzoli G, Cereda E. (2013) Exposure to pesticides or solvents and risk of Parkinson disease. Neurology 80:2035–2041. [DOI] [PubMed] [Google Scholar]
- Pivčević B, Zaja R. (2006) Pesticides and their binary combinations as P-glycoprotein inhibitors in NIH 3T3/MDR1 cells. Environ Toxicol Pharmacol 22:268–276. [DOI] [PubMed] [Google Scholar]
- Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS. (2001) Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther 299:620–628. [PubMed] [Google Scholar]
- Popovic M, Zaja R, Fent K, Smital T. (2014) Interaction of environmental contaminants with zebrafish organic anion transporting polypeptide, Oatp1d1 (Slco1d1). Toxicol Appl Pharmacol 280:149–158. [DOI] [PubMed] [Google Scholar]
- Rapposelli S, Digiacomo M, Balsamo A. (2009) P-gp transporter and its role in neurodegenerative diseases. Curr Top Med Chem 9:209–217. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. (2006) Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. FASEB J 20:1695–1697. [DOI] [PubMed] [Google Scholar]
- Roulet A, Puel O, Gesta S, Lepage JF, Drag M, Soll M, Alvinerie M, Pineau T. (2003) MDR1-deficient genotype in Collie dogs hypersensitive to the P-glycoprotein substrate ivermectin. Eur J Pharmacol 460:85–91. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP, et al. (1994) Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 77:491–502. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. (1992) Parkinson’s disease and exposure to agricultural work and pesticide chemicals. Neurology 42:1328–1335. [DOI] [PubMed] [Google Scholar]
- Sharma H, Zhang P, Barber DS, Liu B. (2010) Organochlorine pesticides dieldrin and lindane induce cooperative toxicity in dopaminergic neurons: role of oxidative stress. Neurotoxicology 31:215–222. [DOI] [PubMed] [Google Scholar]
- Sharom FJ. (2011) The P-glycoprotein multidrug transporter. Essays Biochem 50:161–178. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. (2011) Developmental exposure to organophosphates triggers transcriptional changes in genes associated with Parkinson’s disease in vitro and in vivo. Brain Res Bull 86:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonsalla PK, Zeevalk GD, German DC. (2008) Chronic intraventricular administration of 1-methyl-4-phenylpyridinium as a progressive model of Parkinson’s disease. Parkinsonism Relat Disord 14 (Suppl 2):S116–S118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreeramulu K, Liu R, Sharom FJ. (2007) Interaction of insecticides with mammalian P-glycoprotein and their effect on its transport function. Biochim Biophys Acta 1768:1750–1757. [DOI] [PubMed] [Google Scholar]
- Steece-Collier K, Maries E, Kordower JH. (2002) Etiology of Parkinson’s disease: genetics and environment revisited. Proc Natl Acad Sci USA 99:13972–13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EK, Chan DK, Ng PW, Woo J, Teo YY, Tang K, Wong LP, Chong SS, Tan C, Shen H, et al. (2005) Effect of MDR1 haplotype on risk of Parkinson disease. Arch Neurol 62:460–464. [DOI] [PubMed] [Google Scholar]
- Tan EK, Drozdzik M, Bialecka M, Honczarenko K, Klodowska-Duda G, Teo YY, Tang K, Wong LP, Chong SS, Tan C, et al. (2004) Analysis of MDR1 haplotypes in Parkinson’s disease in a white population. Neurosci Lett 372:240–244. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, et al. (2011) Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect 119:866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiffault C, Langston JW, Di Monte DA. (2000) Increased striatal dopamine turnover following acute administration of rotenone to mice. Brain Res 885:283–288. [DOI] [PubMed] [Google Scholar]
- Uversky VN. (2004) Neurotoxicant-induced animal models of Parkinson’s disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res 318:225–241. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL. (2001) Pesticides directly accelerate the rate of alpha-synuclein fibril formation: a possible factor in Parkinson’s disease. FEBS Lett 500:105–108. [DOI] [PubMed] [Google Scholar]
- Vautier S, Fernandez C. (2009) ABCB1: the role in Parkinson’s disease and pharmacokinetics of antiparkinsonian drugs. Expert Opin Drug Metab Toxicol 5:1349–1358. [DOI] [PubMed] [Google Scholar]
- Wang XF, Li S, Chou AP, Bronstein JM. (2006) Inhibitory effects of pesticides on proteasome activity: implication in Parkinson’s disease. Neurobiol Dis 23:198–205. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Knekt P, O’Reilly EJ, Lyytinen J, Reunanen A, Laden F, Altshul L, Ascherio A. (2010) Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology 74:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund M, Belin AC, Anvret A, Håkansson A, Nissbrandt H, Lind C, Sydow O, Olson L, Galter D. (2009) Association of a polymorphism in the ABCB1 gene with Parkinson’s disease. Parkinsonism Relat Disord 15:422–424. [DOI] [PubMed] [Google Scholar]
- Westerlund M, Belin AC, Olson L, Galter D. (2008) Expression of multi-drug resistance 1 mRNA in human and rodent tissues: reduced levels in Parkinson patients. Cell Tissue Res 334:179–185. [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. (2011) Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol 26 (Suppl 1):S1–S58. [DOI] [PubMed] [Google Scholar]
- Woodahl EL, Yang Z, Bui T, Shen DD, Ho RJ. (2004) Multidrug resistance gene G1199A polymorphism alters efflux transport activity of P-glycoprotein. J Pharmacol Exp Ther 310:1199–1207. [DOI] [PubMed] [Google Scholar]
- Wu KC, Lu YH, Peng YH, Tsai TF, Kao YH, Yang HT, Lin CJ. (2015) Decreased expression of organic cation transporters, Oct1 and Oct2, in brain microvessels and its implication to MPTP-induced dopaminergic toxicity in aged mice. J Cereb Blood Flow Metab 35:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Lee CA, Poirier A, Bentz J, Chu X, Ellens H, Ishikawa T, Jamei M, Kalvass JC, Nagar S, et al. International Transporter Consortium (2013) ITC recommendations for transporter kinetic parameter estimation and translational modeling of transport-mediated PK and DDIs in humans. Clin Pharmacol Ther 94:64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]