Abstract
It is known that 1) elevated serum bile acids (BAs) are associated with decreased body weight, 2) elevated glucagon-like peptide-1 (GLP-1) levels can decrease body weight, and 3) germ-free (GF) mice are resistant to diet-induced obesity. The purpose of this study was to test the hypothesis that a lack of intestinal microbiota results in more BAs in the body, resulting in increased BA-mediated transmembrane G protein–coupled receptor 5 (TGR5) signaling and increased serum GLP-1 as a mechanism of resistance of GF mice to diet-induced obesity. GF mice had 2- to 4-fold increased total BAs in the serum, liver, bile, and ileum. Fecal excretion of BAs was 63% less in GF mice. GF mice had decreased secondary BAs and increased taurine-conjugated BAs, as anticipated. Surprisingly, there was an increase in non–12α-OH BAs, namely, β-muricholic acid, ursodeoxycholic acid (UDCA), and their taurine conjugates, in GF mice. Further, in vitro experiments confirmed that UDCA is a primary BA in mice. There were minimal changes in the mRNA of farnesoid X receptor target genes in the ileum (Fibroblast growth factor 15, small heterodimer protein, and ileal bile acid–binding protein), in the liver (small heterodimer protein, liver receptor homolog-1, and cytochrome P450 7a1), and BA transporters (apical sodium dependent bile acid transporter, organic solute transporter α, and organic solute transporter β) in the ileum of GF mice. Surprisingly, there were marked increases in BA transporters in the large intestine. Increased GLP-1 levels and gallbladder size were observed in GF mice, suggesting activation of TGR5 signaling. In summary, the GF condition results in increased expression of BA transporters in the colon, resulting in 1) an increase in total BA concentrations in tissues, 2) a change in BA composition to favor an increase in non–12α-OH BAs, and 3) activation of TGR5 signaling with increased gallbladder size and GLP-1.
Introduction
Bile acids (BAs) are amphipathic cholesterol metabolites that aid in the absorption of fats and fat-soluble vitamins from the diet. Primary BAs are synthesized in the liver, secreted into bile, and further metabolized by intestinal bacteria to produce secondary BAs. Most of the BAs are actively reabsorbed by the ileum and enter the portal vein. Therefore, the diversity of BAs in the circulation is influenced by their metabolism by the liver as well as intestinal bacteria.
The BA profile is important because BAs also regulate host physiology. Individual BAs have physicochemical properties based on their structure. An increase in BAs that have a –OH group in the 12 position, such as cholic acid (CA) and deoxycholic acid (DCA) (Supplemental Fig. 1), increases cholesterol absorption across the intestine (Li-Hawkins et al., 2002). Taurine conjugation lowers the pKa of BAs, increases their aqueous solubility, and promotes a high intraluminal BA concentration, leading to efficient solubilization of lipids and lipid-soluble vitamins (Setchell et al., 2013). BAs synthesized by the host modify the intestinal bacterial composition, and intestinal bacteria modify the circulating BA composition in the host. Thus, BAs are one of the host factors that help shape the intestinal bacterial community, and thus feeding BAs can alter intestinal microbial composition (Islam et al., 2011).
BAs act as hormones, binding to and activating their receptors: farnesoid X receptor (FXR) and transmembrane G protein–coupled receptor 5 (TGR5) (official name is GPBAR). BAs regulate their own synthesis and transport by binding to and activating FXR (Makishima et al., 1999; Parks et al., 1999; Wang et al., 1999). BAs also activate the membrane-bound G protein–coupled receptor TGR5, leading to an increase in intracellular cAMP production (Maruyama et al., 2002). TGR5 activation in brown adipose tissue increases type 2 iodothyronine deiodinase, which converts T4 to the active T3, which increases energy expenditure (Watanabe et al., 2006). TGR5 activation in the enteroendocrine cells of the intestine increases glucagon-like peptide-1 (GLP-1) secretion (Katsuma et al., 2005; Thomas et al., 2009). Patients treated with a BA-binding resin to reduce cholesterol levels also have improved glycemic control, and these BA-binding resins also improved hyperglycemia in rodent models of obesity (Kobayashi et al., 2007; Chen et al., 2010). TGR5 agonists, such as 6α-ethyl-23(S)-methyl-cholic acid (EMSA or INT-777), are promising drug candidates to treat metabolic disorders, such as type 2 diabetes, obesity, and steatohepatitis, as they improve glucose tolerance and reduce inflammation and steatosis in mice (Keitel and Haussinger, 2012).
Intestinal bacteria contain enzymes that metabolize BAs and change the BA composition in the intestinal content and thus also in the circulation of the host (Narushima et al., 2000, 2006). The capacity of intestinal microbiota to metabolize BAs not only provides intestinal bacteria cellular carbon, nitrogen, and sulfur, but also enables them to be bile resistant and grow in the presence of BAs. Intestinal bacteria perform the following modifications of BAs in the gut lumen: deconjugation of taurine or glycine-conjugated BAs (by bile acid hydrolase), oxidation and epimerization of the 3-, 7-, and 12-hydroxy groups of BAs (by hydroxysteroid dehydrogenases), and 7-dehydroxylation (by 7-dehydratase). Bile acid hydrolase and hydroxysteroid dehydrogenase are present in a broad spectrum of intestinal bacteria, whereas enzymes for 7-dehydratase required to produce DCA and lithocholic acid are restricted to a small number of intestinal anaerobes, such as Clostridium (Ridlon et al., 2006).
Germ-free (GF) mice, which lack intestinal bacteria, are resistant to high-fat diet-induced obesity (Backhed et al., 2007; Rabot et al., 2010), and, in contrast, colonization of GF mice with intestinal bacteria results in increased body fat content and insulin resistance (Backhed et al., 2004). Surgical therapies to treat obesity, such as vertical sleeve gastrectomy, result in weight loss and are associated with elevated circulating BAs (Myronovych et al., 2014; Ryan et al., 2014). It is understood that intestinal bacteria regulate BAs in circulation (Zhang et al., 2014), and elevated BAs in circulation are associated with weight loss. BAs activate TGR5, increase secretion of GLP-1, and improve insulin resistance and weight loss. Thus, it might be possible that GF mice have increased concentrations of bile acids that activate TGR5 and increase GLP-1 levels, which might add in the resistance of GF mice to diet-induced obesity. Therefore, in this study, alterations in BA composition and BA signaling in GF mice were examined to test this hypothesis.
Materials and Methods
Animals.
All mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International–accredited facility at the University of Kansas Medical Center, with a 14-hour light/10-hour dark cycle, in a temperature- and humidity-controlled environment with ad libitum access to feed and water. The initial breeding colony of GF C57BL/6J/UNC mice was established with mice obtained from the National Gnotobiotic Rodent Resource Center (University of North Carolina, Chapel Hill, NC). GF mice were housed in vinyl flexible film isolators (Class Biologically Clean Ltd., Madison, WI), with autoclaved, irradiated Harlan Sani chips (#7990.BG Irradiated Teklad Sani Chips; Harlan Teklad, Madison, WI) as bedding and autoclaved paper towels or autoclaved nestlets as enrichment. All conventional (CV) C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). All mice had free access to the autoclaved rodent diet #5K67 (Labdiet, St. Louis, MO) and autoclaved water. The sterility of the isolator was tested routinely by culturing and performing wet mounts and gram stains of feces. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center.
Chemicals and Reagents.
The sources of individual BA standards and internal standards were described previously (Zhang and Klaassen, 2010; Zhang et al., 2011a). A GLP-1 total enzyme-linked immunosorbent assay kit was purchased from EMD Millipore (St. Charles, MO). Oasis–HLB SPE cartridges for BA extraction were purchased from Waters (Milford, MA). All other chemicals and reagents, unless indicated otherwise, were purchased from Sigma-Aldrich (St. Louis, MO).
Bile Collection.
The protocol for bile-duct cannulation and bile collection has been described previously (Csanaky et al., 2011). Briefly, mice were anesthetized intraperitoneally with a ketamine/midazolam mixture (100 and 5 mg/kg, respectively), and the common bile duct of each mouse was cannulated through a high-abdominal incision with the shaft of a 30-gauge needle attached to PE-10 tubing. Bile was collected in the dark for 40 minutes into preweighed microcentrifuge tubes on ice. The volume of bile was determined gravimetrically using the value 1 for specific gravity.
Tissue and Feces Collection.
Mice were sacrificed with an overdose of pentobarbital and opening the abdominal cavity. Blood was collected from suborbital veins into Microtainer plasma-separating tubes (BD Biosciences, San Jose, CA). Tissues were collected, frozen in liquid nitrogen, and stored at −80°C until further analysis. The small intestine contents were removed, and the tissue was divided into three equal segments, namely, the duodenum, jejunum, and ileum. In this study, the large intestine indicates only the colon and not the cecum. All animal sacrifices and tissue collections were performed between 9:00 AM and noon to minimize variations in BA metabolism due to circadian rhythm (Zhang et al., 2009, 2011b). CV and GF mice were housed individually in metabolic cages, and feces were collected over 24 hours.
Bile Acid Extraction and Quantification by Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry.
Internal standards (40 μg/ml d4-G-CDCA and 20 μg/ml d4-CDCA in MeOH) were added to the samples, and BAs were extracted from the serum, gallbladder bile, livers, biliary bile, ileum, and feces using methods reported previously by our laboratory (Alnouti et al., 2008; Zhang and Klaassen, 2010; Zhang et al., 2011a, 2012). The BAs quantified include taurine-conjugated cholic acid (TCA), taurine-conjugated deoxycholic acid (TDCA), taurine-conjugated chenodeoxycholic acid (TCDCA), taurine-conjugated lithocholic acid (TLCA), taurine-conjugated ursodeoxycholic acid (TUDCA), taurine-conjugated α-muricholic acid, (TαMCA), TMDCA taurine-conjugated β-muricholic acid (TβMCA), taurine-conjugated ω-muricholic acid (TωMCA), cholic acid (CA), deoxycholic acid (DCA) chenodeoxycholic acid (CDCA), lithocholic acid (LCA), ursodeoxycholic acid (UDCA), α muricholic acid βMCA, and ωMCA. TαMCA and TβMCA were summed together and expressed as Tα+βMCA due to a peak separation issue. The concentrations of individual BAs were summed to derive the concentrations of total conjugated, total unconjugated, and total BAs in each tissue compartment.
Preparation of Hepatic Microsomal Fractions.
Hepatic microsomes were prepared following a previously published protocol (MacGeoch et al., 1984). Briefly, livers were homogenized in a buffer (sucrose 250 mM, Tris-HCl 50 mM, pH 7.4, EDTA 1 mM, and Phenylmethanesulfonyl flouride (PMSF) 0.2 mM) with a glass-dounce homogenizer on ice and centrifuged at 10,000g for 25 minutes at 4°C. The supernatant was decanted and centrifuged at 100,000g for 60 minutes at 4°C. The pellet was rinsed with a KCl buffer (KCl 150 mM, Tris-HCl 10 mM, pH 7.4, and EDTA 1 mM) and centrifuged at 100,000g for 60 minutes at 4°C. The microsomes were resuspended in glycerol buffer (Tris-HCl 10 mM, pH 7.4, EDTA 1 mM, phenylmethylsulfonyl flouride (PMSF) 0.2 mM, and glycerol 20% w/v). Protein concentrations were determined by a BCA assay according to the manufacturer’s instructions (Pierce Biotechnology, Rockford, IL).
Microsomal Biotransformation Assay.
Final reaction mixtures contained CDCA (200 µM), 0.5 mg of liver microsomal protein, 100 mM potassium phosphate buffer, pH 7.4, and 2 mM NADPH in a final volume of 250 µl. After preincubation of the microsomal protein and CDCA for 10 minutes at room temperature, reactions were initiated with NADPH and incubated for 60 minutes at 37°C. Reactions were terminated by adding 1 ml of ice-cold acetonitrile, dried under a vacuum, and resuspended in 80 µl of 50% methanol. Contents were mixed and then centrifuged at 20,000g for 10 minutes. The bile acids were quantified as mentioned above.
GLP-1 Quantification.
Total serum GLP-1 was quantified using an enzyme-linked immunosorbent assay kit (EZGLP1T-36K) from EMD Millipore (Billerica, MA) following the manufacturer’s protocol.
RNA Isolation.
Total RNA was isolated from tissues using RNA Bee reagent (Tel-Test Inc., Friendswood, TX) following the manufacturer’s protocol. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm. The quality of RNA was assessed by running the sample on a denaturing agarose gel and visualizing two discrete 18S and 28S ribosomal RNA bands, with the intensity of the 28S band double that of the 18S band.
Multiplex Suspension Assay.
The mRNAs of BA biosynthetic enzymes and BA transporters in the liver and ileum were quantified using Panomics 2.0 QuantiGene Plex technology (Panomics/Affymetrix Inc., Fremont, CA) following the manufacturer’s protocol. Individual gene information can be found on the Panomics web site (http://www.panomics.com), with panel numbers 21330 and 21383. Fluorescence was analyzed using a Bio-Plex 200 system array reader with Luminex 100 X-MAP technology (Bio-Rad, Hercules, CA), and data were acquired using Bio-Plex data manager software 5.0 (Bio-Rad). The mRNAs of the target genes were normalized to the housekeeping gene Rpl13a.
Quantitative Polymerase Chain Reaction Analysis.
Total RNA was transcribed to single-stranded cDNA using the High Capacity cDNA Reverse Transcription Kit 1001073 (Applied Biosystems, Foster City, CA). The resulting cDNA products were amplified by polymerase chain reaction (PCR) using Power SYBR Green PCR Master Mix in a 7900HT Fast Real-Time PCR System (Applied Biosystems). The primers for all real-time PCR reactions were synthesized by Integrated DNA Technologies (Coralville, IA).
cDNA Library Preparation and RNA Sequencing.
The cDNA library preparation and sequencing of the transcriptome were performed with the help of the Kansas University Medical Center Genome Sequencing Facility as described previously (Cui et al., 2012). The cDNA libraries from total RNA samples (n = 3 per group) were prepared using an Illumina TruSeq RNA sample prep kit (Illumina, San Diego, CA). The average size of the cDNAs was approximately 160 bp (excluding the adapters). The cDNA libraries were validated for RNA integrity and quantity using an Agilent 2100 Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA) before sequencing. The cDNA libraries were clustered onto a TruSeq paired-end flow cell and sequenced (2 × 50 bp) using a TruSeq SBS kit (Illumina) on the Illumina HiSeq2000 sequencer (Genome Sequencing Facility, Kansas University Medical Center), with a multiplex strategy of four samples per lane.
RNA-Seq Data Analysis.
After the sequencing platform generated the sequencing images, the pixel-level raw data collection, image analysis, and base calling were performed by Illumina’s real-time analysis software on a personal computer attached to a HiSeq2000 sequencer. The base call files (*.BCL) were converted to qseq files by Illumina’s BCL converter, and the qseq files were subsequently converted to FASTQ files for downstream analysis. The RNA-Seq reads from the FASTQ files were mapped to the mouse mm10 reference genome, and the splice junctions were identified by TopHat. The output files in the binary sequence alignment format were analyzed by Cufflinks to estimate the transcript abundance and differential expression (Cuffdiff, False discovery rate (Benjamini Hochberg) < 0.05). The mRNA abundance was expressed in fragments per kilobase of exon per million reads mapped.
Statistical Analysis.
Data are presented as mean ± S.E.M. Asterisks (*) represent significant differences between CV and GF mice determined by Student’s t test (P < 0.05).
Results
General Characterization of GF Mice
The body weights of adult (3 months old) GF male mice were comparable to the body weights of adult CV mice. However, adult GF female mice were 18% heavier than CV mice at the same age (Fig. 1). Both male and female GF mice had similar liver weights as CV mice (Fig. 1). The liver weight to body weight percentage was similar in male CV and GF mice, but was lower in female GF mice compared with CV mice (5.1% in CV and 4.6% in GF female mice) (data not shown).
Fig. 1.
Body and liver weight of GF mice. Data are presented as mean ± S.E.M., n = 10 per group. Asterisks (*) represent a statistically significant difference between CV and GF mice (P < 0.05) by Student’s t test. Dark blue and light blue bars represent CV and GF male mice, respectively, and red and pink bars represent CV and GF female mice, respectively. F, female; M, male.
BAs in Serum, Liver, Bile, and Ileum of CV and GF Mice
Serum.
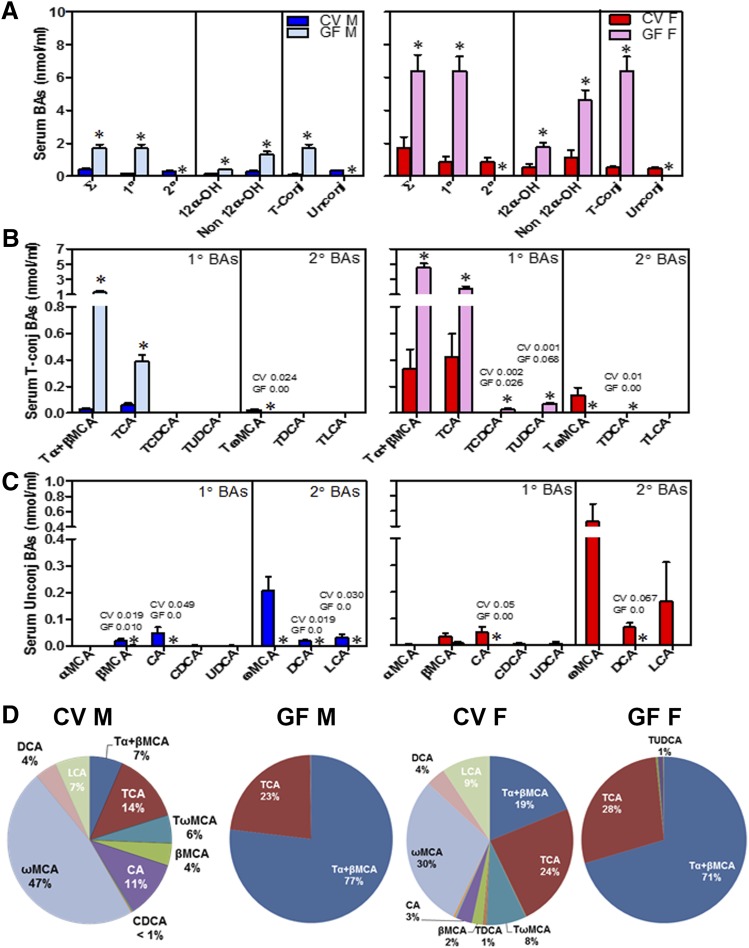
GF male and female mice had a 4-fold increase in total BAs in the serum compared with CV mice (Fig. 2A). The increase in total BAs in the serum of GF mice was due to an increase in primary BAs (11-fold increase in male GF mice and 7-fold increase in female GF mice). Secondary BAs were not detected in GF mice, confirming that intestinal bacteria were absent in these mice. Male and female GF mice had a 3-fold increase in total 12α-OH BAs (CA, DCA, and their taurine conjugates; Fig. 2A) and a 4-fold increase in non–12α-OH BAs (BAs other than CA, DCA, and their taurine conjugates; Fig. 2A) compared with their respective CV mice. Taurine-conjugated BAs increased 15- and 12-fold in GF male and female mice, respectively, compared with CV mice, whereas unconjugated BAs were almost absent in male and female GF mice. This increase in taurine-conjugated BAs was anticipated because of the absence of intestinal bacteria, which are known to deconjugate taurine-conjugated BAs (Fig. 2A).
Fig. 2.
Concentrations of BAs in the serum of CV and GF mice. (A) Concentrations of total BAs (∑), total 1° (primary) and 2° (secondary) BAs, total 12α-OH BAs [CA, TCA, DCA, and taurine-conjugated deoxycholic acid (TDCA)] and non–12α-OH BAs (all other BAs), and total taurine-conjugated (T-Conj) and unconjugated (Unconj) BAs. (B) Concentrations of individual T-conj BAs. (C) Concentrations of individual Unconj BAs. Data are presented as mean ± S.E.M., n = 7–10 mice/group. (D) Proportion of individual BAs in the serum of CV and GF mice. Asterisks (*) represent statistically significant differences between CV and GF mice (P < 0.05) by Student’s t test. Dark blue and light blue bars represent CV and GF male mice, respectively, and red and pink bars represent CV and GF female mice, respectively. F, female; M, male.
Among individual BAs, taurine-conjugated α+β muricholic acid (Tα+βMCA ) Tα+βMCA increased 45- and 14-fold, respectively, in male and female GF mice compared with CV mice (Fig. 2B). Concentrations of TCA were also 7- and 4-fold higher in the serum of male and female GF mice compared with CV mice (Fig. 2B). Female GF mice also had a 13-fold increased concentration of TCDCA and a 68-fold increased concentration of TUDCA compared with CV mice. The concentrations of all unconjugated BAs were lower in the serum of both male and female GF mice compared with CV mice (Fig. 2C).
The proportions of individual BAs in the serum of mice were altered markedly in the GF condition (Fig. 2D). Tα+βMCA made up 7% of BAs in the serum of CV male mice but constituted 77% of BAs in the serum of GF male mice. Similarly, in females, Tα+β MCAs made up 19% of BAs in the serum in CV mice but 71% of BAs in the serum of GF mice.
Liver.
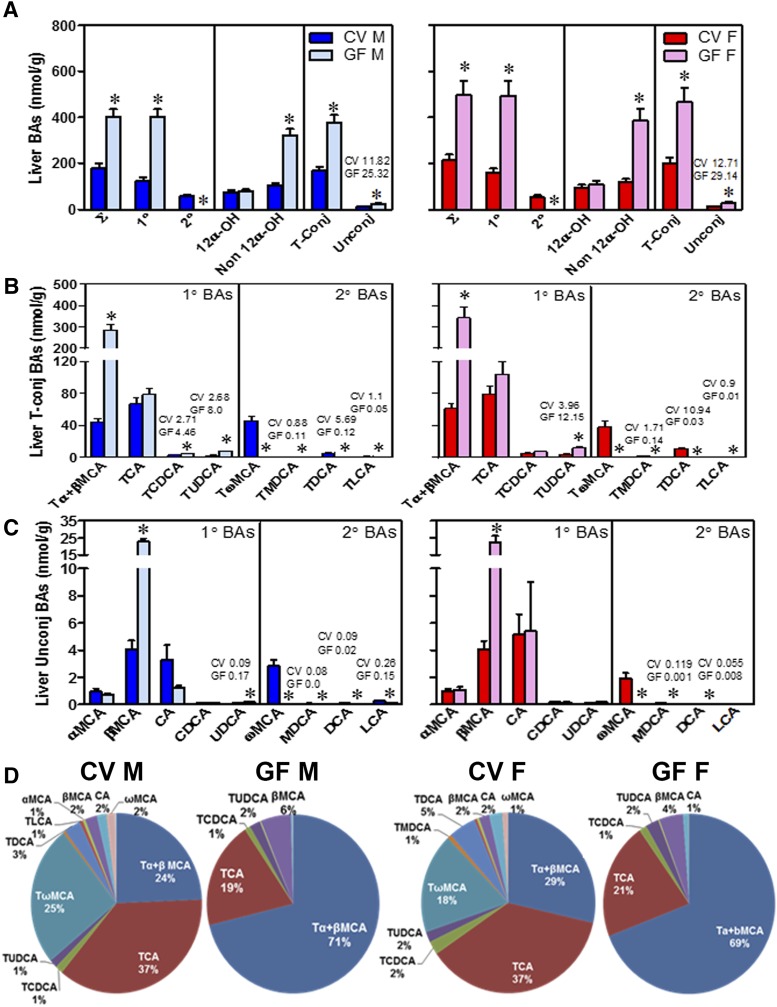
The BA concentrations in the liver are known to increase in pathologic states, such as cholestasis. Interestingly, the absence of intestinal bacteria resulted in a 2-fold increase in total BAs in the livers of male and female mice (Fig. 3A), with no liver damage (data not shown). The increase in total BAs was due to a 3-fold increase in primary BAs and a 3-fold increase in non–12α-OH BAs in both male and female GF mice compared with CV mice. As expected, there was a decrease in secondary BAs in both male and female GF mice. Both taurine-conjugated and unconjugated BAs were 2-fold higher in the livers of GF male and female mice compared with their respective CV mice (Fig. 3A).
Fig. 3.
Concentrations of BAs in the livers of CV and GF mice. (A) Concentrations of total BAs (∑), total 1° (primary) and 2° (secondary) BAs, total 12α-OH BAs [CA, TCA, DCA, and taurine-conjugated deoxycholic acid (TDCA)] and non–12α-OH BAs (all other BAs), and total taurine-conjugated (T-Conj) and unconjugated (Unconj) BAs. (B) Concentrations of individual T-conj BAs. (C) Concentrations of individual Unconj BAs. Data are presented as mean ± S.E.M., n = 7–10 mice/group. (D) Proportion of individual BAs in the livers of CV and GF mice. Asterisks (*) represent statistically significant differences between CV and GF mice (P < 0.05) by Student’s t test. Dark blue and light blue bars represent CV and GF male mice, respectively, and red and pink bars represent CV and GF female mice, respectively. F, female; M, male.
Among conjugated BAs (Fig. 3B), Tα+βMCA increased 6-fold in GF male and female mice compared with CV mice (Fig. 3B). In addition, the livers of GF mice have a 1.7- and 1.4-fold increased TCDCA (although not significant) compared with CV male and female mice, respectively. In contrast, the concentrations of TCA, the other major primary BA in mice, are similar in the livers of GF and CV mice (Fig. 3B).
Interestingly, the concentration of TUDCA, a known therapeutic BA, was increased 3-fold in the livers of both male and female GF mice compared with CV mice (Fig. 3B). This suggests that in mice, TUDCA can be synthesized by the liver and therefore is a primary BA, which is in agreement with a recent report (Sayin et al., 2013). Incubating CDCA with hepatic microsomal protein from GF mice resulted in the appearance of a detectable peak, with the same retention time and mass as the UDCA standard (Supplemental Fig. 2).
Among unconjugated BAs (Fig. 3C), βMCA was increased 5-fold in the livers of male and female GF mice compared with CV mice. In contrast, other unconjugated primary BAs (CA, CDCA, and αMCA) are at similar concentrations in the livers of CV and GF mice.
The proportions of individual BAs were altered in the livers of GF mice (Fig. 3D). Tα+βMCA, which was the predominant BA in the livers of GF mice, was 24% of the total BAs in CV male mice but 71% in GF male mice. Similarly Tα+βMCA were 29% of the total BAs in female CV mice but 69% in female GF mice.
Bile.
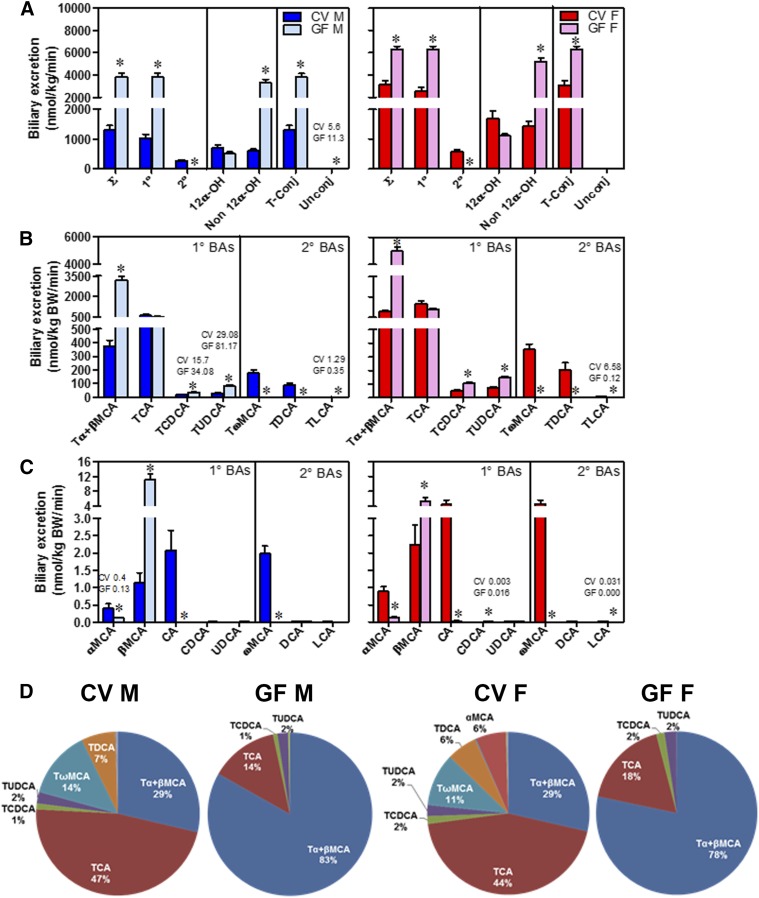
Biliary excretion of total BAs was 3-fold higher in male GF mice and 2-fold higher in female GF mice compared with their respective CV mice (Fig. 4A). The increase in total BAs in the bile of GF mice was due to a 3-fold increase in primary BAs in male GF mice and a 2-fold increase in female GF mice, respectively. Secondary BAs were almost absent in the bile of GF mice. Total non–12α-OH BAs increased 5-fold in male GF mice and 3-fold in female GF mice compared with CV mice. Taurine-conjugated BAs were increased 3- and 2-fold in the bile of male and female GF mice compared with their respective CV mice (Fig. 4A).
Fig. 4.
Biliary excretion of BAs in CV and GF mice. BAs in bile are expressed as nmol/kg body weight per min. (A) Concentrations of total BAs (∑), total 1° (primary) and 2° (secondary) BAs, total 12α-OH BAs [CA, TCA, DCA, and taurine-conjugated deoxycholic acid (TDCA)] and non–12α-OH BAs (all other BAs), and total taurine-conjugated (T-Conj) and unconjugated (Unconj) BAs. (B) Concentrations of individual T-conj BAs. (C) Concentrations of individual Unconj BAs. Bile was collected for 40 minutes from each mouse. Data are presented as mean ± S.E.M., n = 3–6 mice/group. (D) Proportion of individual BAs in the bile of CV and GF mice. Asterisks (*) represent statistically significant differences between CV and GF mice (P < 0.05) by Student’s t test. Dark blue and light blue bars represent CV and GF male mice, respectively, and red and pink bars represent CV and GF female mice, respectively. F, female; M, male.
Tα+βMCA increased 8-fold in male GF mice and 5-fold in female GF mice, although the other major primary BA, TCA, was similar in the bile of GF and CV mice. The concentration of TUDCA increased 2-fold in the bile of male and female GF mice compared with CV mice (Fig. 4B). βMCA increased 9- and 2-fold in the bile of male and female GF mice, respectively, compared with their respective CV mice. CA was minimal in the bile of GF mice (Fig. 4C)
Tα+βMCA constituted 29% of total BAs in the bile of CV male mice but increased to 83% of total BAs in GF male mice (Fig. 4D). Similarly, in females, Tα+βMCA constituted 29% of total BAs in CV mice but increased to 78% of total BAs in GF mice. In contrast, TCA was 47% of total BAs in the bile of male CV mice but decreased to 14% in male GF mice. Similarly, in female mice, TCA was 44% of total BAs in the bile of CV mice but decreased to 18% in GF mice (Fig. 4D).
Ileal Tissue.
Total BA concentrations in ileal tissue were 3-fold higher in male GF mice and 2-fold higher in female GF mice compared with their respective CV controls (Fig. 5A). This is probably due to either increased uptake of BAs from the intestinal lumen or decreased efflux transport across the basolateral membrane. Primary BAs were increased 4- and 2-fold in the ileal tissue of male and female GF mice, respectively, compared with CV mice. Further, non–12α-OH BAs were increased 6- and 4-fold in the ileal tissue of male and female GF mice, respectively, compared with CV mice. Taurine-conjugated BAs were increased 5- and 3-fold in the ileal tissue of GF male and female mice compared with CV mice. Secondary and unconjugated BA concentrations were almost nonexistent in the ileal tissue of GF mice compared with CV mice (Fig. 5A).
Fig. 5.
Concentrations of BAs in the ileal tissue of CV and GF mice. (A) Concentrations of total BAs (∑), total 1° (primary) and 2° (secondary) BAs, total 12α-OH BAs [CA, TCA, DCA, and taurine-conjugated deoxycholic acid (TDCA)] and non–12α-OH BAs (all other BAs), and total taurine-conjugated (T-Conj) and unconjugated (Unconj) BAs. (B) Concentrations of individual T-conj BAs. (C) Concentrations of individual Unconj BAs. Ileal tissue refers to the last one-third of the small intestine. Data are presented as mean ± S.E.M., n = 7–10 mice/group. (D) Proportion of individual BAs in the ileal tissue of CV and GF mice. Asterisks (*) represent statistically significant differences between CV and GF mice (P < 0.05) by Student’s t test. Dark blue and light blue bars represent CV and GF male mice, respectively, and red and pink bars represent CV and GF female mice, respectively. F, female; M, male.
Concentrations of Tα+βMCA increased in the ileal tissue of male GF mice (10-fold) and female GF mice (5-fold) compared with CV mice. TCA concentration increased 3-fold in the ileal tissue of male GF mice and 1.5-fold in the ileal tissue of female GF mice compared with CV mice (Fig. 5B). TUDCA concentration in the ileal tissue was increased 6- and 3-fold in male and female GF mice, respectively, compared with their respective CV mice. In contrast, primary unconjugated BAs were almost absent in the ileal tissue of both male and female GF mice (Fig. 5C).
The proportions of individual BAs in the ileal tissues were altered in GF mice compared with CV mice (Fig. 5D), but were similar to changes in other tissues of GF mice. Tα+βMCA constituted 28% of the total BAs in the ileal tissue of male CV mice but increased to 78% in male GF mice. Similarly, in females, Tα+βMCA constituted 38% of the total BAs in the ileal tissue of CV mice but increased to 75% in GF mice.
Fecal Excretion of BAs in GF Mice
Male CV and GF mice were housed in individual metabolic cages, and feces were collected over a 24-hour period. Due to space restrictions inside the GF isolator, feces were collected only from male GF mice, and we expect the trend of changes in BAs to be similar in male and female GF mice. Generally, more than 90% of BAs in the intestinal lumen are reabsorbed and the remaining BAs are excreted in the feces. There was a 63% decrease in the amount of total BAs in the feces of GF mice compared with CV mice, (Fig. 6A). This suggests an increased intestinal uptake of BAs in the absence of intestinal bacteria in GF mice. Total fecal excretion of 12α-OH BAs in GF mice decreased 75% and non–12α-OH BAs decreased 57% compared with CV mice. Furthermore, the absence of intestinal bacteria that deconjugate BAs leads to a 4-fold increase in total conjugated BAs and a 99% decrease in total unconjugated BAs in the feces of GF mice (Fig. 6A).
Fig. 6.
Concentrations of BAs in the feces of CV and GF mice. BAs are expressed as nmols/gram of feces. (A) Concentrations of total BAs (∑), total 1° (primary) and 2° (secondary) BAs, total 12α-OH BAs [CA, TCA, DCA, and taurine-conjugated deoxycholic acid (TDCA)] and non–12α-OH BAs (all other BAs), and taurine-conjugated (total T-Conj) and unconjugated (Unconj) BAs. (B) Concentrations of individual T-conj BAs. (C) Concentrations of individual Unconj BAs. Mice were housed individually in metabolic chambers, and feces were collected over 24 hours. Data are presented as mean ± S.E.M., n = 4 mice/group. (D) Proportion of individual BAs in the feces of CV and GF mice. Asterisks (*) represent statistically significant differences between CV and GF mice (P < 0.05) by Student’s t test. Dark blue and light blue bars represent CV and GF male mice. M, male.
Fecal excretion of primary taurine-conjugated BAs increased in GF mice compared with CV mice, more specifically, Tα+βMCA increased 6-fold, TCA increased 4-fold, TCDCA increased 2-fold, and TUDCA increased 4-fold (Fig. 6B). Unconjugated BAs were essentially absent in the feces of GF mice compared with CV mice (Fig. 6C).
The proportions of the various BAs in the feces of GF mice were very different than those in CV mice. Tα+βMCA were 5% of the total BAs in the feces of CV mice but increased to 74% of the total BAs in the feces of GF mice (Fig. 6D).
Gene Expression in the Liver
BA Enzymes.
Major enzymes involved in BA biosynthesis include cytochrome P450 (Cyp) 7a1, Cyp7b1, Cyp8b1, Cyp27a1, and the BA conjugation enzymes bile acid–coenzyme A ligase (BAL) and bile acid–coenzyme A:amino acid N-acyltransferase (BAT). Surprisingly, the mRNA of Cyp7a1, the rate-limiting enzyme in BA synthesis, was similar in the livers of CV and GF male mice (Fig. 7A). However, in female GF mice, Cyp7a1 mRNA decreased to about 50% of that in CV mice (Fig. 7A). The mRNA of Cyp27a1 was similar in the livers of male GF and CV mice, but decreased 15% in female GF mice (Fig. 7A). The mRNA of Cyp7b1 was decreased by 30% in the livers of male GF mice and 52% in the livers of female GF mice, respectively, compared with CV mice (Fig. 7A). This may be an attempt to decrease the alternate pathway of BA synthesis to ultimately decrease concentrations of BAs in the livers of GF mice.
Fig. 7.
Gene expression in livers of CV and GF mice. (A) mRNA expression of genes involved in BA synthesis; (B) mRNA expression of genes involved in BA transport; and (C) mRNA expression of genes involved in FXR feedback regulation in the livers of male and female CV and GF mice. mRNA was quantified by beadplex assay; n = 6 per group. Data are presented as mean ± S.E.M. Asterisks (*) represent statistically significant differences between CV and GF mice (P < 0.05) by Student’s t test. Dark blue and light blue bars represent CV and GF male mice, respectively. Red and pink bars represent CV and GF females, respectively. F, female; M, male.
Cyp8b1, the sterol 12α-hydroxylase, is essential for the synthesis of CA. The mRNA of Cyp8b1 was decreased 32% in the livers of male GF mice and 73% in female GF mice compared with their CV controls (Fig. 7A). This correlates with the decrease in concentration of 12α-OH BAs (TCA and CA) and the decrease in the proportion of TCA to Tα+βMCA in the livers of GF mice (Fig. 3, A, B, and D).
Although taurine-conjugated BAs increased in the tissues of GF mice, the mRNA of enzymes involved in the conjugation of BAs, namely, BAL and BAT, were similar in the livers of male GF and CV mice. In the livers of female GF mice, BAL mRNA was decreased by 15% and the mRNA of BAT was decreased by 19% (Fig. 7A).
BA Transporters.
The two BA uptake transporters in the livers are sodium taurocholate cotransporting polypeptide (Ntcp) and organic anion transporting polypeptide (Oatp) 1b2, where Ntcp transports conjugated BAs and Oatp1b2 transports unconjugated BAs. The GF condition increased the mRNA of Ntcp by 42% and Oatp1b2 by 48% in male mice compared with CV male mice (Fig. 7B). This increase in the mRNA of hepatic BA uptake transporters may enable the liver to remove more BAs from the circulation, given that total BAs are higher in the serum of both male and female GF mice. However, in female GF mice, the mRNA of Ntcp and Oatp1b2 is similar to that in female CV mice (Fig. 7B). The mRNA of the major hepatic BA efflux transporter bile salt export pump (Bsep) decreased somewhat (11%) in male GF mice but did not change in female GF mice compared with CV mice (Fig. 7B). The gene expression of multidrug resistance–associated protein (Mrp) 2 increased 25% in the livers of male GF mice but did not change in female GF mice compared with their respective controls (Fig. 7B).
BA Feedback Regulation.
The mRNA of FXR and liver receptor homolog-1 increased minimally (16 and 13%, respectively) in the livers of male GF mice compared with their CV controls (Fig. 7C). However, these small increases may not be biologically significant as there was no subsequent increase in small heterodimer protein (SHP) mRNA or decrease in Cyp7a1 mRNA in the livers of male GF mice compared with CV mice (Fig. 7A). The mRNAs of other important genes involved in BA feedback regulation through FXR activation were similarly expressed in the livers of GF and CV mice (Fig. 7C).
Gene Expression in the Ileum
Transporters.
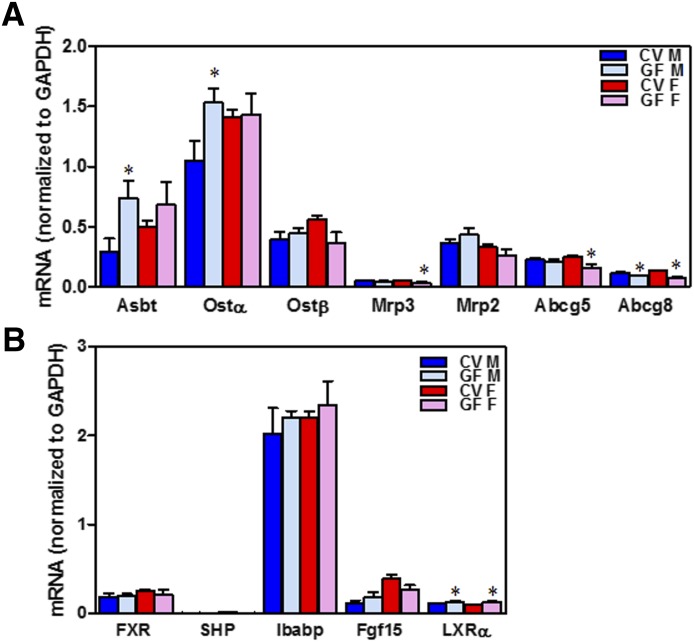
Apical sodium dependent bile acid transporter (Asbt) is the only known apical BA uptake transporter in the ileum. Asbt mRNA increased 154% in the ileum of male GF mice compared with CV mice (Fig. 8A). This correlates with increased total BA concentrations in the ileal tissue and decreased total BA content in the feces observed in GF mice compared with CV mice. However, there was no difference in the expression of Asbt in female CV and GF mice (Fig. 8A). The mRNA of Ostα increased 46% in the ileal tissue of male GF mice compared with CV mice, but there was no change in Ostα mRNA in female GF mice compared with CV mice. The mRNA of Mrp3 was similar in the ileal tissue of male GF and CV mice but decreased minimally in the ileal tissue of female GF mice compared with CV mice. ATP-binding cassette (Abc) subfamily G member 5 mRNA remained similar in male GF and CV mice, but decreased 35% in the ileum of female GF mice compared with female CV mice. However, the mRNA of Abc subfamily G member 8, which is the heterodimer partner of Abc subfamily G member 5, decreased 20% in male GF mice and 40% in female GF mice. The mRNA of the organic solute transporter (Ost) β and Mrp2 were similar in the ileal tissue of male and female GF mice compared with their respective controls (Fig. 8A).
Fig. 8.
Gene expression in ileum of CV and GF mice. (A) mRNA expression of BA transporters in the ileal tissue of male and female CV and GF mice. (B) mRNA expression of genes involved in BA signaling in the ileum of male and female CV and GF mice. mRNA was quantified by beadplex assay; n = 6 per group. Data are presented as mean ± S.E.M. Asterisks (*) represent statistically significant differences between CV and GF mice (P < 0.05) by Student’s t test. Dark blue and light blue bars represent CV and GF male mice, respectively. Red and pink bars represent CV and GF females, respectively. F, female; M, male.
Although the mRNAs of genes involved in BA-FXR signaling in the ileal tissue were similar in GF and CV mice (Fig. 8B), the mRNA of the cholesterol sensor Liver X receptor alpha (LXRα) was minimally induced in both male and female GF mice compared with CV mice (Fig. 8B).
Targets of TGR5 Signaling in CV and GF Mice
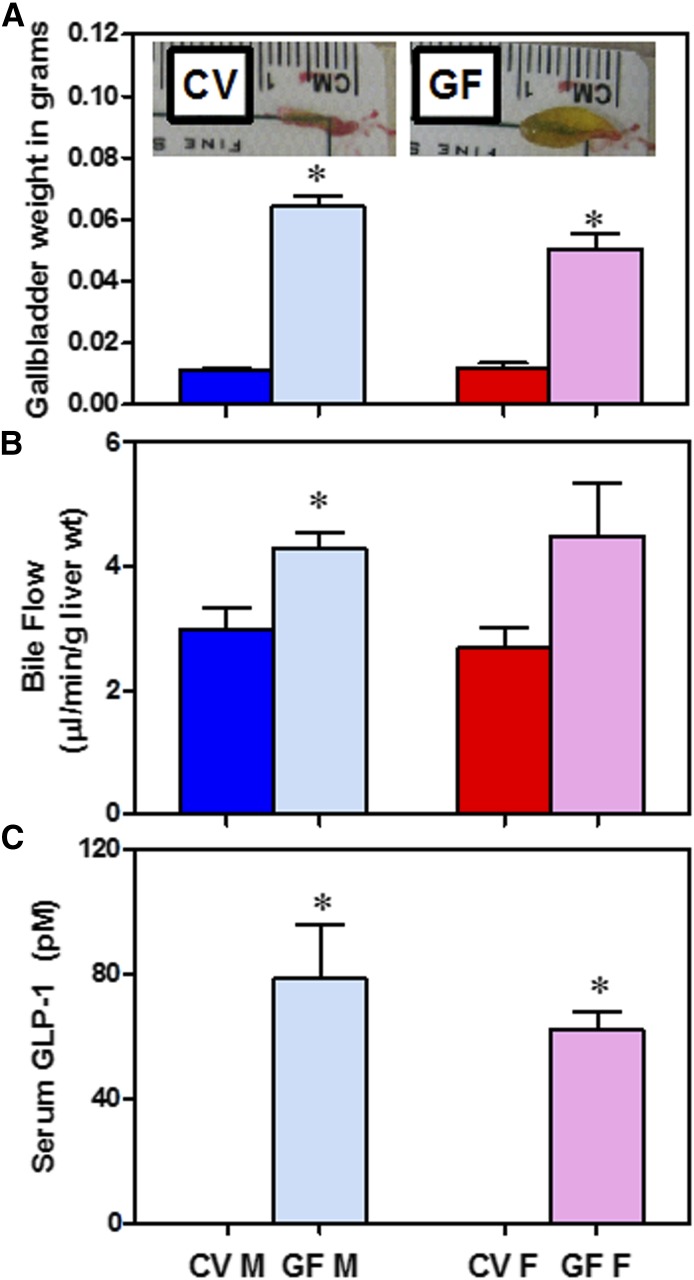
The major effects of TGR5 activation include increased bile flow and gallbladder size (Li et al., 2011) as well as increased GLP-1 secretion from the ileum (Katsuma et al., 2005). The gallbladders in GF mice were approximately 5-fold larger than those in CV mice (Fig. 9A). In addition, bile flow was 44% higher in GF male mice compared with their CV controls (Fig. 9B). GLP-1 was not detectable in the serum of CV mice, but was 78 and 62 ρM in male and female GF mice, respectively (Fig. 9C).
Fig. 9.
Targets of TGR5 signaling. (A) Gallbladder weights of CV and GF mice and (inset) representative pictures. (B) Bile flow in CV and GF mice. (C) Serum GLP-1 quantification by enzyme-linked immunosorbent assay. Data are presented as mean ± S.E.M.; n = 3–6 per group. Asterisks (*) represent statistically significant differences between CV and GF mice (P < 0.05) by Student’s t test. Dark blue and light blue bars represent CV and GF male mice, respectively. Red and pink bars represent CV and GF females, respectively. F, female; M, male.
Gene Expression in Various Parts of the Intestine
Because there was a decrease in fecal excretion of BAs in the feces of GF mice but no consistent increase in the BA transporters in the ileum, the expression of BA transporters in various sections of the intestine of male CV and GF mice were examined by RNA-Seq. The mRNA of the BA uptake transporter Asbt decreased 93% in the jejunum, but increased 69% in the ileum and 96% in the colon of GF mice compared with CV mice (Fig. 10). The mRNA of the BA efflux transporters Ostα and Ostβ increased 94 and 67% in the colon of GF mice compared with CV mice (Fig. 10). The mRNA of FXR increased in the large intestine of GF mice compared with CV mice (Supplemental Fig. 3).
Fig. 10.
Gene expression of BA-related genes across the intestinal sections. Total RNA was isolated from livers of adult male CV and GF C57BL/6 mice (n = 3 per group). The mRNA quantified by RNA-Seq as described in Materials and Methods. Asterisk (*) indicates differential expression determined using Cuffdiff (FDR-BH < 0.05). Dark blue and light blue bars represent CV and GF male mice, respectively. L.Int, large intestine (colon); M, male.
UDCA Synthesis by Enzymes in Mouse Hepatic Microsomes
A striking finding of this study was that GF mice had elevated concentrations of TUDCA. TUDCA in serum was not detectable in male CV mice, but could be quantified in GF mice (Fig. 2B). Compared with CV mice, GF mice had a 3-fold increase in TUDCA in the liver, a 2-fold increase in bile (Fig. 3B; Fig. 4B), a 6-fold increase in ileal tissue, and a 4-fold increase in feces (Fig. 5B; Fig. 6B). Using a traditional biotransformation assay, we found that incubating CDCA with hepatic microsomal protein from GF mice resulted in the appearance of a detectable peak, with the same retention time and mass as the UDCA standard (Supplemental Fig. 2). No UDCA peak was detected when the assay mixture did not contain CDCA or if CA was used instead of CDCA (data not shown), further suggesting that UDCA is a primary BA.
Discussion
The BA profiles of GF mice differ markedly from those of CV mice. In the absence of intestinal bacteria, GF mice have increased conjugated BAs and decreased secondary BAs. GF mice only have a few types of BAs (primary BAs and mainly taurine-conjugated BAs), which indicates that intestinal bacteria play a major role in the diversity of BAs. Somewhat surprisingly, there was an increase in UDCA.
UDCA is an oral drug used to dissolve cholesterol gallstones and treat liver diseases. In the present study, UDCA, which was thought to be a secondary BA made by intestinal bacteria, increased in the tissues of GF mice, similar to a recent report (Sayin et al., 2013). Results from previous studies in our laboratory demonstrate that feeding mice CDCA increases the hepatic concentration of UDCA and TUDCA, suggesting that UDCA is synthesized from CDCA (Zhang and Klaassen, 2010; Song et al., 2011). Indeed, in vitro experiments confirmed that UDCA can be synthesized from CDCA by enzymes in mouse liver microsomes (Supplemental Fig. 2).
The amount of BAs is much higher in GF mice than in CV mice. Total BAs increased 4-fold in the serum (Fig. 2A), 2-fold in the liver (Fig. 3A), 3-fold in the bile (Fig. 4A), and 3-fold in the ileum (Fig. 5A) but decreased 63% in the feces (Fig. 6A) of male GF mice compared with CV mice. These changes are likely due to an increase in the reabsorption of BAs. The ileal BA uptake transporter (Asbt) is thought to be responsible for the reabsorption of most of the BAs from the intestine (Dawson et al., 2003). It is known that antibiotics decrease the number of intestinal bacteria, increase intestinal BA absorption (Miyata et al., 2015), decrease fecal BA excretion, and increase hepatic BA concentrations (Hu et al., 2014; Zhang et al., 2014), with an increase in ileal Asbt expression. On the contrary, Asbt-null mice have limited enterohepatic circulation of BAs, increased fecal BA excretion, and decreased BA pool size (Dawson et al., 2003), which is just the opposite of what is observed in GF mice. GF mice also have decreased gut motility (Kashyap et al., 2013), and thus BAs remain in the intestine longer, providing more time for BAs to enter the enterohepatic circulation. This would also explain the increased half-life of CA in GF rats reported by others (Gustafsson et al., 1957). Although BAs are thought to be primarily reabsorbed by transporters in the ileum, surprisingly, data in the present manuscript (Fig. 10) indicate that the mRNA of the BA transporters Asbt, Ostα, and Ostβ increase mainly in the large intestine of GF mice (Fig. 10). The mRNA of Asbt increases by 96%, Ostα increases by 94%, and Ostβ increases by 67% compared with CV mice (Fig. 10). This suggests that in GF mice, BAs are taken up by transporters in the colon in addition to the small intestine to enter the enterohepatic circulation. Thus, the increase in BA transporters in the large intestine appears to be responsible for the increase in BAs in the tissues and the decrease of BAs in the feces.
The GF condition, as expected, led to an increase in taurine-conjugated BAs: 15-fold in the serum, 2-fold in the liver, 3-fold in the bile, 5-fold in the ileum, and 4-fold in the feces of male GF mice compared with CV mice. Taurine conjugation lowers the pKa of BAs and increases their water solubility. In contrast, unconjugated BAs were decreased in all tissues, except in the liver, where they were 2-fold higher than in CV mice.
BA composition is similar in male and female GF mice, but differs markedly from their respective CV mice. For example, male GF mice have increased total non–12α-OH BAs (MCAs, UDCA, and their taurine conjugates): 4-fold in the serum, 3-fold in the liver, 5-fold in bile, and 6-fold in the ileum. In contrast, there are similar concentrations of 12α-OH BAs (CA, DCA, and their taurine conjugates) in each tissue, except the serum, where they were 3-fold higher in male GF mice than in CV mice.
Tα+βMCA become the major BAs in GF mice as they increase 6-fold in the livers of male and female GF mice; 8-fold in the bile of male GF mice and 5-fold in the bile of female GF mice; 10-fold in the ileum of male GF mice and 5-fold in the ileum of female GF mice; and 6-fold in the feces of male GF mice compared with CV mice. Similarly, TUDCA increased 3-fold in the livers of male and female GF mice; 2-fold in the bile of male and female GF mice; 6-fold in the ileum of male GF mice and 3-fold in the ileum of female GF mice; and 4-fold in the feces of GF male mice compared with CV mice. Increased concentrations of MCA and UDCA make the GF BA pool more hydrophilic, which can protect against cell damage induced by hydrophobic bile acids (Heuman et al., 1991; Rodrigues et al., 1998).
Under normal conditions, FXR regulates hepatic BA synthesis through BA-mediated feedback mechanisms. Increased BAs in the liver activate FXR, which acts via SHP–liver receptor homolog-1 to decrease transcription of Cyp7a1. In addition, increased BAs in the ileum activate FXR, induce the secretion of Fgf15 into the portal circulation, which travels to the liver and via Fibroblast growth factor 4 (Fgfr4)/β-klotho signaling, and down-regulate the transcription of Cyp7a1 (Chiang, 2004; Chiang et al., 2000; Inagaki et al., 2005; Kim et al., 2007). With the increase in total BAs in the liver and intestine of GF mice, one would expect an increase in FXR signaling in the liver and intestine and subsequent down-regulation of Cyp7a1 in the liver. However, the mRNA of Cyp7a1 was not lower in the livers of GF mice, and there was neither an increase in SHP or Fgf15 mRNA in the ileum of GF mice compared with CV mice. TβMCA is an FXR antagonist that decreases the feedback secretion of intestinal fibroblast growth factor 15 (Fgf15) and thus increases hepatic Cyp7a1 mRNA (Li et al., 2013; Sayin et al., 2013; Hu et al., 2014). In the present study, there was an increase in TβMCA (FXR antagonist) but also an increase in other BAs, which are likely FXR agonists in vivo, and thus the mRNA of FXR-target genes in the intestine (Fgf15, SHP, or ileal bile acid–binding protein) and liver (SHP) are similar in GF and CV mice (Fig. 7, A and C; Fig. 8B). As a result, there is no alteration in Cyp7a1.
TGR5 activation by BAs in the intestine induces the secretion of GLP-1, which in turn stimulates insulin secretion and improves insulin sensitivity (Thomas et al., 2009). TGR5 receptor agonists and long-acting GLP-1 receptor agonists improve glucose tolerance and are promising drug candidates to treat metabolic disorders, such as type 2 diabetes and obesity (Nauck, 2011; Keitel and Haussinger, 2012). Although there are minimal changes in FXR signaling in either the liver or intestine of GF mice (Fig. 7, A and C; Fig. 8B), there was an increase in TGR5 signaling. GF mice displayed an increased gallbladder size and increased GLP-1 in the serum (Fig. 9), both of which are endpoints of TGR5 activation (Katsuma et al., 2005; Thomas et al., 2009; Li et al., 2011; Harach et al., 2012). It is interesting to note that the BA composition in TGR5-null mice is hydrophobic (Pean et al., 2013), which is in contrast to the phenotype of GF mice, in which TGR5 is activated.
Increasing GLP-1 is a therapeutic strategy for treating diabetes and obesity. Similar to the GF condition, reducing the number of bacteria in the intestine by antibiotics also alters the BA composition (Zhang et al., 2014) and increases GLP-1 (Hwang et al., 2015). Anionic resins that concentrate BAs in the intestinal lumen also increase GLP-1 secretion via TGR5 (Harach et al., 2012). Further, rectal administration of TCA in humans increases GLP-1 levels in plasma (Wu et al., 2013). The altered BA profile in GF mice might point to a BA that is a potent TGR5 agonist, and thus BAs could be used to increase TGR5 signaling and increase serum GLP-1 concentration. A recent study demonstrated that oral administration of the antioxidant tempol to mice decreases Lactobacillus species in the intestinal lumen, leading to accumulation of TβMCA, inhibition of FXR signaling, and resistance to obesity (Li et al., 2013). Although humans do not make TβMCA, appropriate studies might lead to the establishment of TβMCA as a drug.
Apart from BAs, other intestinal bacterial metabolites, such as short-chain fatty acids (SCFAs) also regulate GLP-1 secretion by activating the SCFA receptors, free fatty acid receptor 2 (FFAR 2) and FFAR3 (Tolhurst et al., 2012). Altering intestinal bacterial composition by dietary fiber administration also induces GLP-1 levels (Reimer and McBurney, 1996; Massimino et al., 1998; Tolhurst et al., 2012). Future experiments utilizing TGR5-null mice would help establish the relative importance of BAs (via TGR5) and SCFA (via FFAR2 or FFAR3) in regulating the secretion of GLP-1 from the intestine.
In conclusion, this study describes changes in BA homeostasis in male and female GF mice. The most notable changes in GF mice are 1) increases in total BAs in the serum, liver, bile, and ileal tissue; 2) a shift in BA composition toward an increase in Tα+βMCA; and 3) a large increase in GLP-1. It appears that these effects are secondary to an increase in BA transporters in the colon.
Supplementary Material
Acknowledgments
The authors thank the National Gnotobiotic Rodent Resource Center at the University of North Carolina for providing the GF C57BL/6J/UNC mice. The authors also thank Dr. Matthew Pratt-Hyatt for his valuable assistance with the ultra-performance liquid chromatography/mass spectrometry and for his help with the microsomal biotransformation assay to study UDCA synthesis; Dr. Julia Yue Cui, Clark Bloomer, and Byunggil Yoo for their technical assistance in RNA-Seq; the members of the Klaassen laboratory for their help in tissue collection; and Dr. Bruno Hagenbuch and Dr. Thomas Pazdernik for careful revision of parts of the manuscript presented in the dissertation.
Abbreviations
- Abc
ATP-binding cassette
- Abcg5/8
ATP-binding cassette sub-family G member 5/8
- Asbt
apical sodium dependent bile acid transporter
- BA
bile acid
- BAAT
Bile acid-CoA:amino acid N-acyltransferase
- BAL
bile acid–coenzyme A ligase
- Bsep
Bile salt export pump
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- CV
conventional
- Cyp
cytochrome P450
- DCA
deoxycholic acid
- FFAR
free fatty acid receptor
- Fgf15
Fibroblast growth factor 15
- Fgfr4
Fibroblast growth factor receptor 4
- Ibabp
Ileal bile acid binding protein
- FXR
farnesoid X receptor
- GF
germ free
- GLP
glucagon-like peptide
- LCA
Lithocholic acid
- L.Int
Large intestine (Colon)
- Lrh-1
Liver receptor homolog-1
- MCA
muricholic acid
- Mrp
multidrug resistance–associated protein
- Ntcp
sodium taurocholate cotransporting polypeptide
- Oatp
organic anion transporting polypeptide
- Ost
organic solute transporter
- PCR
polymerase chain reaction
- SCFA
short-chain fatty acid
- SHP
small heterodimer protein
- TαMCA
Taurine-conjugated α-muricholic acid
- TCA
taurine-conjugated cholic acid
- TCDCA
taurine-conjugated chenodeoxycholic acid
- TDCA
Taurine-conjugated deoxycholic acid
- TGR5
transmembrane G protein–coupled receptor 5
- TβMCA
taurine-conjugated β-muricholic acid
- TωMCA
Taurine-conjugated ω-muricholic acid
- TLCA
Taurine-conjugated lithocholic acid
- TUDCA
taurine-conjugated ursodeoxycholic acid
- UDCA
ursodeoxycholic acid
Authorship Contributions
Participated in research design: Selwyn, Csanaky, Zhang, Klaassen.
Conducted experiments: Selwyn, Csanaky, Zhang, Klaassen.
Performed data analysis: Selwyn, Csanaky, Zhang, Klaassen.
Wrote or contributed to the writing of the manuscript: Selwyn, Csanaky, Zhang, Klaassen.
Footnotes
This research was supported by the National Institutes of Health [Grants ES09649, ES019487, and GM111381].
This research is part of the thesis of Dr. Felcy Pavithra Selwyn titled “Alterations in bile acid homeostasis and drug metabolism in germ-free mice.” A portion of this research has been presented at the following meetings: Selwyn F and Klaassen CD (2012) Characterization of bile acid homeostasis in germ-free mice Selwyn FP, Zhang Y and Klaassen CD. Experimental Biology 2012; 2012 Apr 21–25; San Diego, CA. American Society of Pharmacotherapy and Experimental Therapeutics, Bethesda, MD; (2012) Characterization of bile acid homeostasis in germ-free mice. Bile Acid-Mediated Integration of Metabolism and Liver Disease: A Research Workshop; 2012 Jun 8; Bethesda, MD. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Alnouti Y, Csanaky IL, Klaassen CD. (2008) Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 873:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101:15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. (2007) Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 104:979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, McNulty J, Anderson D, Liu Y, Nystrom C, Bullard S, Collins J, Handlon AL, Klein R, Grimes A, et al. (2010) Cholestyramine reverses hyperglycemia and enhances glucose-stimulated glucagon-like peptide 1 release in Zucker diabetic fatty rats. J Pharmacol Exp Ther 334:164–170. [DOI] [PubMed] [Google Scholar]
- Chiang JY. (2004) Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol 40:539–551. [DOI] [PubMed] [Google Scholar]
- Chiang JY, Kimmel R, Weinberger C, Stroup D. (2000) Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Biol Chem 275:10918–10924. [DOI] [PubMed] [Google Scholar]
- Csanaky IL, Lu H, Zhang Y, Ogura K, Choudhuri S, Klaassen CD. (2011) Organic anion-transporting polypeptide 1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated bile acids: studies in Oatp1b2-null mice. Hepatology 53:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui JY, Gunewardena SS, Yoo B, Liu J, Renaud HJ, Lu H, Zhong XB, Klaassen CD. (2012) RNA-Seq reveals different mRNA abundance of transporters and their alternative transcript isoforms during liver development. Toxicol Sci 127:592–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, Maeda N, Parks JS. (2003) Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem 278:33920–33927. [DOI] [PubMed] [Google Scholar]
- Gustafsson BE, Bergstrom S, Lindstedt S, Norman A. (1957) Turnover and nature of fecal bile acids in germfree and infected rats fed cholic acid-24-14C; bile acids and steroids 41. Proc Soc Exp Biol Med 94:467–471. [DOI] [PubMed] [Google Scholar]
- Harach T, Pols TW, Nomura M, Maida A, Watanabe M, Auwerx J, Schoonjans K. (2012) TGR5 potentiates GLP-1 secretion in response to anionic exchange resins. Sci Rep 2:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuman DM, Mills AS, McCall J, Hylemon PB, Pandak WM, Vlahcevic ZR. (1991) Conjugates of ursodeoxycholate protect against cholestasis and hepatocellular necrosis caused by more hydrophobic bile salts. In vivo studies in the rat. Gastroenterology 100:203–211. [DOI] [PubMed] [Google Scholar]
- Hu X, Bonde Y, Eggertsen G, Rudling M. (2014) Muricholic bile acids are potent regulators of bile acid synthesis via a positive feedback mechanism. J Intern Med 275:27–38. [DOI] [PubMed] [Google Scholar]
- Hwang I, Park YJ, Kim YR, Kim YN, Ka S, Lee HY, Seong JK, Seok YJ, Kim JB. (2015) Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J 29:2397–2411. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, et al. (2005) Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2:217–225. [DOI] [PubMed] [Google Scholar]
- Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. (2011) Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141:1773–1781. [DOI] [PubMed] [Google Scholar]
- Kashyap PC, Marcobal A, Ursell LK, Larauche M, Duboc H, Earle KA, Sonnenburg ED, Ferreyra JA, Higginbottom SK, Million M, et al. (2013) Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 144:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuma S, Hirasawa A, Tsujimoto G. (2005) Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 329:386–390. [DOI] [PubMed] [Google Scholar]
- Keitel V, Häussinger D. (2012) Perspective: TGR5 (Gpbar-1) in liver physiology and disease. Clin Res Hepatol Gastroenterol 36:412–419. [DOI] [PubMed] [Google Scholar]
- Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. (2007) Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res 48:2664–2672. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ikegami H, Fujisawa T, Nojima K, Kawabata Y, Noso S, Babaya N, Itoi-Babaya M, Yamaji K, Hiromine Y, et al. (2007) Prevention and treatment of obesity, insulin resistance, and diabetes by bile acid-binding resin. Diabetes 56:239–247. [DOI] [PubMed] [Google Scholar]
- Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. (2013) Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 4:2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Holmstrom SR, Kir S, Umetani M, Schmidt DR, Kliewer SA, Mangelsdorf DJ. (2011) The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol 25:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Hawkins J, Gåfvels M, Olin M, Lund EG, Andersson U, Schuster G, Björkhem I, Russell DW, Eggertsen G. (2002) Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest 110:1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGeoch C, Morgan ET, Halpert J, Gustafsson JA. (1984) Purification, characterization, and pituitary regulation of the sex-specific cytochrome P-450 15 beta-hydroxylase from liver microsomes of untreated female rats. J Biol Chem 259:15433–15439. [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. (1999) Identification of a nuclear receptor for bile acids. Science 284:1362–1365. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. (2002) Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 298:714–719. [DOI] [PubMed] [Google Scholar]
- Massimino SP, McBurney MI, Field CJ, Thomson AB, Keelan M, Hayek MG, Sunvold GD. (1998) Fermentable dietary fiber increases GLP-1 secretion and improves glucose homeostasis despite increased intestinal glucose transport capacity in healthy dogs. J Nutr 128:1786–1793. [DOI] [PubMed] [Google Scholar]
- Miyata M, Hayashi K, Yamakawa H, Yamazoe Y, Yoshinari K. (2015) Antibacterial drug treatment increases intestinal bile acid absorption via elevated levels of ileal apical sodium-dependent bile acid transporter but not organic solute transporter α protein. Biol Pharm Bull 38:493–496. [DOI] [PubMed] [Google Scholar]
- Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, Dexheimer PJ, Aronow B, Seeley RJ, Kohli R. (2014) Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring) 22:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narushima S, Ito K, Kuruma K, Uchida K. (2000) Composition of cecal bile acids in ex-germfree mice inoculated with human intestinal bacteria. Lipids 35:639–644. [DOI] [PubMed] [Google Scholar]
- Narushima S, Itoha K, Miyamoto Y, Park SH, Nagata K, Kuruma K, Uchida K. (2006) Deoxycholic acid formation in gnotobiotic mice associated with human intestinal bacteria. Lipids 41:835–843. [DOI] [PubMed] [Google Scholar]
- Nauck MA. (2011) Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med 124 (Suppl)S3–S18. [DOI] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, et al. (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284:1365–1368. [DOI] [PubMed] [Google Scholar]
- Péan N, Doignon I, Garcin I, Besnard A, Julien B, Liu B, Branchereau S, Spraul A, Guettier C, Humbert L, et al. (2013) The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology 58:1451–1460. [DOI] [PubMed] [Google Scholar]
- Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M, Raymond F, Mansourian R, Chou CJ. (2010) Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J 24:4948–4959. [DOI] [PubMed] [Google Scholar]
- Reimer RA, McBurney MI. (1996) Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology 137:3948–3956. [DOI] [PubMed] [Google Scholar]
- Ridlon JM, Kang DJ, Hylemon PB. (2006) Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47:241–259. [DOI] [PubMed] [Google Scholar]
- Rodrigues CMP, Fan G, Ma X, Kren BT, Steer CJ. (1998) A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest 101:2790–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, et al. (2014) FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. (2013) Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17:225–235. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Heubi JE, Shah S, Lavine JE, Suskind D, Al-Edreesi M, Potter C, Russell DW, O’Connell NC, Wolfe B, Jha P, Zhang W, Bove KE, Knisely AS, Hofmann AF, Rosenthal P, Bull LN. (2013) Genetic defects in bile acid conjugation cause fat-soluble vitamin deficiency. Gastroenterology 144:945–955.e6. [DOI] [PMC free article] [PubMed]
- Song P, Zhang Y, Klaassen CD. (2011) Dose-response of five bile acids on serum and liver bile acid concentrations and hepatotoxicty in mice. Toxicol Sci 123:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, et al. (2009) TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. (2012) Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chen J, Hollister K, Sowers LC, Forman BM. (1999) Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3:543–553. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al. (2006) Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439:484–489. [DOI] [PubMed] [Google Scholar]
- Wu T, Bound MJ, Standfield SD, Gedulin B, Jones KL, Horowitz M, Rayner CK. (2013) Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes Metab 15:474–477. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Csanaky IL, Lehman-McKeeman LD, Klaassen CD. (2011a) Loss of organic anion transporting polypeptide 1a1 increases deoxycholic acid absorption in mice by increasing intestinal permeability. Toxicol Sci 124:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Klaassen CD. (2010) Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J Lipid Res 51:3230–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Limaye PB, Lehman-McKeeman LD, Klaassen CD. (2012) Dysfunction of organic anion transporting polypeptide 1a1 alters intestinal bacteria and bile acid metabolism in mice. PLoS One 7:e34522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Limaye PB, Renaud HJ, Klaassen CD. (2014) Effect of various antibiotics on modulation of intestinal microbiota and bile acid profile in mice. Toxicol Appl Pharmacol 277:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YK, Guo GL, Klaassen CD. (2011b) Diurnal variations of mouse plasma and hepatic bile acid concentrations as well as expression of biosynthetic enzymes and transporters. PLoS One 6:e16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YK, Yeager RL, Klaassen CD. (2009) Circadian expression profiles of drug-processing genes and transcription factors in mouse liver. Drug Metab Dispos 37:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.