Abstract
The tryptophan microbiota metabolites indole-3-acetate, indole-3-aldehyde, indole, and tryptamine are aryl hydrocarbon receptor (AhR) ligands, and in this study we investigated their AhR agonist and antagonist activities in nontransformed young adult mouse colonocyte (YAMC) cells. Using Cyp1a1 mRNA as an Ah-responsive end point, we observed that the tryptophan metabolites were weak AhR agonists and partial antagonists in YAMC cells, and the pattern of activity was different from that previously observed in CaCo2 colon cancer cells. However, expansion of the end points to other Ah-responsive genes including the Cyp1b1, the AhR repressor (Ahrr), and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-inducible poly(ADP-ribose) polymerase (TiParp) revealed a highly complex pattern of AhR agonist/antagonist activities that were both ligand- and gene-dependent. For example, the magnitude of induction of Cyp1b1 mRNA was similar for TCDD, tryptamine, and indole-3-acetate, whereas lower induction was observed for indole and indole-3-aldehyde was inactive. These results suggest that the tryptophan metabolites identified in microbiota are selective AhR modulators.
Introduction
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that was initially identified as the intracellular protein that bound the environmental toxicant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), related halogenated aromatics, and polynuclear aromatic hydrocarbons (Nebert et al., 1972; Poland et al., 1976; Gu et al., 2000). The role of the AhR in mediating the biochemical and toxic responses induced by TCDD and related compounds has been confirmed in AhR knockout (AhR−/−) mice which are resistant to the effects of TCDD (Nebert et al., 1972; Fernandez-Salguero et al., 1995; Schmidt et al., 1996; Mimura et al., 1997). Ligand-dependent activation of the AhR results in formation of a nuclear complex with the AhR nuclear translocator (Arnt) protein which binds cis-xenobiotic response elements (XREs) in the Cyp1a1 and other target gene promoters (Poland et al., 1976; Hankinson, 1995; Whitlock, 1999). However, several nonclassic pathways have been discovered, and these include AhR interactions with other nuclear partners, binding to nonconsensus cis-promoter elements, and also responses that are associated with the extranuclear AhR (Blankenship and Matsumura, 1997; Kim et al., 2000; Singh et al., 2007; Vogel et al., 2007; Li and Matsumura, 2008; Dong and Matsumura, 2009; Denison et al., 2011; Jackson et al., 2014; Vogel et al., 2014).
Since the initial discovery that the AhR binds toxic polychlorinated and polynuclear aromatic hydrocarbons, it has subsequently been shown that the AhR also binds structurally and functionally diverse ligands, including health-promoting phytochemicals such indole-3-carbinol, flavonoids and extracts from fruits and vegetables, and a growing list of pharmaceuticals including omeprazole and other benzimidazoles (Bjeldanes et al., 1991; Denison et al., 1998; Song et al., 2002; Jeuken et al., 2003; Henry et al., 2006; Hu et al., 2007; Safe et al., 2012). In addition, structurally diverse “endogenous” biochemicals have been identified as AhR ligands, and there is evidence that the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) and kynurenine may function as an endogenous ligand for the AhR (Song et al., 2002; Oberg et al., 2005; Henry et al., 2006; Wincent et al., 2009; Opitz et al., 2011).
The development of AhR−/− and tissue-specific AhR knockout mice has been instrumental in showing that this receptor plays an essential role in various tissues and is a critical regulator of inflammation, autoimmune and immune responses and is a potential drug target for treating multiple diseases including cancer (Kerkvliet, 2009; Stevens et al., 2009; Marshall and Kerkvliet, 2010; Busbee et al., 2013; Safe et al., 2013). For example, there is extensive evidence that the AhR and its agonists including AhR-active cruciferous vegetables play a protective role in mouse models of intestinal cancer and inflammation (Kawajiri et al., 2009; Arsenescu et al., 2011; Benson and Shepherd, 2011; Furumatsu et al., 2011; Kiss et al., 2011; Li et al., 2011; Monteleone et al., 2011; Singh et al., 2011; Lee et al., 2012).
Several studies have reported that the gut microbiota produces metabolites that include AhR-active compounds that could potentially modulate AhR-mediated intestinal resiliency and responses to inflammatory stimuli (Li et al., 2009; Bansal et al., 2010; Zelante et al., 2013; Fukumoto et al., 2014; Venkatesh et al., 2014). Research in our laboratories has previously investigated the AhR activities of the tryptophan metabolites indole, indole-3-acetate, tryptamine, and 3-indoxyl sulfate using CYP1A1 induction as a prototypical AhR-dependent response in human CaCo2 colon cancer cells (Jin et al., 2014). In this report, we determined the AhR activity of tryptophan metabolites in a nontransformed young adult mouse coloncyte (YAMC) cell line (D’Abaco et al., 1996), and there were significant differences between YAMC versus CaCo2 cells with respect to the gene-specific AhR agonist and antagonist activities of tryptophan metabolites.
Materials and Methods
Cell Lines, Antibodies, and Reagents.
The YAMC cell line was initially generated from the Immorto mouse (Whitehead et al., 1993) and has been previously used in our studies (Kolar et al., 2007; Weige et al., 2009; Turk et al., 2011). Cells were maintained in RPMI 1640 medium with 5% fetal bovine serum, 5 units/ml mouse interferon-γ (IF005) (EMD Millipore, Billerica, MA), 1% ITS “−” minus (insulin, transferrin, selenium) (41-400-045; Life Technologies, Grand Island, NY) at 33°C (permissive conditions). In preparation for experiments, cells were transferred to 37°C (nonpermissive conditions).
AhR antibody (BML-SA210) was purchased from Enzo Life Sciences (Farmingdale, NY). The assay for metabolic activity of the tryptophan metabolites was determined using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay essentially as described by Jin et al. (2014) (Supplemental Fig. 1). β-Actin (A1978) was purchased from Sigma-Aldrich (St. Louis, MO), and Cyp1a1 antibody was kindly provided by Dr. Paul Thomas (Rutgers University). Indole (>99%), indole-3-acetate (98%), indole-3-aldehyde (97%), and tryptamine (99%) were purchased from Sigma-Aldrich, and TCDD (99%) was synthesized in our laboratory. CH-223191 (2-methyl-N-[2-methyl-4-[(2-methylphenyl)diazenyl]phenyl]pyrazole-3-carboxamide) (cat. no. 3858) was purchased from Tocris Bioscience (Bristol, United Kingdom). GelRed Nucleic Acid Stain (RGB-4103) was purchased from Phenix Research Products (Candler, NC).
Chromatin Immunoprecipitation Assay.
The chromatin immunoprecipitation (ChIP) assay was performed using the ChIP-IT Express Magnetic Chromatin Immunoprecipitation kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. YAMC cells (1.2 × 107 cells) were treated with TCDD and/or compounds for 2 or 24 hours. The cells were then fixed with 1% formaldehyde, and the cross-linking reaction was stopped by addition of 0.125 M glycine. After washing with phosphate-buffered saline, cells were scraped and pelleted. The collected cells were hypotonically lysed, and nuclei were collected and then sonicated to the desired chromatin length (200–1500 base pairs).
The sonicated chromatin was immunoprecipitated with normal rabbit IgG or AhR antibodies and protein A-conjugated magnetic beads at 4°C for overnight. After the magnetic beads were extensively washed, the protein-DNA crosslinks were reversed and eluted. DNA was prepared by proteinase K digestion followed by polymerase chain reaction (PCR) amplification. The Cyp1a1 primers were 5′-AGG CTC TTC TCA CGC AAC TC-3′ (sense) and 5′-CGG GTG CAG AGC TAT CTA AGT-3′ (antisense); we then amplified a 207-base pair region of mouse Cyp1a1 promoter, which contained the AhR-binding sequences. The PCR products were analyzed on a 2% agarose gel in the presence of GelRed Nucleic Acid Stain.
Quantitative Real-Time PCR.
Total RNA was isolated using Zymo Quick RNA MiniPrep Kit (Zymo Research, Irvine, CA) according to the manufacturer’s protocol. RNA was eluted with 35 μl of RNase-free water and stored at −80°C. Real-time PCR was performed using iTaq Universal SYBR Green One-Step Kit (Bio-Rad Laboratories, Hercules, CA). The primers used are shown in Table 1.
TABLE 1.
Primers used in quantitative real-time PCR
| Name | Forward Primer | Reverse Primer |
|---|---|---|
| TBP | GAACAATCCAGACTAGCAGCA | GGGAACTTCACATCACAGCTC |
| Cyp1a1 | CTGAAGTGGTTCTGAGCGG | TCCACTCCATCTTCCGACTT |
| Cyp1b1 | GGATATCAGCCACGACGAAT | ATTATCTGGGCAAAGCAACG |
| TiParp | GCCAGACTGTGTAGTACAGCC | GGGTTCCAGTTCCCAATCTTTT |
| Ahrr | ACATACGCCGGTAGGAAGAGA | GGTCCAGCTCTGTATTGAGGC |
Western Blot Analysis.
Cells (1 × 105) were plated in six-well plates in RPMI media containing 2.5% fetal bovine serum for 16 hours and then treated with different concentrations of the compounds for 24 hours. Cells were collected using high-salt buffer (50 mM HEPES, 0.5 mol/l NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, and 1% Triton-X-100) and 10 μl/ml protease inhibitor cocktail (Sigma-Aldrich). Protein lysates were incubated for 5 minutes at 95°C before electrophoresis and then separated on 10% SDS-polyacrylamide gel electrophoresis 120 V for 2 to 3 hours. Proteins were transferred onto polyvinylidene difluoride membranes by wet electroblotting in a buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol for 1.5 hours at 180 mA. Membranes were then blocked for 30 minutes with specific antibodies. Specific proteins were detected by the use of chemiluminescence and then were exposed to Kodak Image Station 4000-mm Pro (Carestream Health, Rochester, NY)
Statistical Analysis.
Statistical significance of differences between the treatment groups was determined by an analysis of variance and/or Student’s t test, and the levels of probability were noted. At least three repeated experiments were determined for each data point, and results are expressed as mean ± S.E.
Results
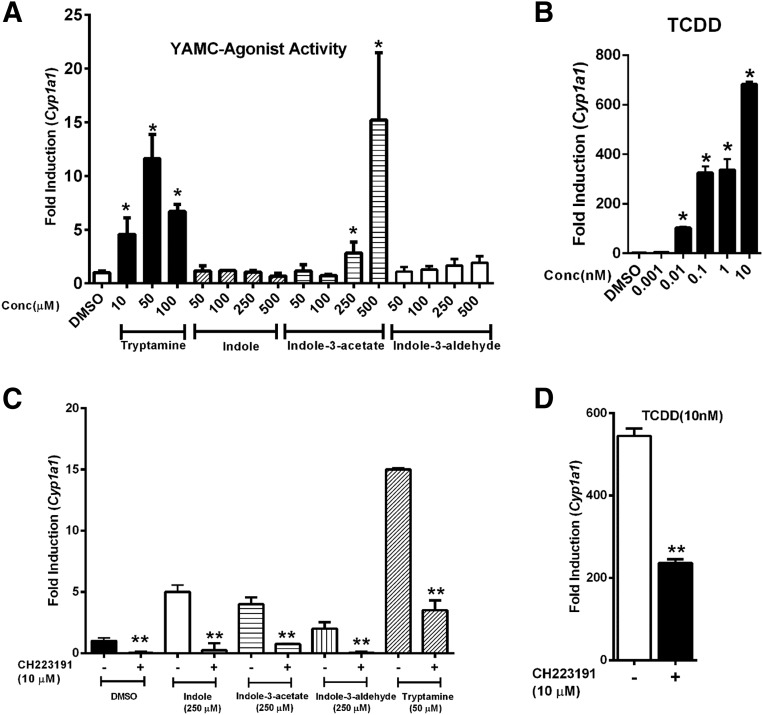
YAMC cells were treated with different concentrations of tryptamine (10–100 μM), indole (50–500 μM), indole-3-acetate (50–500 μM), and indole-3-aldehyde (50–500 μM), and the induction of Cyp1a1 mRNA was determined (Fig. 1A). Tryptamine and indole-3-acetate significantly induced Cyp1a1 mRNA levels (>10-fold) at concentrations of 50 and 500 μM, respectively, whereas indole and indole-3-aldehyde were inactive.
Fig. 1.
Tryptophan metabolites and TCDD as inducers of Cyp1a1 in YAMC cells. YAMC cells were treated for 24 hours with (A) tryptophan metabolites, (B) TCDD, (C) tryptophan metabolites plus CH, or (D) TCDD plus CH. Expression of Cyp1a1 mRNA was determined by real-time PCR. Results are expressed as mean ± S.E. for three replicate determinations, and significant (P < 0.05) induction (*) (A and B) or inhibition by CH (**) (C and D) is indicated.
In contrast 0.01–10 nM TCDD, the most potent AhR agonist, induced a >600-fold increase in Cyp1a1 mRNA levels with maximal induction by 10 nM TCDD (Fig. 1B), as previously observed in CaCo2 cells (Jin et al., 2014). Induction of Cyp1a1 mRNA by the tryptophan metabolites (Fig. 1C) and TCDD was inhibited after cotreatment with the AhR antagonist CH-223191 (CH) (Fig. 1D). In the inhibition experiment we observed some induction of Cyp1a1 by indole and indole-3-aldehyde alone (Fig. 1C), and over several experiments low-level induction responses by these compounds were variable (0- to 4-fold) but <1% of the response observed for TCDD.
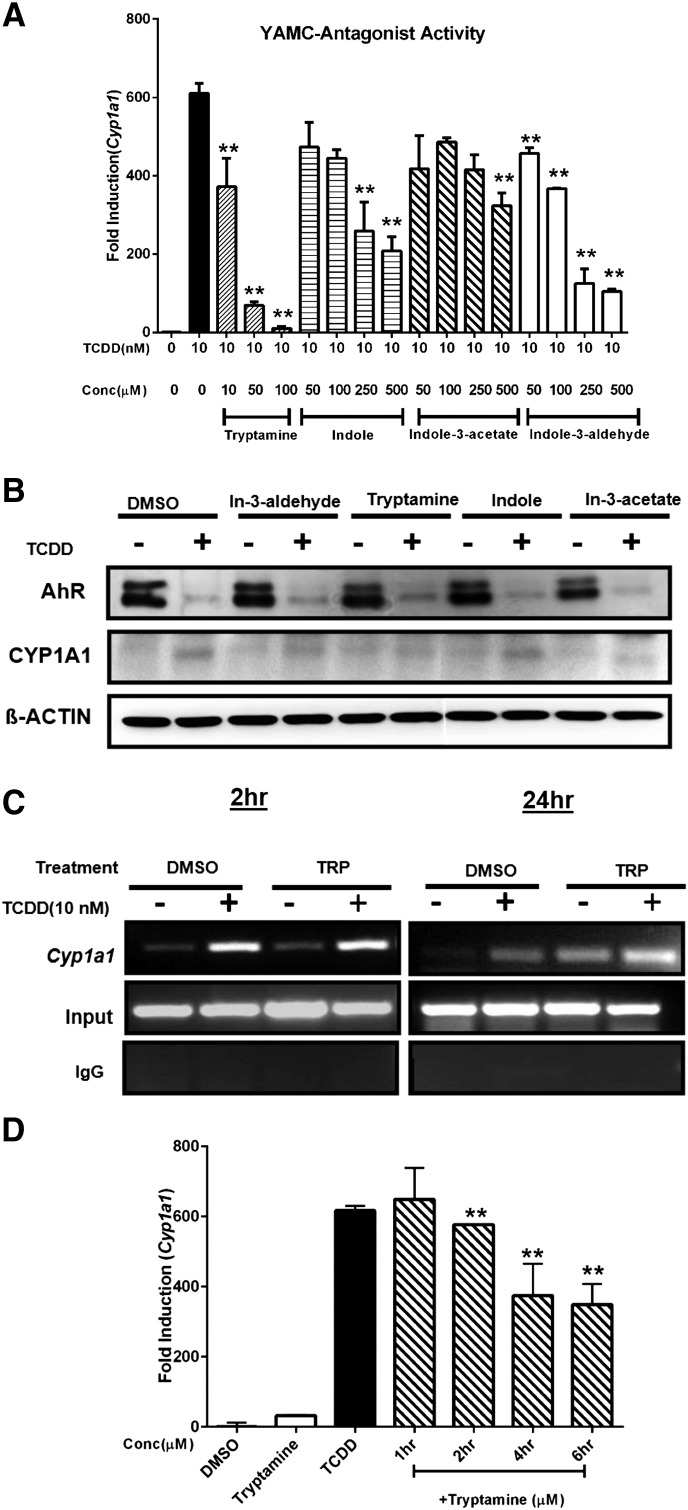
Previous studies in CaCo2 cells showed that indole was an AhR antagonist (Jin et al., 2014), and we further investigated the inhibitory effect of the tryptophan metabolites on induction of Cyp1a1 by TCDD (Fig. 2A). All four compounds exhibited AhR antagonist activity, and both tryptamine and indole-3-aldehyde decreased induction of Cyp1a1 mRNA by TCDD by >75%, which was more effective than observed for CH (Fig. 1D). Western blot analysis (Fig. 2B) showed that TCDD but not the tryptophan metabolites decreased AhR protein expression, and in combination experiments AhR levels resembled that observed for TCDD alone.
Fig. 2.
Tryptophan metabolites as AhR antagonists. YAMC cells were treated with tryptophan metabolites, TCDD, and their combination, and the effects on (A) Cyp1a1 mRNA and (B) CYP1A1/AhR proteins were determined by real-time PCR and Western blot analysis. (C) YAMC cells were treated with 10 nM TCDD, 50 μM tryptamine, or their combination for 2 and 24 hours and real-time PCR was used to determine interactions of the AhR with the Cyp1a1 promoter (containing XRE) in a ChIP assay. (D) YAMC cells were treated with dimethylsulfoxide or 10 nM TCDD for 24 hours and also cotreated with 50 μM tryptamine after 18, 20, 22, and 23 hours, and Cyp1a1 mRNA was determined by real-time PCR. The results (A and D) are expressed as mean ± S.E. (three replicates), and significant (P < 0.05) inhibition is indicated (**).
TCDD induced Cyp1a1 protein in YAMC cells, whereas minimal induction was observed for the tryptophan metabolites and in combination experiments indole-3-acetate appeared to be the most effective inhibitor of TCDD-induced CYP1A1 protein. Unfortunately, CYP1A1 protein levels in YAMC cells were low, and the results of the TCDD + tryptophan metabolites studies were difficult to interpret.
We also examined the effects of TCDD, tryptamine, and their combination on recruitment of the AhR to the Cyp1a1 XRE in a ChIP assay. After treatment of 2 hours, TCDD alone or in combination with tryptamine induced AhR interactions with the Cyp1a1 promoter, whereas minimal effects were observed in YAMC cells treated with tryptamine alone (Fig. 2C). These results contrasted to those observed in CaCo2 cells where indole, the most effective AhR antagonist, blocked TCDD-induced AhR interactions with the ICyp1a1 promoter (Jin et al., 2014).
Analysis of these interactions were also investigated after treatment for 24 hours; significant AhR recruitment to the Cyp1a1 promoter was observed after treatment with tryptamine alone, and a comparison of the results of 2- and 24-hour treatments suggested that the tryptamine-induced AhR recruitment was a relatively slow process. In contrast, after treatment with TCDD for 24 hours, the AhR binding to the Cyp1a1 promoter was decreased, and this was consistent with the observed TCDD-induced degradation of the AhR protein (Fig. 2B).
Despite the inhibition of TCDD-induced Cyp1a1 mRNA levels by 24 hours of tryptamine treatment (Fig. 2A), AhR binding to the Cyp1a1 promoter in the combined treatment group was essentially additive (Fig. 2C). Therefore, it is possible that the inhibition of TCDD-induced Cyp1a1 by tryptamine is posttranscriptional and AhR-independent.
YAMC cells were treated with TCDD alone for 24 hours and cotreated with tryptamine after 18, 20, 22 and 23 hours after addition of TCDD. The results showed that there was a time-dependent decrease in induced Cyp1a1 mRNA (Fig. 2D), suggesting that some of the inhibitory effects of tryptamine were posttranscriptional and may be due to destabilization of Cyp1a1 mRNA.
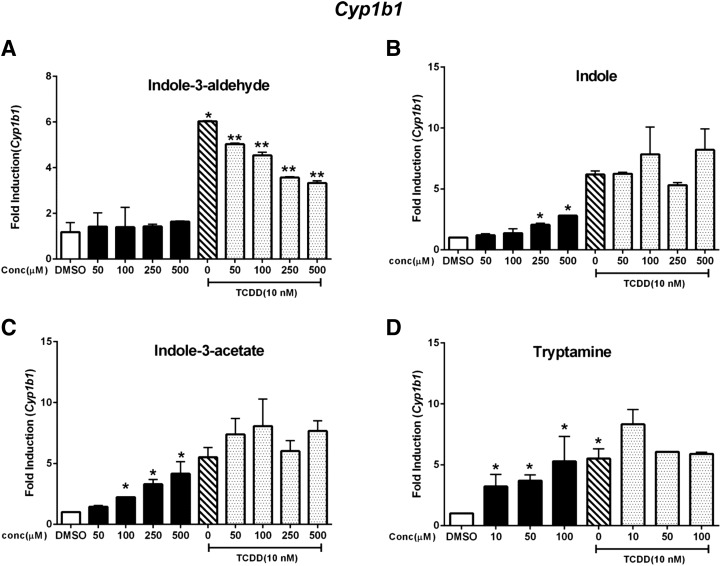
The tryptophan metabolites exhibited structure-dependent AhR agonist/antagonist activities with respect to induction of Cyp1a1 in YAMC cells, and this pattern of activity was investigated with other Ah-responsive genes (Savas et al., 1994; D’Abaco et al., 1996; Baba et al., 2001; Diani-Moore et al., 2010). The results in Fig. 3A show that TCDD but not indole-3-aldehyde induced Cyp1b1 expression in YAMC cells, and in combination studies indole-3-aldehyde partially inhibited TCDD-induced Cyp1b1 expression. Indole was a partial agonist for induction of Cyp1b1 but did not inhibit TCDD-induced Cyp1b1 mRNA levels (Fig. 3B). Indole-3-acetate (Fig. 3C) and tryptamine (Fig. 3D) induced Cyp1b1 mRNA levels similar to that of TCDD and did not inhibit induction by TCDD, indicating that both compounds were full AhR agonists for induction of Cyp1b1.
Fig. 3.
Induction of Cyp1b1. YAMC cells were treated with TCDD (alone), indole-3-aldehyde (A), indole (B), indole-3-acetate (C), and tryptamine (D) alone and in combination with TCDD for 24 hours, and Cyp1b1 was determined by real-time PCR. Results are expressed as mean ± S.E. (three replicates), and significant (P < 0.05) induction (*) or inhibition (**) of TCDD-induced Cyp1b1 is indicated.
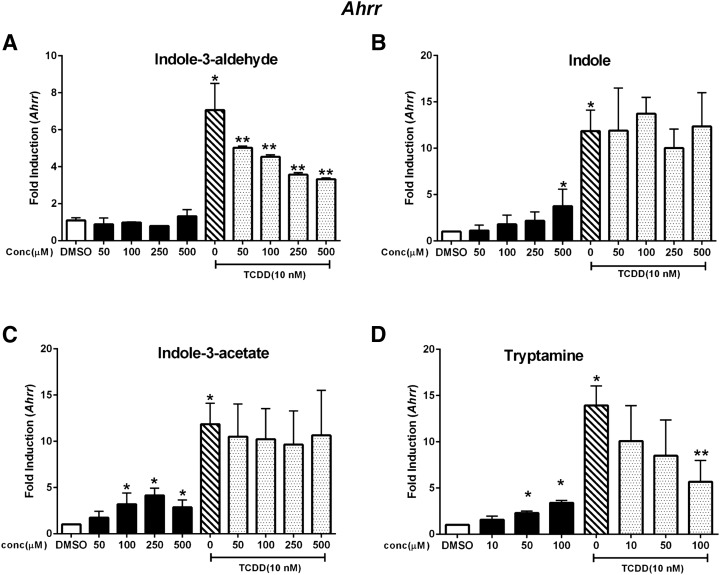
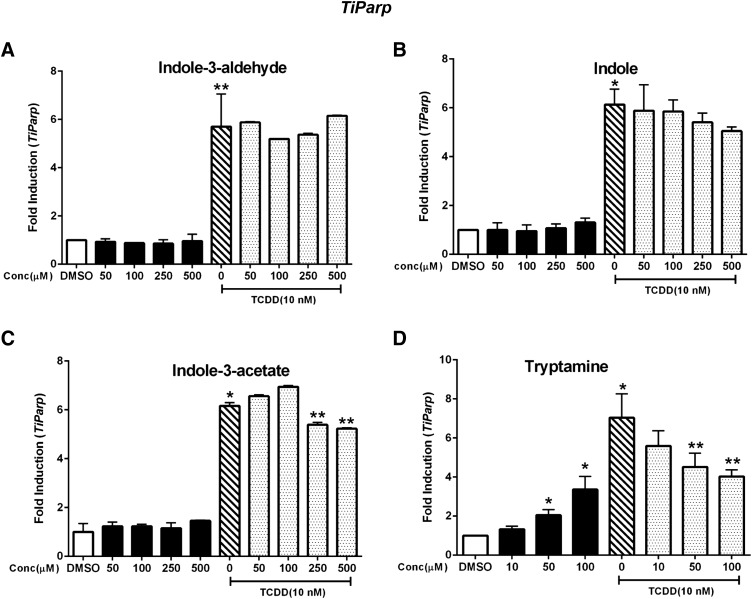
A similar approach was used to examine the AhR agonist/antagonist activities of the tryptophan metabolites with respect to regulation of Ahrr and TiParp gene expression. Indole-3-aldehyde minimally induced Ahrr (<2-fold) at the highest concentration (500 μM); TCDD induced Ahrr (<7- fold) and in combination indole-3-aldehyde was a weak AhR antagonist (Fig. 4A). Indole (Fig. 4B), indole-3-acetate (Fig. 4C), and tryptamine (Fig. 4D) were partial agonists and induced Ahrr, and only tryptamine exhibited partial AhR antagonist activity. Indole-3-aldehyde, indole, and indole-3-acetate (Fig. 5, A–C) did not induce TiParp or inhibit induction of TiParp by TCDD, whereas tryptamine (Fig. 5D) exhibited partial agonist/antagonist activity. Thus, the effects of the tryptophan metabolites as AhR agonist and antagonists were highly gene specific in YAMC cells, and these difference are summarized in Table 2.
Fig. 4.
Induction of Ahrr. YAMC cells were treated with TCDD (alone), indole-3-aldehyde (A), indole (B), indole-3-acetate (C), or tryptamine (D) alone and in combination with TCDD for 24 hours, and Cyp1b1 was determined by real-time PCR. Results are expressed as mean ± S.E. (three replicates), and significant (P < 0.05) induction (*) or inhibition (**) of TCDD-induced Ahrr is indicated.
Fig. 5.
Induction of TiParp. YAMC cells were treated with TCDD (alone), indole-3-aldehyde (A), indole (B), indole-3-acetate (C), or tryptamine (D) alone and in combination with TCDD for 24 hours, and TiParp was determined by real-time PCR. Results are expressed as mean ± S.E. (three replicates), and significant (P < 0.05) induction (*) or inhibition (**) of TCDD-induced TiParp is indicated.
TABLE 2.
Effects of the tryptophan metabolites as AhR agonists and antagonists are highly gene specific in YAMC cells
| Metabolite |
Cyp1a1 |
Cyp1b1 |
Ahrr |
TiParp |
||||
|---|---|---|---|---|---|---|---|---|
| Ag | Ant | Ag | Ant | Ag | Ant | Ag | Ant | |
| TCDD | + | − | + | − | + | − | + | − |
| Indole-3-aldehyde | −a | + | − | + | − | + | − | − |
| Indole | −a | + | + | − | + | − | − | − |
| Indole-3-acetate | + | + | + | − | + | − | − | − |
| Tryptamine | + | + | + | − | + | − | + | + |
Weak agonist activity and somewhat variable.
Discussion
The AhR is expressed in the gastrointestinal tract, and studies in animal models demonstrate that this receptor and its ligands play an important role in gut health and response to stressors and disease (Kawajiri et al., 2009; Arsenescu et al., 2011; Benson and Shepherd, 2011; Furumatsu et al., 2011; Kiss et al., 2011; Li et al., 2011; Monteleone et al., 2011; Singh et al., 2011; Lee et al., 2012). Loss of the AhR results in formation of colon tumors at the cecum, and this is accompanied by increased expression of β-catenin in the small intestine, whereas wild-type AhR+/+ mice do not develop tumors or overexpress β-catenin (Kawajiri et al., 2009). Apcmin/+ mice, which express a mutation in the Apc tumor suppressor gene, were crossed with AhR+/− (heterozygote) mice, and the resulting Apcmin/+/AhR+/− mice were more susceptible to cecal tumorigenesis (Kawajiri et al., 2009). However, AhR-active botanic compounds such as indole-3-carbinol and diindolylmethane (from cruciferous vegetables) significantly suppressed intestinal tumorigenesis in Apcmin/+ and Apcmin/+ AhR+/− mice (Kawajiri et al., 2009).
Gut interleukin (IL)-22–producing innate lymphoid cells (ILC22) cells and postnatal lymphoid tissue-inducer-like subsets express the AhR which is essential for many of their functions. For example, the loss of the AhR results in decreased expression of ILC22 and decreased protection against bacterial infections (Lee et al., 2012). The AhR agonist TCDD has been shown to induce Notch1, which is differentially required for the development of various ILC22 and lymphoid tissue-inducer-like cell subtypes.
The important functions of the AhR in maintaining intestinal function and health and protection against bacterial infections has been described in several reports showing that the AhR and its ligands also protect against intestinal damage/inflammation in experimental models of colitis and Crohn’s disease (Arsenescu et al., 2011; Furumatsu et al., 2011; Monteleone et al., 2011; Singh et al., 2011). The severity of the effects of 2,4-trinitrobenzene sulfonic acid-induced colitis (which resembles Crohn’s disease) in mice was significantly decreased by treatment with the AhR agonists FICZ (Lee et al., 2012) and TCDD (Benson and Shepherd, 2011), and this was accompanied by suppression of several markers of inflammation. The severity of dextran sodium sulfate-induced colitis in mice was also decreased by the AhR agonists β-naphthoflavone (Furumatsu et al., 2011), TCDD (Benson and Shepherd, 201), and FICZ (Lee et al., 2012); in the latter study, the AhR antagonist 2-methyl-2H-pyrazole-3-carboxylic acid enhanced the severity of the colitis (Lee et al., 2012).
Previously, we investigated the tryptophan metabolites in CaCo2 human colon cancer cells and demonstrated their ligand-dependent AhR agonist and antagonist activities based primarily on modulation of CYP1A1 gene expression (Jin et al., 2014). We also observed similar responses in nontransformed YAMC cells where the most active AhR agonists for induction of Cyp1a1 were tryptamine and indole-3-acetate; however, the fold induction by both compounds was <3% of that observed for TCDD. In contrast, both tryptamine and indole-3-aldehyde were potent inhibitors of TCDD-induced Cyp1a1 in YAMC cells, whereas tryptamine was primarily a full AhR agonist in CaCo2 cells using CYP1A1 mRNA as an end point, demonstrating the importance of cell context.
We also investigated the AhR activity of the four tryptophan metabolites using three additional Ah-responsive genes, namely, Ahrr, Cyp1b1, and TiParp, and the results indicated that the AhR agonist and antagonist activities were both compound- and gene-specific (Table 2). For example, tryptamine was a weak AhR agonist and partial antagonist for Cyp1a1 mRNA expression; however, examination of the ligand-dependent recruitment of the AhR complex to the Cyp1a1 promoter (Fig. 2C) did not readily explain a mechanism for the activity of tryptamine as an AhR agonist (Fig. 2A).
In a separate experiment, we observed that treatment of YAMC cells with TCDD alone for 24 hours maximally induced Cyp1a1 mRNA, which could then be significantly decreased by addition of tryptamine 18, 20, or 22 hours after treatment with TCDD, suggesting that some of the inhibitory effects of tryptamine on induced Cyp1a1 mRNA may be posttranscriptional. In contrast, tryptamine and TCDD induced similar levels of Cyp1b1 mRNA, and tryptamine did not affect TCDD-induced Cyp1b1, indicating that tryptamine was a full AhR agonist for this response.
The cell context- and gene-specific AhR agonist and antagonist activities of the tryptophan metabolites are not unique and have been observed for other AhR ligands including 6-methyl-1,3,8-trichlorobenzofuran, flavonoids, and pharmaceuticals (Astroff et al., 1988; Lu et al., 1996; McDougal et al., 2001; Zhou and Gasiewicz, 2003; Jin et al., 2012; Safe et al., 2012). We also observed that the AhR antagonist CH inhibited Cyp1a1 induction by TCDD and the tryptophan metabolites, indicating that CH inhibited induction of Cyp1a1 by a diverse spectrum of AhR ligands, as previously reported elsewhere (Choi et al., 2012). Ongoing studies show that CH also antagonized induction of Cyp1b1 by TCDD and the tryptophan metabolites; however, CH did not antagonize induction of Ahrr or TiParp by TCDD and tryptophan metabolites (data not shown), and this is currently being investigated.
In summary, our results show that for a limited set of Ah-responsive genes the AhR agonist and antagonist activities of the tryptophan metabolites are gene specific in nontransformed YAMC cells and are different from that previously observed in CaCo2 cancer cells (Jin et al., 2014). Differences in the AhR agonist and antagonist activities of the tryptophan metabolites are due not only to the transformed versus nontransformed phenotype of CaCo2 and YAMC cells but also to their different human versus mouse origins. These results suggest that indole-3-aldehyde, indole, indole-3-acetate, and tryptamine are selective AhR modulators (Safe et al., 2013; Murray et al., 2014) based on the results observed in this study.
Although indole-3-aldehyde exhibited minimal AhR agonist activity, a recent report indicated that AhR-dependent induction of IL-22 by indole-3-aldehyde plays a key role in microbiota-mediated protection from fungal infection and colitis (Zelante et al., 2013); however, indole-3-aldehyde did not induce IL-22 in YAMC cells (data not shown). Current studies are evaluating the contributions of AhR-active tryptophan metabolites in YAMC and other mouse- and human-derived cell lines to identify in vitro models that mimic in vivo effects of these compounds and identify relevant end points such as IL-22 induction that will predict the effects of AhR-active microbiota metabolites on gut health.
Supplementary Material
Abbreviations
- AhR
aryl hydrocarbon receptor
- Ahrr
AhR repressor
- Arnt
aryl hydrocarbon receptor nuclear translocator
- CH223191
CH, 2-methyl-N-[2-methyl-4-[(2-methylphenyl)diazenyl]phenyl]pyrazole-3-carboxamide
- ChIP
chromatin immunoprecipitation
- FICZ
6-formylindolo-[3,2-b]-carbazole
- IL-22
interleukin-22
- ILC22
interleukin-22–producing innate lymphoid cells
- PCR
polymerase chain reaction
- SAhRMs
selective aryl hydrocarbon receptor modulators
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TiParp
2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible poly(ADP-ribose) polymerase
- XRE
xenobiotic response element
- YAMC
young adult mouse colonocyte
Authorship Contributions
Participated in research design: Cheng, Jin, Allred, Jayaraman, Chapkin, Safe.
Conducted experiments: Cheng, Jin, Allred, Safe.
Contributed new reagents or analytic tools: Cheng, Jin, Allred, Jayaraman, Chapkin, Safe.
Performed data analysis: Cheng, Jin, Safe.
Wrote or contributed to the writing of the manuscript: Cheng, Jin, Allred, Jayaraman, Chapkin, Safe.
Footnotes
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences [P30-ES023512], Texas AgriLife Research, and the Sid Kyle Chair Endowment.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Arsenescu R, Arsenescu V, Zhong J, Nasser M, Melinte R, Dingle RW, Swanson H, de Villiers WJ. (2011) Role of the xenobiotic receptor in inflammatory bowel disease. Inflamm Bowel Dis 17:1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astroff B, Zacharewski T, Safe S, Arlotto MP, Parkinson A, Thomas P, Levin W. (1988) 6-Methyl-1,3,8-trichlorodibenzofuran as a 2,3,7,8-tetrachlorodibenzo-p-dioxin antagonist: inhibition of the induction of rat cytochrome P-450 isozymes and related monooxygenase activities. Mol Pharmacol 33:231–236. [PubMed] [Google Scholar]
- Baba T, Mimura J, Gradin K, Kuroiwa A, Watanabe T, Matsuda Y, Inazawa J, Sogawa K, Fujii-Kuriyama Y. (2001) Structure and expression of the Ah receptor repressor gene. J Biol Chem 276:33101–33110. [DOI] [PubMed] [Google Scholar]
- Bansal T, Alaniz RC, Wood TK, Jayaraman A. (2010) The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci USA 107:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Shepherd DM. (2011) Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol Sci 120:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA. (1991) Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci USA 88:9543–9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship A, Matsumura F. (1997) 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) causes an Ah receptor-dependent and ARNT-independent increase in membrane levels and activity of p60(Src). Environ Toxicol Pharmacol 3:211–220. [DOI] [PubMed] [Google Scholar]
- Busbee PB, Rouse M, Nagarkatti M, Nagarkatti PS. (2013) Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr Rev 71:353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Lee H, Dingle RW, Kim KB, Swanson HI. (2012) Implications and development of AHR-based therapeutic agents. Mol Cell Pharmacol 4:53–62. [Google Scholar]
- D’Abaco GM, Whitehead RH, Burgess AW. (1996) Synergy between Apc min and an activated ras mutation is sufficient to induce colon carcinomas. Mol Cell Biol 16:884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Seidel SD, Rogers WJ, Ziccardi MH, Winter GM, Heath-Pagliuso S. (1998) Natural and synthetic ligands for the Ah receptor, in Molecular Biology Approaches to Toxicology (Puga A, Kendall KB, eds) pp 3–33, Taylor and Francis, London. [Google Scholar]
- Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. (2011) Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci 124:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diani-Moore S, Ram P, Li X, Mondal P, Youn DY, Sauve AA, Rifkind AB. (2010) Identification of the aryl hydrocarbon receptor target gene TiPARP as a mediator of suppression of hepatic gluconeogenesis by 2,3,7,8-tetrachlorodibenzo-p-dioxin and of nicotinamide as a corrective agent for this effect. J Biol Chem 285:38801–38810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Matsumura F. (2009) The conversion of rapid TCCD nongenomic signals to persistent inflammatory effects via select protein kinases in MCF10A cells. Mol Endocrinol 23:549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. (1995) Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 268:722–726. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Toshimitsu T, Matsuoka S, Maruyama A, Oh-Oka K, Takamura T, Nakamura Y, Ishimaru K, Fujii-Kuriyama Y, Ikegami S, et al. (2014) Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol Cell Biol 92:460–465. [DOI] [PubMed] [Google Scholar]
- Furumatsu K, Nishiumi S, Kawano Y, Ooi M, Yoshie T, Shiomi Y, Kutsumi H, Ashida H, Fujii-Kuriyama Y, Azuma T, et al. (2011) A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig Dis Sci 56:2532–2544. [DOI] [PubMed] [Google Scholar]
- Gu YZ, Hogenesch JB, Bradfield CA. (2000) The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol 40:519–561. [DOI] [PubMed] [Google Scholar]
- Hankinson O. (1995) The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol 35:307–340. [DOI] [PubMed] [Google Scholar]
- Henry EC, Bemis JC, Henry O, Kende AS, Gasiewicz TA. (2006) A potential endogenous ligand for the aryl hydrocarbon receptor has potent agonist activity in vitro and in vivo. Arch Biochem Biophys 450:67–77. [DOI] [PubMed] [Google Scholar]
- Hu W, Sorrentino C, Denison MS, Kolaja K, Fielden MR. (2007) Induction of cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol Pharmacol 71:1475–1486. [DOI] [PubMed] [Google Scholar]
- Jackson DP, Li H, Mitchell KA, Joshi AD, Elferink CJ. (2014) Ah receptor-mediated suppression of liver regeneration through NC-XRE-driven p21Cip1 expression. Mol Pharmacol 85:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeuken A, Keser BJ, Khan E, Brouwer A, Koeman J, Denison MS. (2003) Activation of the Ah receptor by extracts of dietary herbal supplements, vegetables, and fruits. J Agric Food Chem 51:5478–5487. [DOI] [PubMed] [Google Scholar]
- Jin UH, Lee SO, Safe S. (2012) Aryl hydrocarbon receptor (AHR)-active pharmaceuticals are selective AHR modulators in MDA-MB-468 and BT474 breast cancer cells. J Pharmacol Exp Ther 343:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S. (2014) Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol 85:777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri K, Kobayashi Y, Ohtake F, Ikuta T, Matsushima Y, Mimura J, Pettersson S, Pollenz RS, Sakaki T, Hirokawa T, et al. (2009) Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci USA 106:13481–13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet NI. (2009) AHR-mediated immunomodulation: the role of altered gene transcription. Biochem Pharmacol 77:746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Gazourian L, Quadri SA, Romieu-Mourez R, Sherr DH, Sonenshein GE. (2000) The RelA NF-κB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene 19:5498–5506. [DOI] [PubMed] [Google Scholar]
- Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. (2011) Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334:1561–1565. [DOI] [PubMed] [Google Scholar]
- Kolar SS, Barhoumi R, Lupton JR, Chapkin RS. (2007) Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. Cancer Res 67:5561–5568. [DOI] [PubMed] [Google Scholar]
- Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, et al. (2012) AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol 13:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Hullar MA, Schwarz Y, Lampe JW. (2009) Human gut bacterial communities are altered by addition of cruciferous vegetables to a controlled fruit- and vegetable-free diet. J Nutr 139:1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Matsumura F. (2008) Significance of the nongenomic, inflammatory pathway in mediating the toxic action of TCDD to induce rapid and long-term cellular responses in 3T3-L1 adipocytes. Biochemistry 47:13997–14008. [DOI] [PubMed] [Google Scholar]
- Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. (2011) Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147:629–640. [DOI] [PubMed] [Google Scholar]
- Lu YF, Santostefano M, Cunningham BD, Threadgill MD, Safe S. (1996) Substituted flavones as aryl hydrocarbon (Ah) receptor agonists and antagonists. Biochem Pharmacol 51:1077–1087. [DOI] [PubMed] [Google Scholar]
- Marshall NB, Kerkvliet NI. (2010) Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann N Y Acad Sci 1183:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal A, Wormke M, Calvin J, Safe S. (2001) Tamoxifen-induced antitumorigenic/antiestrogenic action synergized by a selective aryl hydrocarbon receptor modulator. Cancer Res 61:3902–3907. [PubMed] [Google Scholar]
- Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, et al. (1997) Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 2:645–654. [DOI] [PubMed] [Google Scholar]
- Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, MacDonald TT, Pallone F, Monteleone G. (2011) Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 141:237–248,248e231. [DOI] [PubMed] [Google Scholar]
- Murray IA, Patterson AD, Perdew GH. (2014) Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer 14:801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Goujon FM, Gielen JE. (1972) Aryl hydrocarbon hydroxylase induction by polycyclic hydrocarbons: simple autosomal dominant trait in the mouse. Nat New Biol 236:107–110. [DOI] [PubMed] [Google Scholar]
- Oberg M, Bergander L, Håkansson H, Rannug U, Rannug A. (2005) Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol Sci 85:935–943. [DOI] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, et al. (2011) An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478:197–203. [DOI] [PubMed] [Google Scholar]
- Poland A, Glover E, Kende AS. (1976) Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem 251:4936–4946. [PubMed] [Google Scholar]
- Safe S, Chadalapaka G, Jutooru I. (2012) AHR-reactive compounds in the human diet, in The Ah Receptor in Biology and Toxicology (Pohjanvirta R, ed) pp 331–342, John Wiley & Sons, Hoboken, NJ. [Google Scholar]
- Safe S, Lee SO, Jin UH. (2013) Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol Sci 135:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas U, Bhattacharyya KK, Christou M, Alexander DL, Jefcoate CR. (1994) Mouse cytochrome P-450EF, representative of a new 1B subfamily of cytochrome P-450s. Cloning, sequence determination, and tissue expression. J Biol Chem 269:14905–14911. [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. (1996) Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA 93:6731–6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Nagarkatti M, Nagarkatti PS. (2007) Role of dioxin response element and nuclear factor-κB motifs in 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated regulation of Fas and Fas ligand expression. Mol Pharmacol 71:145–157. [DOI] [PubMed] [Google Scholar]
- Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. (2011) Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS ONE 6:e23522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Clagett-Dame M, Peterson RE, Hahn ME, Westler WM, Sicinski RR, DeLuca HF. (2002) A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci USA 99:14694–14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens EA, Mezrich JD, Bradfield CA. (2009) The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology 127:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk HF, Kolar SS, Fan YY, Cozby CA, Lupton JR, Chapkin RS. (2011) Linoleic acid and butyrate synergize to increase Bcl-2 levels in colonocytes. Int J Cancer 128:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, et al. (2014) Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41:296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Khan EM, Leung PS, Gershwin ME, Chang WL, Wu D, Haarmann-Stemmann T, Hoffmann A, Denison MS. (2014) Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-κB. J Biol Chem 289:1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. (2007) RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol 21:2941–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weige CC, Allred KF, Allred CD. (2009) Estradiol alters cell growth in nonmalignant colonocytes and reduces the formation of preneoplastic lesions in the colon. Cancer Res 69:9118–9124. [DOI] [PubMed] [Google Scholar]
- Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. (1993) Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci USA 90:587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JP., Jr (1999) Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol 39:103–125. [DOI] [PubMed] [Google Scholar]
- Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, Rannug A, Rannug U. (2009) The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem 284:2690–2696. [DOI] [PubMed] [Google Scholar]
- Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, et al. (2013) Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39:372–385. [DOI] [PubMed] [Google Scholar]
- Zhou J, Gasiewicz TA. (2003) 3′-methoxy-4′-nitroflavone, a reported aryl hydrocarbon receptor antagonist, enhances Cyp1a1 transcription by a dioxin responsive element-dependent mechanism. Arch Biochem Biophys 416:68–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.