Abstract
AT-1001 [N-(2-bromophenyl)-9-methyl-9-azabicyclo[3.3.1] nonan-3-amine] is a high-affinity and highly selective ligand at α3β4 nicotinic cholinergic receptors (nAChRs) that was reported to decrease nicotine self-administration in rats. It was initially reported to be an antagonist at rat α3β4 nAChRs heterologously expressed in HEK293 cells. Here we compared AT-1001 actions at rat and human α3β4 and α4β2 nAChRs similarly expressed in HEK 293 cells. We found that, as originally reported, AT-1001 is highly selective for α3β4 receptors over α4β2 receptors, but its binding selectivity is much greater at human than at rat receptors, because of a higher affinity at human than at rat α3β4 nAChRs. Binding studies in human and rat brain and pineal gland confirmed the selectivity of AT-1001 for α3β4 nAChRs and its higher affinity for human compared with rat receptors. In patch-clamp electrophysiology studies, AT-1001 was a potent partial agonist with 65–70% efficacy at both human and rat α3β4 nAChRs. It was also a less potent and weaker (18%) partial agonist at α4β2 nAChRs. Both α3β4 and α4β2 nAChRs are upregulated by exposure of cells to AT-1001 for 3 days. Similarly, AT-1001 desensitized both receptor subtypes in a concentration-dependent manner, but it was 10 and 30 times more potent to desensitize human α3β4 receptors than rat α3β4 and human α4β2 receptors, respectively. After exposure to AT-1001, the time to recovery from desensitization was longest for the human α3β4 nAChR and shortest for the human α4β2 receptor, suggesting that recovery from desensitization is primarily related to the dissociation of the ligand from the receptor.
Introduction
Neuronal nicotinic acetylcholine receptors (nAChRs) mediate important functions across the central and peripheral nervous systems. These receptors are pentameric cation channels comprised of 12 subunits, α2–10 and β2–4, the combinations of which define the nAChR subtypes. Although there are a large number of theoretically possible nAChR subtypes, with possibly differing properties and characteristics, the predominant subtypes found in the central and peripheral nervous systems are α4β2*, α7*, and α3β4* (where * indicates that an additional subunit might be incorporated in the receptor). These nAChR subtypes are differentially expressed, with α4β2* and α7* receptors predominating in most areas of the forebrain and α3β4* receptors predominating in certain areas of the midbrain and hindbrain, as well as in the pineal gland, adrenal gland, and most autonomic ganglia (Perry et al., 2002; Hernandez et al., 2004; Gotti et al., 2006; Mao et al., 2006).
Most known ligands and drugs that target nAChRs have much higher affinity for the α4β2* subtypes, and these receptors appear to be involved in several important functions, including essential aspects of nicotine addiction. However, increasing evidence suggests that the α3β4* subtypes also may be important in nicotine addiction and withdrawal (Salas et al., 2004; Glick et al., 2011; McCallum et al., 2012; Jackson et al., 2013). Moreover, the genes coding for both of these constituent subunits are part of a gene cluster in which allelic variations have been associated with heavy smoking (Bierut et al., 2007, 2008; Berrettini et al., 2008; Saccone et al., 2009). The possibility that the α3β4* nAChR subtype contributes to nicotine addiction, and even to other addictions (Muldoon et al., 2014), as well as its importance in crucial physiologic functions such as ganglionic neurotransmission, underscores the need for selective ligands for this receptor that can be used as research tools and/or eventually as therapeutic drugs.
Recently, a new compound, AT-1001 [N-(2-bromophenyl)-9-methyl-9-azabicyclo[3.3.1] nonan-3-amine], was found to be a selective, high-affinity ligand at rat α3β4 nAChRs (Toll et al., 2012; Zaveri et al., 2015). Furthermore, AT-1001 was reported to be a potent antagonist or partial agonist at α3β4 nAChRs (Toll et al., 2012; Zaveri et al., 2015), and it effectively reduced nicotine self-administration in rats (Toll et al., 2012). Rat and human nAChR subunits are, in general, very similar, but an important difference between the two species is found within the extracellular D loop of the β4 subunits (Young et al., 2007). This structural variation confers differences in the pharmacological responses to certain drugs (Young et al., 2007; Zwart et al., 2008; Stokes and Papke, 2012). To further explore the pharmacological properties of AT-1001, we carried out comparative studies on the pharmacology of binding and function for rat and human α3β4 and α4β2 nAChRs expressed in HEK-293 cells, as well as binding in rat and human brain, which express primarily β2-containing nAChRs, and pineal gland, which express exclusively (rat) or primarily (human) α3β4 nAChRs. Our results indicate that there are substantial species differences in the binding and functional characteristics of AT-1001 at rat and human α3β4 nAChRs and, most important, that AT-1001 is a potent partial agonist with an efficacy of 65–70% at these receptors. In addition, we found that AT-1001 desensitizes both α3β4 and α4β2 nAChRs but is much more potent at the α3β4 subtype.
Materials and Methods
AT-1001 (N-(2-bromophenyl)-9-methyl-9-azabicyclo[3.3.1] nonan-3-amine) was synthesized as described by Zaveri et al. (2010). [3H]epibatidine ([3H]EB) was purchased from PerkinElmer Life Sciences (Boston, MA). Unless otherwise indicated, all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Lines.
Tissue culture medium and antibiotics were obtained from Invitrogen (Carlsbad, CA), unless otherwise stated. Fetal bovine serum and horse serum were provided by Gemini Bio-Products (Woodland, CA). The cell lines KXα3β42R2 and KXα4β2R2, expressing rat α3β4 and α4β2 nAChRs, respectively, were established and characterized previously (Xiao et al., 1998; Meyer et al., 2001). These cell lines were maintained in medium consisting of Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin G, 100 μg/ml streptomycin, and 0.7 mg/ml Geneticin (G418). The cell lines YXα3β4H1 and YXα4β2H1, expressing human α3β4 and α4β2 nAChRs, respectively, were established by introducing human α3 and β4 subunit genes or human α4 and β2 subunit genes into HEK293 cells. These cell lines were maintained in a medium consisting of minimum essential medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin G, 100 μg/ml streptomycin, 0.7 mg/ml G418 (Geneticin), and 0.1 mg/ml hygromycin B.
Brain and Pineal Gland.
Frozen rat brains and pineal glands from adult Sprague-Dawley rats (approximately 50% males and 50% females) were purchased from Zivic Miller Laboratories (Portersville, PA). Human frontal cerebral cortex and pineal glands were obtained at autopsy from psychiatrically normal individuals (men, average age 57). All procedures were carried out in compliance with an approved protocol from the University of Mississippi Medical Center Institutional Review Board. Written informed consent was obtained from legally defined next of kin for tissue collection and informant-based retrospective diagnostic interviews. Psychiatric assessments were performed as previously described (Cobb et al., 2013). Toxicology screens revealed nothing remarkable.
Radioligand Binding.
Cells were harvested at 90–100% confluency by first aspirating culture media and replacing it with 25 ml of 50 mM Tris-HCl buffer (pH 7.4), gently scraping cells from the culture flask surface, and centrifuging this cell suspension at 1000g for 10 minutes at 4°C. Cell membrane homogenates were then prepared by first resuspending the whole cells in 50 mM Tris-HCl buffer, homogenizing them with a Polytron homogenizer (Kinematica AG, Luzern, Switzerland), followed by centrifugation at 33,000g for 10 minutes at 4°C. The supernatant was discarded, and the pellet was resuspended in fresh buffer and treated as above two more times. The final pellet was resuspended in 50 mM Tris-HCl buffer and used in subsequent experiments. Brain and pineal glands were homogenized in 50 mM Tris-HCl buffer (pH 7.4) and membranes were then prepared as described above for cells.
Radioligand competition and saturation binding assays were performed with [3H]EB as described previously (Xiao et al., 1998) in 0.25 ml 50 mM Tris-HCl buffer, pH 7.4 at 23°C. The affinities of AT-1001 at the rat and human versions of the α3β4 and α4β2 nAChR subtypes expressed in HEK293 cells were determined in competition binding assays. Cell membrane homogenates were incubated for 4 hours with ∼0.4 nM [3H]EB in the absence or presence of increasing concentrations of AT-1001. The homogenates were then filtered through Whatman GF/C filters (GE Healthcare Life Sciences, Pittsburgh, PA) treated with 0.5% polyethylenimine and counted in a Beckman-Coulter LS6500 Scintillation Counter (Franklin Lakes, NJ). Nonspecific binding was measured in the presence of 300 μM (−)nicotine, and specific binding was defined as the difference between total and nonspecific binding. The AT-1001 binding competition curves were fit to one-site and two-site binding competition models by nonlinear least square regression analysis using GraphPad Prism 5 (San Diego, CA). The Ki of AT-1001 at each receptor was then calculated with the Cheng-Prusoff equation (Cheng and Prusoff, 1973) using the [3H]EB Kd for each receptor.
To determine how well AT-1001 binding affinities for rat and human α3β4 and α4β2 nAChR subtypes heterologously expressed in HEK293 cells correspond to its binding affinities in native tissues, we measured its competition for nAChRs labeled by [3H]EB in membrane homogenates from rat and human cerebral cortex and pineal gland. Immunoprecipitation studies with subunit-specific antibodies have established that the heteromeric nAChRs in rat cerebral cortex are predominantly (∼90%) the α4β2 subtype (Whiting et al., 1987; Flores et al., 1992; Mao et al., 2008), with ∼16% of these receptors also containing an α5 subunit (Mao et al., 2008). The predominant nAChRs in human cerebral cortex are also the α4β2 subtype (Gotti et al., 2006). The heteromeric nAChRs in rat pineal gland are virtually all the α3β4 subtype (Hernandez et al., 2004). The human pineal gland appears to contain the α3β4 nAChR subtype predominantly, but also one that includes the β2 subunit (unpublished data). Therefore, to measure binding competition at the α3β4 subtype alone in human pineal gland, 10 nM sazetidine-A was included in the assay to block binding to essentially all β2-containing nAChRs. Under these conditions, AT-1001 competition curves for [3H]EB binding sites in the human pineal fit best to a model for one class of binding site consistent with α3β4 nAChRs.
To investigate the type of binding inhibition exerted by AT-1001, [3H]EB binding saturation assays in membranes from HEK cells expressing α3β4 and α4β2 nAChRs were compared in the absence or presence of several concentrations of AT-1001. Cell membrane homogenates were incubated for 4 hours with increasing concentrations of [3H]EB (from 0.5 pM to 3 nM for rat receptors or up to 20 nM for human receptors). The homogenates were then filtered through Whatman GF/C filters and counted as above. Nonspecific binding of [3H]EB was determined in the presence of 300 μM (−)nicotine, and specific binding was defined as the difference between total and nonspecific binding. Free ligand concentrations were adjusted for amount bound, and the receptor densities (Bmax) and dissociation constants (Kd) were then determined using nonlinear least-square regression analysis in GraphPad Prism 5.
Upregulation of Nicotinic Receptors.
The effect of chronic exposure to AT-1001 on nAChR density was examined by culturing cells for 3 days in media containing either 0, 0.3, or 10 μM AT-1001 or, for comparison, 10 μM (−)nicotine. After 3 days, when the cells were 90–100% confluent, they were collected, homogenized, and washed, as described above, except that the cell membranes were incubated at 37°C for 30 minutes after each homogenization and then centrifuged at 33,000g and resuspended in buffer. This procedure was repeated five times to reduce the possibility of any AT-1001 remaining in the membranes. The cell homogenates were then resuspended, and receptor binding site density was measured with a single, saturating concentration (∼3 nM) of [3H]EB.
Electrophysiology.
Nicotinic receptor function was evaluated in HEK cell lines in the whole cell voltage-clamp configuration using an Axopatch 200B amplifier (Axon Instruments, Burlingame, CA). Cells plated on a glass coverslip were placed in the recording chamber of a microfluidic chip (Dynaflow Resolve, Cellectricon, Sweden) containing extracellular solution comprised of (in millimolar): 130 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 dextrose, and 10 HEPES. Voltage-clamp recordings (Vhold = −70 mV) were made with patch electrodes (5–8 MΩ) with internal solution (pH 7.2) comprised of the following (in millimolar): 145 K-gluconate, 5 EGTA, 5 MgCl2, 10 HEPES, 5 ATP.Na, and 0.2 GTP.Na. Series resistance was typically <10 MΩ and was not compensated but was continuously monitored with a hyperpolarizing 5-mV pulse. Generation of voltage-clamp protocols and acquisition of data were carried out using pCLAMP 10 software (Axon Instruments, Inc.). Sampling frequency was 20 kHz and current signals were filtered at 5 kHz before digitization and storage. All experiments were performed at room temperature (23–25°C).
Drug Application.
In conjunction with whole cell voltage-clamp recording, drugs were applied to cells via a microfluidic laminar stream solution exchange system (MLSSE; Dynaflow Resolve) as described previously (Fedorov et al., 2012). Briefly, after establishing a steady-state laminar flow, a drug application protocol was initiated, which consisted of exposing a single voltage-clamped cell to various drugs at the indicated concentrations (200-millisecond drug exposure; 1-minute interstimulus interval). Peak elicited currents in each individual cell were normalized to the peak current elicited by 1 mM ACh in the same cell. This concentration of ACh reliably stimulates maximum currents in these cells.
Rubidium Efflux Assays.
The effect of AT-1001 on nAChR function was also examined by assessing 86Rb+ efflux through the receptor channel, as described previously (Xiao et al., 1998; Meyer et al., 2001). Cells were first loaded with 86RbCl by incubating them for 2–4 hour with 0.5 ml media containing ∼100,000 dpm 86Rb+. To test agonist activity of AT-1001, the cells were rinsed gently four times with 1 ml buffer over 10 minutes, and then either buffer alone or buffer containing 100 μM nicotine or different concentrations of AT-1001 was added for 2 minutes. The background efflux was determined in the cells that received buffer alone, whereas maximal response was defined as the efflux elicited by 100 μM nicotine. The 86Rb+ efflux was assessed using Cerenkov counting on a Beckman-Coulter LS6500 Scintillation Counter. After subtracting background efflux, AT-1001 stimulation was expressed as the percent of efflux elicited by 100 μM nicotine, which stimulates maximum 86Rb+ efflux in these cells.
To examine concentration-dependent desensitization, cells were loaded with 86Rb+, rinsed gently three times over ∼2 minutes with 1 ml buffer each time, and then treated for 10 minutes with different concentrations of AT-1001 in 0.5 ml buffer. After this 10-minute treatment, the drug-containing buffer was aspirated, and the cells were immediately stimulated with 100 µM nicotine in 0.5 ml buffer for 2 minutes. For analysis, nicotine-stimulated 86Rb+ efflux in cells exposed to AT-1001 was compared with stimulated 86Rb+ efflux in cells not exposed to AT-1001, which was set as 100%.
Results
Binding of AT-1001 to Human and Rat α3β4 and α4β2 nAChRs Expressed in HEK293 Cells.
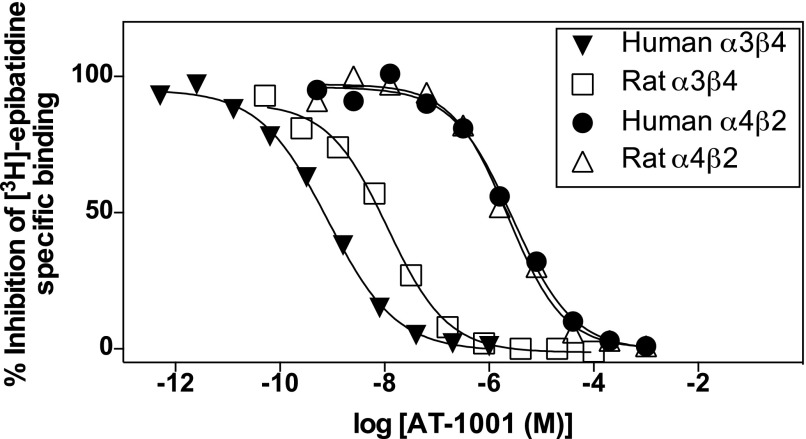
The binding affinities of AT-1001 were examined in membrane homogenates from four separate HEK293 cell lines expressing either human or rat α3β4 nAChRs or human or rat α4β2 nAChRs. AT-1001 competed effectively with [3H]EB for binding at all of the nAChR subtypes tested. Figure 1 shows the competition curves from a representative experiment, and the results of five such binding studies are summarized in Table 1. As shown previously (Toll et al., 2012), AT-1001 competes with much higher affinity for α3β4 than for α4β2 nAChRs. Importantly, however, its affinity for the human α3β4 receptor expressed in these cells is approximately 20-fold higher than its affinity for the rat α3β4 receptor (P < 0.001), whereas its affinities for the human and rat α4β2 nAChRs are similar. Consequently, the relative affinity (selectivity ratio) of AT-1001, calculated as the ratio of its Ki for α4β2 nAChRs over its Ki for α3β4 nAChRs, is ∼40 for the rat receptors but more than 980 for the human receptors.
Fig. 1.
Representative competition binding curves of AT-1001 competing against 0.4 nM [3H]EB at human and rat α3β4 nAChRs and human and rat α4β2 nAChRs heterologously expressed in HEK cells. See Table 1 for Ki values and selectivity ratios from five similar studies.
TABLE 1.
The binding affinities of AT-1001 at human and rat α3β4 and α4β2 nAChRs expressed in HEK293 cells determined by competition binding with [3H]epibatidine
The Ki values were calculated from binding competition curves such as those shown in Fig. 1, using the Cheng-Prusoff equation and the Kd of [3H]epibatidine at each receptor. The selectivity ratio is the ratio of Ki α4β2/Ki α3β4 for each species. Values represent the mean ± standard error of 5 experiments.
| Ki | Selectivity Ratio (Ki α4β2/Ki α3β4) | |
|---|---|---|
| nM | ||
| Human α3β4 | 0.092 ± 0.008 | 989 |
| Human α4β2 | 91 ± 8 | |
| Rat α3β4 | 1.9 ± 0.1*** | 41 |
| Rat α4β2 | 78 ± 10 |
The Ki value of AT-1001 at the rat α3β4 nAChR is significantly higher than at the human α3β4 receptor (P < 0.001), whereas the Ki values at the α4β2 nAChRs are not significantly different from each other.
Binding of AT-1001 to Human and Rat α3β4 and α4β2 nAChRs in Native Tissues.
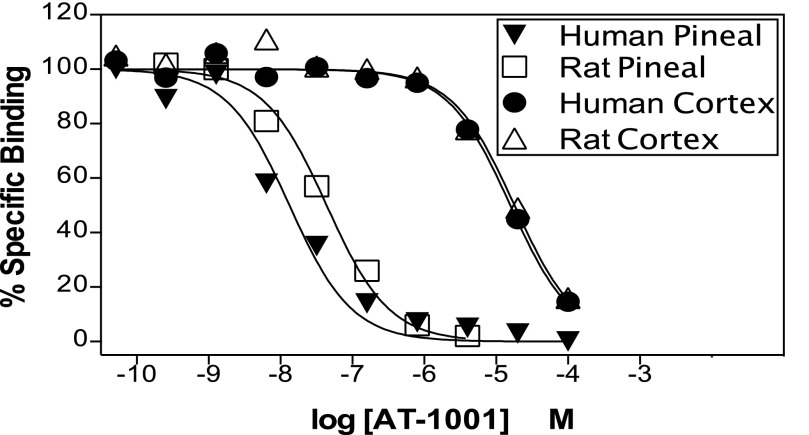
To determine how closely AT-1001 binding in these heterologously expressed rat and human α3β4 and α4β2 nAChRs corresponds to its binding in native tissues, we examined AT-1001 competition for receptors in rat and human pineal gland, which in the rat are exclusively and in the human predominantly α3β4 receptors, and in rat and human cerebral cortex, which are predominantly α4β2 nAChRs in both species. As shown in Fig. 2 and summarized in Table 2, consistent with its higher affinity for α3β4 nAChRs, AT-1001 competes with much higher affinity for nAChRs in rat and human pineal gland than for the receptors in rat and human cortex. Interestingly, the affinities of AT-1001 in these native tissues were 8- to 4-fold lower than the affinities found in the HEK cells heterologously expressing the corresponding nAChRs (compare Tables 1 and 2). This might be because of differences such as lipids or accessory proteins in the cell types, or to differences in the stoichiometry of the receptors expressed in HEK cells versus the native cells. Nevertheless, as shown in Table 2, the relative selectivity ratio between human pineal (α3β4) and human cortex (α4β2) and rat pineal and rat cortex were similar to the selectivity ratios measured in the heterologously expressed receptors.
Fig. 2.
Representative competition binding curves of AT-1001 competing against 0.4 nM [3H]EB at nAChRs in human and rat pineal gland and in human and rat cerebral cortex. See Table 2 for Ki values and selectivity ratios from 2 to 3 similar studies.
TABLE 2.
Binding affinities of AT-1001 at human and rat pineal gland (predominantly α3β4 nAChRs) and human and rat cerebral cortex (predominantly α4β2 nAChRs) determined by competition binding with [3H]epibatidine
The Ki values were calculated from the binding competition curves such as those shown in Fig. 2, using the Cheng-Prusoff equation and the Kd of [3H]epibatidine at each receptor. The selectivity ratio is the ratio of Ki cortex/Ki pineal for each species. Values are the mean ± standard error of 2 independent assays for pineal and 3 for cortex.
| Ki | Selectivity Ratio (Ki cortex/Ki pineal) | |
|---|---|---|
| nM | ||
| Human pineal | 1.2 ± 0.6 | 917 |
| Human cortex | 1100 ± 300 | |
| Rat pineal | 16 ± 1** | 69 |
| Rat cortex | 1100 ± 90 |
The Ki value of AT-1001 at the rat pineal nAChR is significantly higher than at the human pineal nAChR (P < 0.01), whereas the Ki values at the human and rat cortex nAChRs do not differ.
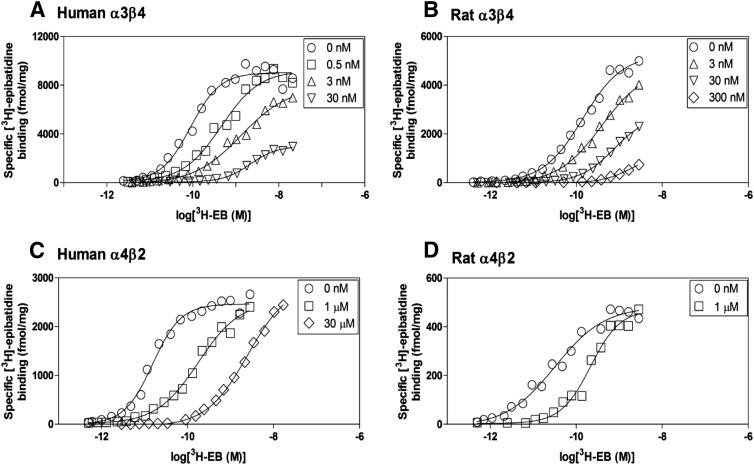
To determine the type of mechanism by which AT-1001 inhibits binding at nAChRs, [3H]EB binding saturation curves were compared in the absence and presence of AT-1001. To account for its higher affinity at the human α3β4 receptor, we extended the concentration of [3H]EB used for the human receptors. As shown in Fig. 3, A and B, at both human and rat α3β4 nAChRs, low concentrations of AT-1001 shifted the [3H]EB saturation curves to the right, consistent with simple competitive inhibition; but at higher concentrations, AT-1001 appeared to also decrease the Bmax of the saturation curves, which is usually indicative of a noncompetitive mechanism. For example, at the highest AT-1001 concentration used here, the Bmax of the human α3β4 nAChR was decreased to 28 ± 3% of the control Bmax and it was decreased to about 20% at the rat α3β4 nAChR. Even when the incubation time for the [3H]EB binding assays in the presence of 30 nM AT-1001 was extended to 24 hours to ensure that equilibrium would be reached, the saturation curves were again shifted to the right, but the full Bmax was still decreased (unpublished data). A possible explanation for the apparent decrease in Bmax of the α3β4 nAChRs is related to the high affinity of AT-1001, which is similar to that of [3H]EB at these receptors; thus, even at the highest concentration, AT-1001 is probably acting competitively, but at these high concentrations it may simply out-compete [3H]EB for rebinding to any unoccupied receptors, resulting in the appearance of a noncompetitive mechanism.
Fig. 3.
Effects of AT-1001 on [3H]epibatidine binding saturation curves. [3H]epibatidine saturation binding was measured in the absence and presence of AT-1001 in human α3β4 (A), rat α3β4 (B), human α4β2 (C), and rat α4β2 (D) nAChRs with a 4-hour incubation. These saturation curves are representative of 3–5 experiments.
In contrast to its apparently complex binding profile at α3β4 nAChRs, at the α4β2 nAChRs, where it has much lower affinity (and [3H]EB has higher affinity), AT-1001 shifted the [3H]EB saturation curves to the right without altering the Bmax (Fig. 3, C and D). This right shift, consistent with simple competitive inhibition, was found even in the presence of 30 μM AT-1001, a concentration used to achieve the receptor occupancy at α4β2 nAChRs that would approximate the occupancy by the 30 nM concentration at human α3β4 nAChRs, which have nearly 1000-fold higher affinity.
Upregulation of nAChRs by AT-1001.
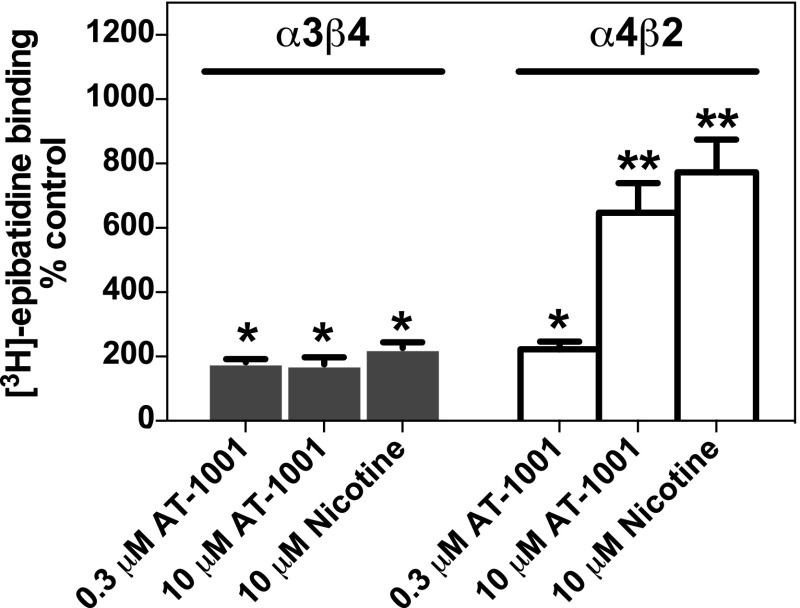
Chronic administration of most nicotinic agonists and partial agonists in vivo upregulate α4β2 nAChRs in brain (Schwartz and Kellar; 1983; Marks et al., 1983, 2011; Flores et al., 1992; Mao et al., 2008). In contrast, the α3β4* receptors found in autonomic ganglia, adrenal gland, retina, and pineal gland are not upregulated by chronic nicotine (Flores et al., 1997; Dávila-García et al., 2003). However, in cell lines that heterologously express various nAChR subunit combinations, α3β4 nAChRs as well as all other putative nAChR subtypes tested are increased by exposure for 1 to 5 days to several nicotinic ligands that bind to the agonist binding site (Wang et al., 1998; Meyer et al., 2001; Xiao and Kellar, 2004). To determine if AT-1001 also upregulates heterologously expressed nAChRs, we measured [3H]EB binding to human α3β4 and α4β2 nAChRs from cells treated for 3 days with 0.3 or 10 μM AT-1001, concentrations that would be expected to occupy virtually all α3β4 nAChRs and 77 or 99% of the α4β2 nAChRs. These treatments upregulated both the α3β4 and α4β2 nAChRs (Fig. 4). The α3β4 receptors were increased approximately 2-fold by AT-1001 at both the 0.3 and 10 μM concentrations, similar to the increase induced by 10 μM nicotine. The α4β2 nAChRs, which are more responsive than α3β4 receptors to upregulation by agonists, were increased 2-fold by the 0.3 μM concentration of AT-1001 but 6-fold by the 10 μM concentration. The increase in α4β2 nAChRs induced by 10 μM AT-1001 was similar to that induced by 10 μM nicotine (Fig. 4).
Fig. 4.
AT-1001 induces upregulation of human α3β4 and human α4β2 nAChRs. Cells were incubated for 3 days with either 0.3 or 10 μM AT-1001 or 10 μM nicotine before membranes were prepared and nAChRs measured with 3 nM [3H]EB. Each bar represents the mean ± S.E.M. from 3 to 5 experiments. Significantly different from control cells incubated without added drug: *P < 0.05; **P < 0.01.
Agonist Activity of AT-1001.
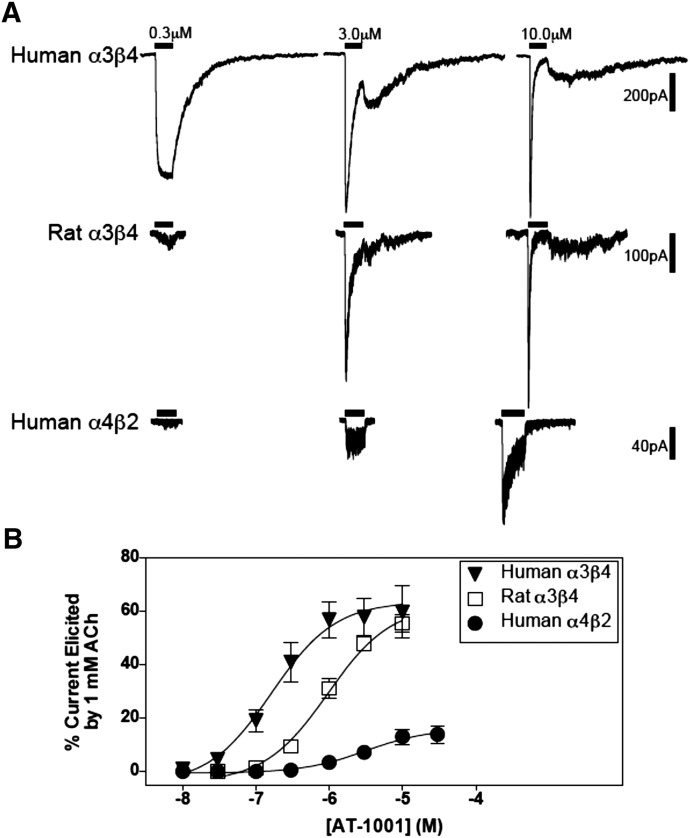
Agonist activity of AT-1001 at nAChRs was determined in patch-clamp electrophysiology studies and 86Rb+ efflux studies. Examples of the response traces elicited by AT-1001 in patch-clamp experiments with human and rat α3β4 and human α4β2 receptors are shown in Fig. 5A. AT-1001 concentration-response curves for these three receptors are shown in Fig. 5B and summarized in Table 3. The responses elicited by AT-1001 were normalized to the response of each cell to 1 mM ACh, which reliably elicited maximum responses in these cells. AT-1001 demonstrated 65–70% partial agonist activity at the two α3β4 nAChRs and 18% agonist activity at the human α4β2 receptor (see legend to Fig. 5 for the maximal current responses for each of these receptors). It also showed partial agonist activity at rat α4β2 receptors, but because these receptors have a low maximal response to 1 mM ACh (Emax = 48 ± 7 pA, n = 3) and an even lower response to AT-1001 (Emax 7.2 ± 5 pA), we could not determine a reliable EC50 value for AT-1001 at this rat receptor.
Fig. 5.
AT-1001 is a partial agonist at human α3β4, rat α3β4, and human α4β2 nAChRs as assessed with patch-clamp electrophysiology. (A) Representative whole cell current traces elicited by AT-1001 from human and rat α3β4 and human α4β2 nAChRs expressed in HEK cells (the horizontal bar = 1-second drug application). (B) Concentration-response curves of AT-1001 stimulated currents in the three nAChRs. The points in the curves were normalized to 1 mM ACh and are the mean ± S.E.M. of 6–9 experiments. The maximal responses to 1 mM ACh for human and rat α3β4 and human α4β2 nAChRs were, respectively: 1142 ± 246, 1648 ± 382, and 312 ± 129 pA. See Table 3 for the EC50 values. (The maximal response of rat α4β2 receptors to 1 mM ACh was 48 ± 7 pA, and the response to AT-1001 was only 7.2 pA, which was considered too small for a reliable calculation of an EC50 value for AT-1001.).
TABLE 3.
Functional parameters of AT-1001 at expressed human and rat α3β4 nAChRs and human α4β2 nAChRs measured in patch clamp and 86Rb+-efflux studies
| Patch-Clamp Electrophysiology |
86Rb+ Efflux |
|||||
|---|---|---|---|---|---|---|
| EC50 | Emax | Hill slope | EC50 | Emax | Hill slope | |
| μM | % | μM | % | |||
| Human α3β4 | 0.4 ± 0.1** | 70 ± 6** | 1.8 ± 0.2* | 0.1 ± .003** | 59 ± 0.3** | 2.0 ± 0.2* |
| Rat α3β4 | 1.4 ± 0.2** | 65 ± 4** | 1.8 ± 0.1* | 1.5 ± 0.3 | 36 ± 3 | 2.3 ± 0.4* |
| Human α4β2 | 4.3 ± 1.2** | 18 ± 3 | 1.3 ± 0.1* | N.D. | N.D. | N.D. |
| Rat α4β2 | N.D. | 16 ± 9 | N.D. | N.D. | N.D. | N.D. |
N.D., not determined because responses to AT-1001 at rat α4β2 receptors in patch clamp studies and at both rat and human α4β2 receptors in 86Rb+-efflux studies were too small to calculate a reliable EC50.
In patch clamp studies, the EC50 values are statistically different from one another (P < 0.01); and the Emax values for both human and rat α3β4 nAChRs are statistically greater than the values for the human α4β2 receptors (P < 0.01). In the 86Rb+-efflux studies, both the EC50 and the Emax values for human α3β4 receptors are statistically different from the rat receptors (P < 0.01).
The Hill coefficients are statistically greater than 1 (P < 0.05).
As shown in Table 3, of the three receptors at which we were able to calculate EC50 values in these patch-clamp studies, AT-1001 has the highest potency at human α3β4 receptors (EC50 = 0.4 μM); its next highest potency is at rat α3β4 (EC50 = 1.4 μM), and its lowest potency is at human α4β2 receptors (EC50 = 4.3 μM). Thus, the agonist potency of AT-1001 is 3 times higher at human α3β4 nAChRs than at the rat version, and it is ∼10 times higher than at human α4β2 nAChRs.
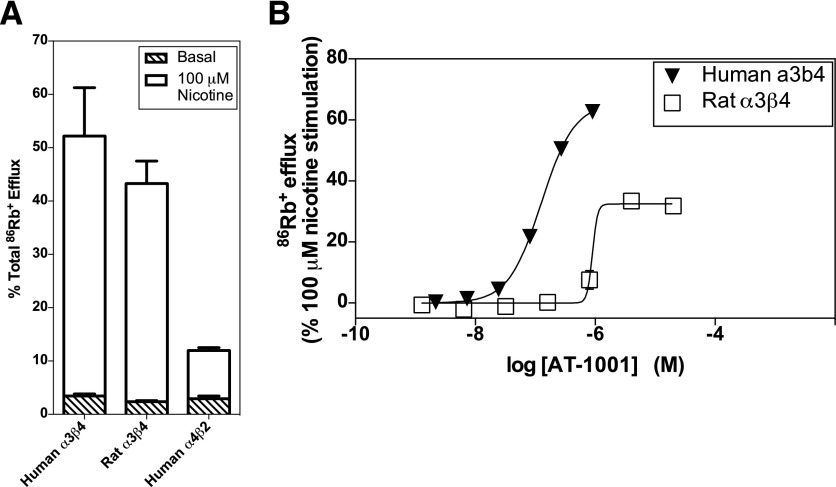
Responses elicited by AT-1001 in 86Rb+ efflux studies were normalized to the responses elicited by 100 μM nicotine; these nicotine-stimulated responses are shown in Fig. 6A. Consistent with the electrophysiological measurements, AT-1001 demonstrated partial agonist activity in these 86Rb+ efflux measurements at both human and rat α3β4 receptors (Fig. 6B). Although nicotine reliably induces 86Rb+ efflux via the human α4β2 nAChR channels (see Fig. 6A), AT-1001 did not induce a measurable response in these receptors with this assay. Neither nicotine nor AT-1001 elicited responses at the rat α4β2 receptor in this cell line. The EC50 and Emax values for the human and rat α3β4 receptors calculated from these 86Rb+ efflux studies are summarized in Table 3. Consistent with the electrophysiological studies, AT-1001 demonstrated significantly higher potency at the human α3β4 nAChR than at the rat receptor (P < 0.01). In these 86Rb+ efflux assays, the Emax value for the human receptor was significantly greater than for the rat receptor (P < 0.01).
Fig. 6.
AT-1001 stimulates 86Rb+ efflux at human and rat α3β4 nAChRs. (A) Nicotine-stimulated 86Rb+ efflux at human α3β4, rat α3β4 and human α4β2 nAChRs. Efflux was measured in the absence (basal efflux) and presence (stimulated efflux) of 100 μM nicotine. Values are the mean ± S.E.M. of 3 or 4 independent experiments. (B) Partial agonist response to AT-1001 at human and rat α3β4 nAChRs measured in the 86Rb+ efflux assay. Although nicotine reliably stimulates 86Rb+ efflux at human α4β2 nAChRs (see A), AT-1001 did not elicit a measurable response at these receptors in this assay. Curves are representative of 3 or 4 independent experiments. See Table 3 for EC50 and Emax values.
Desensitization.
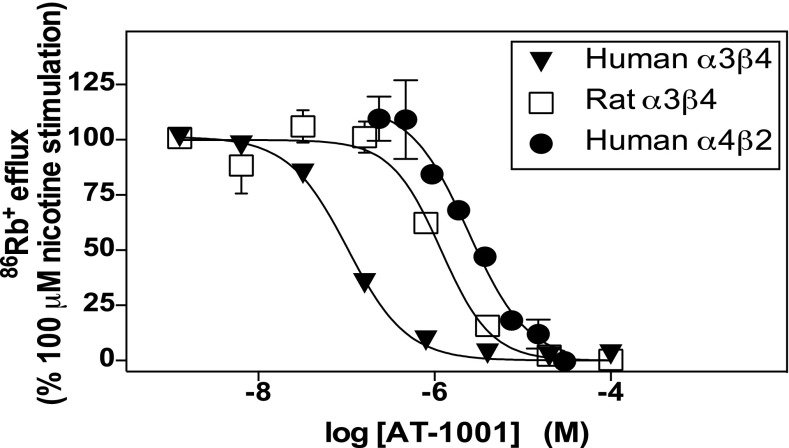
Nicotinic agonists and partial agonists induce nAChR desensitization, which appears to depend primarily on a ligand’s occupancy of the agonist binding site rather than its efficacy at the receptor. The potencies of AT-1001 to desensitize human and rat α3β4 nAChRs and human α4β2 nAChRs were determined using the 86Rb+ efflux assay, which we find is a more suitable method than patch clamp for assessment of desensitization because it measures the responses in a large population of cells. Thus, nicotine-stimulated 86Rb+ efflux was measured immediately after a 10-minute exposure to different concentrations of AT-1001. The 10-minute exposure to the highest concentrations of AT-1001 fully desensitized all three nAChRs tested, but the desensitization potency of AT-1001 varied across the receptors (Fig. 7; Table 4). The concentration that decreased the nicotine-stimulated 86Rb+-efflux response by 50% is referred to here as the DC50.
Fig. 7.
AT-1001 potently desensitizes human and rat α3β4 and human α4β2 nAChRs. Desensitization was determined in 86Rb+-efflux measurements after a 10-minute exposure to AT-1001 at the concentrations shown. Values are the mean ± S.E.M. from 3 or 4 independent measurements. Where no S.E.M. bar is evident, it is within the symbol. See Table 4 for the calculated parameters from these curves.
TABLE 4.
Desensitization parameters of AT-1001 at α3β4 and α4β2 nAChRs as determined in 86Rb+-efflux assays
Desensitization of the receptor responses to 100 μM nicotine was determined after exposing cells to AT-1001 for 10 minutes at concentrations of 1 nM to 100 μM (see Fig. 7). The DC50 is the concentration of AT-1001 that decreases the response to nicotine by 50%.
| DC50 | Hill Slope | |
|---|---|---|
| μM | ||
| Human α3β4 | 0.11 ± 0.01** | 1.4 ± 0.05** |
| Rat α3β4 | 1.2 ± 0.1** | 1.5 ± 0.2 |
| Human α4β2 | 3.3 ± 0.4** | 1.6 ± 0.1** |
The differences between the DC50 values among the three receptors are all statistically significant, (P < 0.01). The Hill coefficients for human α3β4 and α4β2 receptors are greater than 1 (P < 0.01). The Hill coefficient for rat α3β4 was not statistically different from 1, but trended in that direction (P < 0.1).
The order of potency for desensitization of these three receptors followed the same order of potency for activating the receptors and as the affinity for binding; but in the studies that allowed accurate assessments of the EC50 values (all three receptors in patch-clamp studies and two of the three receptors in 86Rb+ efflux assays), the potencies to desensitize the receptors were closer to the potencies to activate than to the affinities for the agonist binding sites. Thus, under these conditions, the DC50 concentrations of AT-1001 were similar to its EC50 for activation at each of these receptors; whereas in contrast, these DC50 concentrations of AT-1001 were 1200 to 600 times higher than its binding Ki values at the human and rat α3β4 receptors, respectively, and 36 times higher than the Ki at the human α4β2 receptor. It should be noted, however, that the similarities between the potencies to desensitize and to activate may, at least in part, reflect the similar incubation times under which the measurements were made, i.e., seconds and minutes for activation and desensitization, compared with hours for equilibrium binding.
As shown in Table 4, the Hill slopes for these desensitization measurements at the human α3β4 and α4β2 receptors were statistically greater than 1 (P < 0.01), whereas measurement at the rat α3β4 receptors trended in that direction (P < 0.1). This suggests that desensitization involves more than simple receptor occupancy by a ligand at a single site; it could, for example, indicate that two or more binding sites have to be occupied to induce full desensitization and that there is a cooperative step in the process.
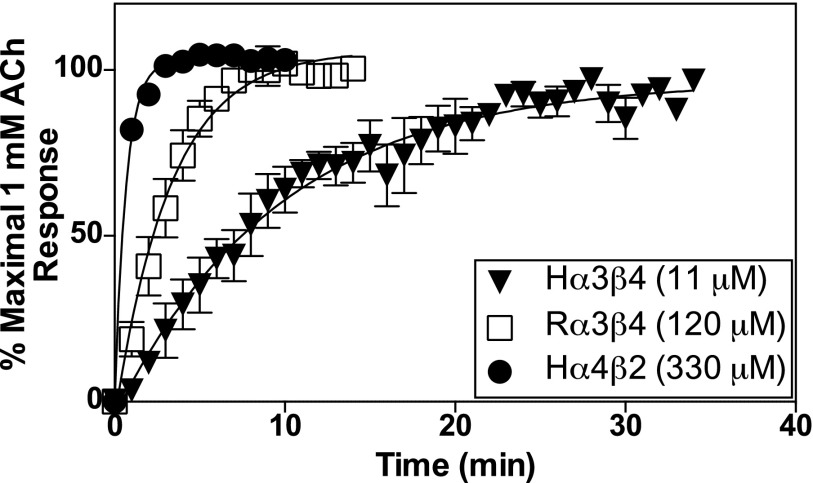
AT-1001 at high concentrations completely desensitizes all of these nAChRs (Fig. 7). To determine the rates of recovery from AT-1001–induced desensitization for each receptor subtype, we measured responses to ACh using the patch-clamp method because it allows a high degree of temporal resolution. Responses to 1 mM ACh in cells expressing human or rat α3β4 or human α4β2 receptors were recorded, and the cells were then exposed for 10 minutes to AT-1001 at a concentration equal to 100 times its DC50. After a rapid wash in buffer, the cells were exposed to a rapid pulse of 1 mM ACh each minute followed by a wash in buffer to observe the recovery of function for up to ∼35 minutes. As shown in Fig. 8, the human α4β2 receptors display the fastest rate of recovery, with a half-time for recovery of 0.68 minute; the rat α3β4 receptors recovered next fastest, with a half-time of 3.3 minutes, and the human α3β4 receptors displayed the slowest rate of recovery, with a half-time of 9.7 minutes. These results suggest that the rate of recovery from desensitization after exposure to AT-1001 is a function of the binding affinity of the ligand—that is, directly related to its dissociation rate.
Fig. 8.
Recovery of function of nAChRs measured by patch-clamp electrophysiology after desensitization. Currents stimulated by 1 mM ACh were measured each minute after a 10-minute exposure to AT-1001 at a concentration 100 times the DC50 of the respective receptor (shown in the figure inset). Results were normalized to the maximal response to 1 mM ACh in the cells before exposure to AT-1001. Each curve fit to a one-phase exponential model with a t1/2 of 9.7 minutes, 3.3 minutes, and 0.68 minutes for human α3β4, rat α3β4, and human α4β2 receptors, respectively. The points in the curves represent the mean ± S.E.M. of 3–5 experiments.
Discussion
AT-1001 is likely to be an important tool for studying nAChRs because it is the first ligand with high selectivity for α3β4 nAChR binding sites. Nevertheless, in assigning nAChR subtype(s) that mediate the effects of AT-1001, especially its effects in vivo, it is important to have as complete a pharmacological profile as possible.
Our results confirm earlier studies that AT-1001 binds with high affinity and selectivity to α3β4 nAChRs (Toll et al., 2012) and that it displays partial agonist activity (Zaveri et al., 2015). In HEK cells heterologously expressing the rat versions of the α3β4 and α4β2 nAChR subtypes, we found that AT-1001 displays 41-fold selectivity for the α3β4 subtype, which also is in general agreement with the initial report (Toll et al., 2012). Interestingly, however, in cells expressing the human versions of these nAChR subtypes, AT-1001 exhibits nearly 1000-fold selectivity for the α3β4 subtype. This was due to approximately 20-fold higher affinity of AT-1001 for the human than for the rat α3β4 receptor, while exhibiting similar affinities for the heterologously expressed rat and human α4β2 receptors.
To further explore this difference between rat and human nAChRs, we compared the affinities of AT-1001 in rat and human pineal gland, which under the conditions used here represent nearly exclusively the α3β4 nAChR subtype, and rat and human cerebral cortex, which represent predominantly (≥90%) the α4β2* nAChR subtype. Although the binding affinities of AT-1001 for the heterologously expressed receptors and their corresponding subtypes in native tissues differed, possibly because of different subunit stoichiometries or differences in accessory proteins or lipids between the cells in native tissues and HEK cells, the selectivity of AT-1001 for α3β4 nAChRs was still very clear; in fact, the selectivity of AT-1001 for both human and rat α3β4 over α4β2 nAChRs was similar in the native tissues and in the cell lines.
An iodinated analog of AT-1001, [125I]AT-1012, was recently synthesized and shown to be an excellent radioligand for autoradiography studies of α3β4 nAChRs in rat brain (Wu et al., 2014). If the same difference in affinity between human and rat α3β4 nAChRs holds for [125I]AT-1012, it should be an even better probe for α3β4 nAChRs in human central nervous system and peripheral tissues, where the density of nAChRs is much lower than in these cell lines.
At low concentrations of AT-1001 (up to about 3 nM), there is a clear rightward shift of [3H]EB saturation curves, consistent with a competitive mode of inhibition; but at higher concentrations of AT-1001 (30 and 300 nM), which are approximately 300 times and 150 times its Ki at human and rat α3β4 nAChRs, respectively, there is also an apparent decrease in the Bmax of binding, suggesting a noncompetitive mode of inhibition at the receptor. However, this more likely results from a very slow dissociation rate of AT-1001 at α3β4 nAChRs, which would be consistent with its very high affinity and the high concentrations of AT-1001 out-competing [3H]EB for rebinding to the open receptors. In contrast to its inhibition of [3H]EB binding to α3β4 nAChRs, AT-1001 inhibition of binding to α4β2 nAChRs, where it has a much lower affinity, was consistent with a standard and simple competitive mechanism only.
The initial paper describing AT-1001 pharmacology determined that it was a selective antagonist at rat α3β4 nAChRs (Toll et al., 2012). In contrast, we found that it is a potent and relatively efficacious partial agonist at both human and rat α3β4 nAChRs. Its efficacy relative to ACh was 65–70% in whole cell patch-clamp measurements and 36–59% relative to nicotine in 86Rb+ efflux assays. The difference between the efficacies found in these two types of assays may be related to receptor desensitization, which is more evident during the much longer stimulation period required in the 86Rb+ efflux assay than in patch-clamp measurements (see below). In our patch-clamp measurements of human α3β4 nAChRs expressed in HEK cells, AT-1001 displayed a potency of 0.4 μM, which is nearly identical to the potency found in a recent study in Xenopus oocytes expressing human α3β4 nAChRs (Zaveri et al., 2015). The potency of AT-1001 was 3.5-fold higher at the human than the rat α3β4 receptor in patch-clamp measurements and 15-fold higher in 86Rb+ efflux assays. These species differences in AT-1001 agonist potencies are consistent with, although somewhat lower, than its 21-fold difference in binding affinity at these α3β4 receptors. AT-1001 also displayed partial agonist activity at the human α4β2 nAChR in patch-clamp studies, but its efficacy was only 18% of the ACh response and its potency was 10-times lower than at the human α3β4 receptor.
The differences in binding and function of AT-1001 at the α3β4 and α4β2 nAChRs probably reflect the strong influence of both the α and β subunits on the pharmacology of nAChRs (Luetje and Patrick, 1991; Papke and Heinemann, 1994; Parker et al., 1998), whereas the differences between the human and rat α3β4 nAChRs may result from differences in two adjacent amino acids within the extracellular D loop of the β4 subunit (Young et al., 2007). Several other drugs have now been found to display differences in affinity, potency, and/or efficacy between human and rat α3β4 nAChRs (Young et al., 2007; Zwart et al., 2008; Stokes and Papke, 2012).
AT-1001 has one of the highest affinities for α3β4 nAChRs that has been reported, similar to or only slightly lower than the affinity of epibatidine for these receptors. In contrast, its affinity for the α4β2 subtype is one to three orders of magnitude lower than such ligands as nicotine, cytisine, A-85380 [3-((2S)-azetidinylmethoxy)pyridine dihydrochloride], and epibatidine. Despite this difference in binding affinity at these two receptors, a 3-day incubation of cells with AT-1001 at concentrations designed to occupy nearly all of the α3β4 and most of the α4β2 receptors increased both receptor subtypes. Both the lower (0.3 μM) and higher (10 μM) concentrations of AT-1001 increased human α3β4 nAChRs by about 2-fold, which was similar to the increase induced by 10 μM nicotine measured here, as well as in rat α3β4 receptors expressed in HEK cells in previous studies (Meyer et al., 2001; Xiao and Kellar, 2004). In contrast, although incubation with 0.3 μM AT-1001 increased human α4β2 nAChRs approximately 2-fold, incubation with 10 μM AT-1001 increased these receptors approximately 6-fold, which was similar to the increase induced by 10 μM nicotine. Thus, despite its much higher affinity for α3β4 nAChRs, AT-1001 can clearly affect the regulation of α4β2 receptors as well. These results are consistent with the idea that occupancy of the receptor’s orthosteric binding site, independent of channel function, is the trigger for upregulation in these cells. However, because there appear to be differences between the mechanisms for upregulation of nAChRs in heterologous systems and neurons (Lomazzo et al., 2011), it is not known whether AT-1001 would upregulate either α3β4 or α4β2 nAChRs in vivo.
Nicotinic agonists desensitize nAChRs. Moreover, nicotine is usually 10 to 40 times more potent to desensitize than to activate nAChRs, both in vivo (Sharp and Beyer, 1986; Hulihan-Giblin et al., 1990) and in vitro (Grady et al., 1994; Marks et al., 1994; Lester and Dani, 1995; Meyer et al., 2001). In contrast, the potencies of AT-1001 to desensitize both α3β4 and α4β2 nAChRs are similar to its potencies to activate these receptors. Although this apparent difference between AT-1001 and other nicotinic ligands might be due to the time frame or other conditions of the measurements, it also suggests that the mechanisms triggering or underlying nAChR desensitization might differ among ligands. The Hill slopes for desensitization of human α3β4 and α4β2 nAChRs were significantly greater than 1, and that for rat α3β4 nAChRs trended in that direction, suggesting that desensitization might involve more than one step and include a cooperative process, such as binding of the ligand to a second orthosteric site or, less likely, to an allosteric site.
Although the DC50 values of AT-1001 for desensitization by AT-1001 are similar to its EC50 values for activation at each of the three receptors, the time to recovery from desensitization follows the reverse order of its binding affinity; thus, the receptor with the highest affinity recovers slowest and the receptor with the lowest affinity recovers fastest. This is consistent with the time to recovery from desensitization being dependent on the dissociation of the ligand from the receptor. The α4β2 nAChR subtype is usually found to exhibit the longest recovery times after desensitization, and our data here suggest this is probably a function of the slower dissociation rates of most nicotinic ligands, which usually have highest affinity at α4β2 nAChRs. AT-1001 is clearly an exception.
In conclusion, we examined the binding affinities and functional effects of AT-1001 at human and rat α3β4 and α4β2 nAChRs, as well as its effects on the regulation of these receptors. We found AT-1001 to be a 65–70% partial agonist at α3β4 nAChRs, with higher binding affinity and potency at the human than the rat version of these receptors. Moreover, it is highly selective in its binding affinity (989-fold), its functional potency (10-fold), and its desensitization potency (30-fold) for human α3β4 over α4β2 nAChRs.
Acknowledgments
The authors thank Drs. Stefano Vicini and Ruixi Luo for helpful discussions about the agonist activity of AT-1001 and Teresa Xie for her skillful help with 86Rb+ efflux assays. The authors also thank the families who consented to donate the human brain tissues and pineal glands used in these studies and to be interviewed. We gratefully acknowledge the support of The Cuyahoga County Medical Examiner’s Office, Cleveland, OH. The assistance of Drs. James C. Overholser and George Jurjus and of Lesa Dieter in the psychiatric assessments is also acknowledged.
Abbreviations
- A-85380
3-((2S)-azetidinylmethoxy)pyridine dihydrochloride
- AT-1001
N-(2-bromophenyl)-9-methyl-9-azabicyclo[3.3.1] nonan-3-amine
- [3H]EB
[3H]epibatidine
- nAChR
nicotinic acetylcholine receptor
Authorship Contributions
Participated in research design: Tuan, Wolfe, Sahibzada, Xiao, Kellar.
Conducted experiments: Tuan, Olsen, Al-Muhtasib, Bowman Dalley, Lewin.
Contributed new reagents or analytic tools: Horti, Gao, Stockmeier.
Performed data analysis: Tuan, Wolfe, Sahibzada, Kellar.
Wrote or contributed to the writing of the manuscript: Tuan, Wolfe, Sahibzada, Kellar.
Footnotes
This work was supported by the National Institutes of Health National Institute of Drug Abuse [Grants R01-DA012976, U19-DA027990] and the National Institutes of Health National Institute of General Medical Sciences to the COBRE Center for Psychiatric Neuroscience (P30GM103328).
References
- Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L, et al. (2007) Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet 16:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, et al. (2008) Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 165:1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. (2008) Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry 13:368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108. [DOI] [PubMed] [Google Scholar]
- Cobb JA, Simpson J, Mahajan GJ, Overholser JC, Jurjus GJ, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA. (2013) Hippocampal volume and total cell numbers in major depressive disorder. J Psychiatr Res 47:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dávila-García MI, Musachio JL, Kellar KJ. (2003) Chronic nicotine administration does not increase nicotinic receptors labeled by [125I]epibatidine in adrenal gland, superior cervical ganglia, pineal or retina. J Neurochem 85:1237–1246. [DOI] [PubMed] [Google Scholar]
- Fedorov N, Benson L, Graef JD, Hyman J, Sollenberger J, Pettersson F, Lippiello PM, Bencherif M. (2012) A method for bidirectional solution exchange--“liquid bullet” applications of acetylcholine to α7 nicotinic receptors. J Neurosci Methods 206:23–33. [DOI] [PubMed] [Google Scholar]
- Flores CM, Dávila-García MI, Ulrich YM, Kellar KJ. (1997) Differential regulation of neuronal nicotinic receptor binding sites following chronic nicotine administration. J Neurochem 69:2216–2219. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. (1992) A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol 41:31–37. [PubMed] [Google Scholar]
- Glick SD, Sell EM, McCallum SE, Maisonneuve IM. (2011) Brain regions mediating α3β4 nicotinic antagonist effects of 18-MC on nicotine self-administration. Eur J Pharmacol 669:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. (2006) Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 27:482–491. [DOI] [PubMed] [Google Scholar]
- Grady SR, Marks MJ, Collins AC. (1994) Desensitization of nicotine-stimulated [3H]dopamine release from mouse striatal synaptosomes. J Neurochem 62:1390–1398. [DOI] [PubMed] [Google Scholar]
- Hernandez SC, Vicini S, Xiao Y, Dávila-García MI, Yasuda RP, Wolfe BB, Kellar KJ. (2004) The nicotinic receptor in the rat pineal gland is an α3β4 subtype. Mol Pharmacol 66:978–987. [DOI] [PubMed] [Google Scholar]
- Hulihan-Giblin BA, Lumpkin MD, Kellar KJ. (1990) Acute effects of nicotine on prolactin release in the rat: agonist and antagonist effects of a single injection of nicotine. J Pharmacol Exp Ther 252:15–20. [PubMed] [Google Scholar]
- Jackson KJ, Sanjakdar SS, Muldoon PP, McIntosh JM, Damaj MI. (2013) The α3β4* nicotinic acetylcholine receptor subtype mediates nicotine reward and physical nicotine withdrawal signs independently of the α5 subunit in the mouse. Neuropharmacology 70:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RA, Dani JA. (1995) Acetylcholine receptor desensitization induced by nicotine in rat medial habenula neurons. J Neurophysiol 74:195–206. [DOI] [PubMed] [Google Scholar]
- Lomazzo E, Hussmann GP, Wolfe BB, Yasuda RP, Perry DC, Kellar KJ. (2011) Effects of chronic nicotine on heteromeric neuronal nicotinic receptors in rat primary cultured neurons. J Neurochem 119:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetje CW, Patrick J. (1991) Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci 11:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. (2008) The α4β2α5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem 104:446–456. [DOI] [PubMed] [Google Scholar]
- Mao D, Yasuda RP, Fan H, Wolfe BB, Kellar KJ. (2006) Heterogeneity of nicotinic cholinergic receptors in rat superior cervical and nodose Ganglia. Mol Pharmacol 70:1693–1699. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. (1983) Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther 226:817–825. [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Yang JM, Lippiello PM, Collins AC. (1994) Desensitization of nicotine-stimulated 86Rb+ efflux from mouse brain synaptosomes. J Neurochem 63:2125–2135. [DOI] [PubMed] [Google Scholar]
- Marks MJ, McClure-Begley TD, Whiteaker P, Salminen O, Brown RW, Cooper J, Collins AC, Lindstrom JM. (2011) Increased nicotinic acetylcholine receptor protein underlies chronic nicotine-induced up-regulation of nicotinic agonist binding sites in mouse brain. J Pharmacol Exp Ther 337:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum SE, Cowe MA, Lewis SW, Glick SD. (2012) α3β4 nicotinic acetylcholine receptors in the medial habenula modulate the mesolimbic dopaminergic response to acute nicotine in vivo. Neuropharmacology 63:434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EL, Xiao Y, Kellar KJ. (2001) Agonist regulation of rat alpha 3 beta 4 nicotinic acetylcholine receptors stably expressed in human embryonic kidney 293 cells. Mol Pharmacol 60:568–576. [PubMed] [Google Scholar]
- Muldoon PP, Jackson KJ, Perez E, Harenza JL, Molas S, Rais B, Anwar H, Zaveri NT, Maldonado R, Maskos U, et al. (2014) The α3β4* nicotinic ACh receptor subtype mediates physical dependence to morphine: mouse and human studies. Br J Pharmacol 171:3845–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Heinemann SF. (1994) Partial agonist properties of cytisine on neuronal nicotinic receptors containing the beta 2 subunit. Mol Pharmacol 45:142–149. [PubMed] [Google Scholar]
- Parker MJ, Beck A, Luetje CW. (1998) Neuronal nicotinic receptor beta2 and beta4 subunits confer large differences in agonist binding affinity. Mol Pharmacol 54:1132–1139. [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Dávila-García MI, Kellar KJ. (2002) Measuring nicotinic receptors with characteristics of α4β2, α3β2 and α3β4 subtypes in rat tissues by autoradiography. J Neurochem 82:468–481. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, Grucza RA, Sun L, Duan W, Budde J, et al. (2009) The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res 69:6848–6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. (2004) Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci 24:10035–10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp BM, Beyer HS. (1986) Rapid desensitization of the acute stimulatory effects of nicotine on rat plasma adrenocorticotropin and prolactin. J Pharmacol Exp Ther 238:486–491. [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. (1983) Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science 220:214–216. [DOI] [PubMed] [Google Scholar]
- Stokes C, Papke RL. (2012) Use of an α3β4 nicotinic acetylcholine receptor subunit concatamer to characterize ganglionic receptor subtypes with specific subunit composition reveals species-specific pharmacologic properties. Neuropharmacology 63:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Zaveri NT, Polgar WE, Jiang F, Khroyan TV, Zhou W, Xie XS, Stauber GB, Costello MR, Leslie FM. (2012) AT-1001: a high affinity and selective α3β4 nicotinic acetylcholine receptor antagonist blocks nicotine self-administration in rats. Neuropsychopharmacology 37:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Nelson ME, Kuryatov A, Olale F, Cooper J, Keyser K, Lindstrom J. (1998) Chronic nicotine treatment up-regulates human alpha3 beta2 but not alpha3 beta4 acetylcholine receptors stably transfected in human embryonic kidney cells. J Biol Chem 273:28721–28732. [DOI] [PubMed] [Google Scholar]
- Whiting P, Esch F, Shimasaki S, Lindstrom J. (1987) Neuronal nicotinic acetylcholine receptor beta-subunit is coded for by the cDNA clone alpha 4. FEBS Lett 219:459–463. [DOI] [PubMed] [Google Scholar]
- Wu J, Perry DC, Bupp JE, Jiang F, Polgar WE, Toll L, Zaveri NT. (2014) [¹²⁵I]AT-1012, a new high affinity radioligand for the α3β4 nicotinic acetylcholine receptors. Neuropharmacology 77:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Kellar KJ. (2004) The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther 310:98–107. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, Kellar KJ. (1998) Rat α3/β4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Mol Pharmacol 54:322–333. [DOI] [PubMed] [Google Scholar]
- Young GT, Broad LM, Zwart R, Astles PC, Bodkin M, Sher E, Millar NS. (2007) Species selectivity of a nicotinic acetylcholine receptor agonist is conferred by two adjacent extracellular beta4 amino acids that are implicated in the coupling of binding to channel gating. Mol Pharmacol 71:389–397. [DOI] [PubMed] [Google Scholar]
- Zaveri NT, Bertrand S, Yasuda D, Bertrand D. (2015) Functional characterization of AT-1001, an α3β4 nicotinic acetylcholine receptor ligand, at human α3β4 and α4β2 nAChR. Nicotine Tob Res 17:361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri N, Jiang F, Olsen C, Polgar W, Toll L. (2010) Novel alpha 3 Beta 4 nicotinic acetylcholine receptor-selective ligands. Discovery, structure-activity studies, and pharmacological evaluation. J Med Chem 53:8187–8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, Broad LM, Williams AC, Zhang D, Ding C, et al. (2008) Sazetidine-A is a potent and selective agonist at native and recombinant α 4 β 2 nicotinic acetylcholine receptors. Mol Pharmacol 73:1838–1843. [DOI] [PubMed] [Google Scholar]