Abstract
Combination drug therapies under development for cystic fibrosis caused by the ∆F508 mutation in cystic fibrosis transmembrane conductance regulator (CFTR) include a “corrector” to improve its cellular processing and a “potentiator” to improve its chloride channel function. Recently, it was reported that the approved potentiator N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide (Ivacaftor) reduces ∆F508-CFTR cellular stability and the efficacy of investigational correctors, including 3-(6-[([1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl) amino]-3-methyl-2-pyridinyl)-benzoic acid and 1-(2,2-difluoro-1,3-benzodioxol-5-yl)-N-(1-[(2R)-2,3-dihydroxypropyl]-6-fluoro-2-(2-hydroxy-1,1-dimethylethyl)-1H-indol-5-yl), which might contribute to the modest reported efficacy of combination therapy in clinical trials. Here, we report the identification and characterization of potentiators that do not interfere with ∆F508-CFTR stability or corrector action. High-throughput screening and structure-activity analysis identified several classes of potentiators that do not impair corrector action, including tetrahydrobenzothiophenes, thiooxoaminothiazoles, and pyrazole-pyrrole-isoxazoles. The most potent compounds have an EC50 for ∆F508-CFTR potentiation down to 18 nM and do not reduce corrector efficacy in heterologous ∆F508-CFTR–expressing cells or primary cultures of ∆F508/∆F508 human bronchial epithelia. The ΔF508-CFTR potentiators also activated wild-type and G551D CFTR, albeit weakly. The efficacy of combination therapy for cystic fibrosis caused by the ∆F508 mutation may be improved by replacement of Ivacaftor with a potentiator that does not interfere with corrector action.
Introduction
Mutations in the cAMP-activated chloride channel cystic fibrosis transmembrane conductance regulator (CFTR) cause the genetic disease cystic fibrosis (CF), in which defective epithelial fluid secretion and viscous mucus impairs the function of the lung, pancreas, and other organs. The most common CFTR mutation, ∆F508, is present in at least one allele in ∼90% of CF patients in North America. The ∆F508 mutation causes CFTR misfolding, with retention at the endoplasmic reticulum, accelerated degradation, and impaired chloride channel function (Collins, 1992; Boucher, 2004; Davis, 2006; Riordan, 2008).
There has been great interest in the development of small-molecule, CFTR-targeted therapeutics to treat the underlying CFTR defect responsible for the clinical manifestations in CF (Ashlock and Olson, 2011; Hanrahan et al., 2013; Rowe and Verkman, 2013). N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide (VX-770; Ivacaftor) is approved for therapy of CF caused by mutations that affect CFTR gating but not cellular processing, including G551D and nine other point mutations (Ramsey et al., 2011; Davis et al., 2012; O’Reilly and Elphick, 2013). A combination drug approach has been adopted for therapy of CF caused by the ∆F508-CFTR mutation: a “corrector” to improve its cellular processing and a “potentiator” to improve its chloride channel function. Two reported phase 3 clinical trials of combination therapy of the investigational corrector 3-(6-[([1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl)amino]-3-methyl-2-pyridinyl)-benzoic acid (VX-809) with VX-770 showed modest clinical benefit in ∆F508 homozygous CF patients, with a range of 2.6–4% improvement in lung function as measured by forced expiratory volume in 1 second (NCT01807949; NCT01807923; Boyle et al., 2014; Vertex Pharmaceuticals press release, Cambridge, MA). Two recent papers reported that VX-770 reduces ∆F508-CFTR cellular stability and the efficacy of VX-809 and a second investigational corrector, 1-(2,2-difluoro-1,3-benzodioxol-5-yl)-N-(1-[(2R)-2,3-dihydroxypropyl]-6-fluoro-2-(2-hydroxy-1,1-dimethylethyl)-1H-indol-5-yl) (VX-661) (Cholon et al., 2014; Veit et al., 2014). The reduced corrector efficacy in cells chronically exposed to VX-770 results from impaired ∆F508-CFTR folding efficiency at the endoplasmic reticulum and accelerated plasma membrane turnover (Veit et al., 2014), which might account, in part, for the modest clinical efficacy of combination therapy.
We reasoned that a potentiator that does not reduce ∆F508-CFTR stability and corrector efficacy may be superior to VX-770 for combination therapy. The study here was done to identify potentiators that do not impair corrector action. This project was motivated by the observation that some potentiators, such as P5, do not destabilize ∆F508-CFTR or reduce corrector efficacy (Veit et al., 2014). The tetrahydrobenzothiophene P5 was the first reported CFTR potentiator, as identified by our laboratory using a cell-based functional high-throughput screen (Yang et al., 2003). Here, we screened 273 analogs of P5 as well as 60,000 unrelated drug-like synthetic small molecules to identify potentiators that do not interfere with corrector action. Nanomolar-potency potentiators were identified and characterized, which do not interfere with corrector action or impair ∆F508-CFTR cellular stability.
Materials and Methods
Cell Lines.
Fisher rat thyroid (FRT) epithelial cells stably expressing ∆F508, wild-type, or G551D CFTR, together with halide-sensitive green fluorescent protein yellow fluorescence protein (YFP)–H148Q/I152L/F46L (Galietta et al., 2001) were as reported (Ma et al., 2002; Pedemonte et al., 2005). FRT cells were cultured in Coon’s modified Ham’s F12 medium, supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. For primary screening, FRT cells expressing ΔF508-CFTR were plated at 50,000 cells/well 18–24 hours prior to screening.
CFBE41o− TetON (CFBE) cells expressing ΔF508-CFTR–horseradish peroxidase (HRP) or ΔF508-CFTR-3HA were as described (Veit et al., 2012, 2014). CFBE cells were grown in minimal essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 10 mM HEPES. For propagation, cells were cultured in plastic flasks coated with 10 μg/ml human fibronectin, 30 μg/ml calf-skin collagen, and 100 μg/ml bovine serum albumin diluted in LHC-8 basal medium (Invitrogen). For chemiluminescence assay, CFBE cells were plated in black, 96-well microplates (Costar, Corning Inc., Lowell, MA) at 15,000 cells/well. ∆F508-CFTR-HRP expression was induced 24 hours after plating with 0.5 μg/ml doxycycline for 2 days before assay.
Primary Cultures of ∆F508/∆F508 Human Bronchial Epithelial Cells.
Primary human ∆F508/∆F508 CF bronchial epithelial cells were isolated and then cultured on semipermeable inserts (Snapwell, Corning Inc.) in ALI media at an air-liquid interface, as described (Yamaya et al., 1992; Fulcher and Randell, 2013). Media was changed every 2–3 days. All protocols involving the collection and use of human tissues and cells were reviewed and approved by the University of California, San Francisco Institutional Review Board.
Compounds.
Sixty thousand chemically diverse, drug-like synthetic compounds (>90% with a molecular size of 250–500 Da; ChemDiv Inc., San Diego, CA) were used for primary screening. For optimization, 387 commercially available analogs of two chemical classes were tested as well as 273 analogs of P5. Analogs of active compounds (purity > 95%) were purchased from ChemDiv Inc.
Screening Procedures.
For the assay of potentiator activity, FRT cells expressing ΔF508-CFTR and YFP were grown at 37°C/5% CO2 for 18–24 hours and then for 18–24 hours at 27°C, as described (Yang et al., 2003). At the time of the assay, cells were washed with phosphate-buffered saline (PBS) and then incubated for 10 minutes with PBS (60 µl) containing forskolin (20 μM) and the test compound (2.5 μM final concentration). Each well was assayed individually for iodide influx by recording fluorescence continuously (200 milliseconds per point) for 2 seconds (baseline) and then for 12 seconds after rapid addition of 180 μl PBS, in which 137 mM chloride was replaced by iodide. Fluorescence was recorded using a TECAN Infinite M1000 plate reader (TECAN Groups Ltd., Mannedorf, Switzerland), equipped with an automated stacker and dual syringe pumps (excitation: 500 ± 10 nm; emission: 535 ± 10 nm). The initial iodide influx rate was computed as described (Ma et al., 2002). All compound plates contained negative controls (dimethylsulfoxide vehicle) and positive controls (50 µM genistein).
Chemiluminescence Assays.
CFBE cells expressing ∆F508-CFTR-HRP were incubated with 100 µl of medium containing the test compound, 3 µM VX-809, and 0.5 μg/ml doxycycline for 24 hours at 37°C. All plates contained negative controls (dimethylsulfoxide vehicle) and positive controls (3 µM VX-809). Cells were washed 4 times with PBS, and HRP activity was assayed by the addition of 50 µl/well of the HRP substrate (WesternBright Sirius Kit; Advansta Corp., Menlo Park, CA), as described (Phuan et al., 2014). After shaking for 5 minutes, chemiluminescence was measured using a TECAN Infinite M1000 plate reader (integration time of 100 milliseconds).
Short-Circuit Current Measurements.
Short-circuit current measurements were done as previously described (Phuan et al., 2014). FRT cells expressing ΔF508, wild-type, or G551D CFTR were cultured on inserts (Snapwell, Corning) for 5–7 days and used when the transepithelial resistance was >2000 μΩ/cm2. For potentiator testing, the ΔF508-CFTR–expressing FRT cells were incubated for 18–24 hours at 27°C before measurements. The basolateral solution contained 120 mM NaCl, 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 25 mM NaHCO3, and 5 mM HEPES, pH 7.4. In the apical bathing solution, 60 mM NaCl was replaced by Na gluconate and CaCl2 was increased to 2 mM. Solutions were bubbled with 95% O2/5% CO2 and maintained at 37°C. The basolateral membrane was permeabilized with 250 μg/ml amphotericin B. Primary cultures of human bronchial epithelial cells were measured after 21–28 days of culture when cells reached full differentiation, and the transepithelial resistance was >1000 μΩ/cm2. The primary cultures were incubated with VX-809 (10 µM) at the basolateral side for 18–24 hours at 37°C prior to measurements. The apical and basolateral chambers contained identical solutions: 130 mM NaCl, 0.38 mM KH2PO4, 2.1 mM K2HPO4, 1 mM MgCl2, 1 mM CaCl2, 25 mM NaHCO3, and 10 mM glucose. Solutions were bubbled with 5% CO2/95% O2 and maintained at 37°C. Hemichambers were connected to a DVC-1000 voltage clamp (World Precision Instruments Inc., Sarasota, FL) via Ag/AgCl electrodes and 1 M KCl agar bridges for recording of the short-circuit current.
CFTR Plasma Membrane Density Measurements.
The plasma membrane density of triple human influenza hemagglutinin (3HA)–tagged CFTR was determined by cell surface enzyme-linked immunosorbent assay, as described (Okiyoneda et al., 2010). Plasma membrane density measurements were normalized to cell viability determined by Alamar Blue assay (Invitrogen).
Immunoblotting.
Immunoblotting was performed as described (Okiyoneda et al., 2010). The 3HA-tagged ΔF508-CFTR was detected by monoclonal mouse anti-HA antibody (MMS101R; Covance, Princeton, NJ), and monoclonal mouse anti–Na+/K+-ATPase antibody (H3; Santa Cruz Biotechnology, Santa Cruz, CA) was used to confirm equal loading.
Results
Screening Strategy.
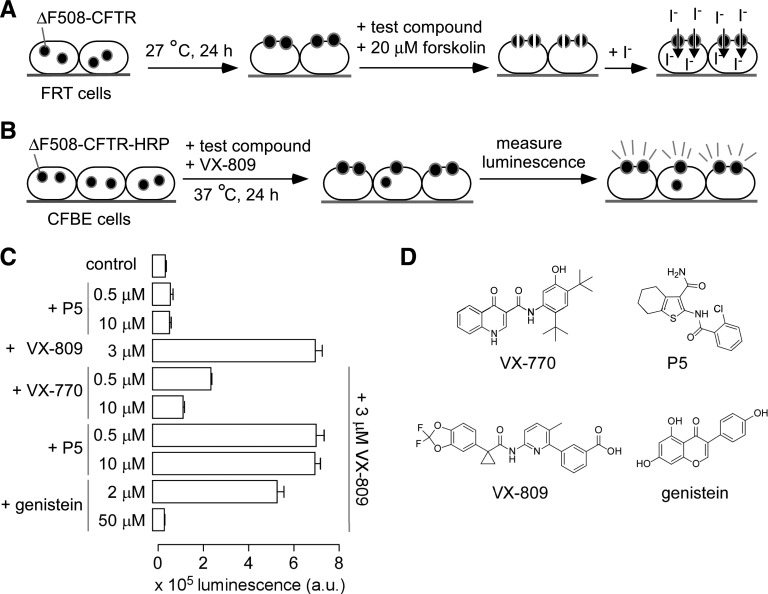
Figure 1, A and B, diagrams the assays used to identify potentiators that do not interfere with ∆F508-CFTR corrector action. The primary high-throughput screen for potentiators was done using FRT cells stably expressing human ΔF508-CFTR and an iodide-sensitive YFP. The cells were cultured for 24 hours at 27°C to target ΔF508-CFTR to the plasma membrane (low-temperature rescue). Iodide influx was measured by extracellular addition of iodide in the presence test compound (at 2.5 μM) and cAMP agonist (20 μM forskolin). Active potentiators identified in the primary screen were counter-screened for the effect on the corrector action using a human lung epithelium–derived (CFBE41o−) cell line (Ehrhardt et al., 2006) stably transfected with human ΔF508-CFTR containing an HRP inserted in its fourth extracellular loop (Phuan et al., 2014; Veit et al., 2014). The expression of wild-type CFTR in CFBE is under the control of the same expression vector as in the ΔF508-3HA and ΔF508-HRP cells reported previously (Veit et al., 2012), in which CFTR expression was shown to be similar to endogenous levels in Calu-3 cells. The ΔF508-HRP CFBE cells were cultured on 96-well plates and then potentiators (10 μM) were added for 18–24 hours together with VX-809 (3 μM). Cell surface ∆F508-CFTR was quantified by a luminescence readout. Potentiators that interfere with ∆F508-CFTR cellular stability or corrector action reduce the luminescence signal. Figure 1C shows the reduced VX-809 corrector efficacy in cells following chronic exposure to VX-770 or genistein, but not P5, as reported before (Veit et al., 2014). Chemical structures are shown in Fig. 1D.
Fig. 1.
Screens for identification of ∆F508-CFTR potentiators that do not interfere with corrector action. (A) Potentiator screen using FRT cells coexpressing ∆F508-CFTR and YFP iodide–sensing protein. Cells were incubated at 27°C for 24 hours before assay to target ΔF508-CFTR to the plasma membrane. Test compounds at 2.5 µM were added for 10 minutes at room temperature in the presence of forskolin (20 μM) before iodide addition. (B) Cell-surface expression screen using ΔF508-CFTR-HRP CFBE cells. Cells were incubated with test compounds and 3 μM VX-809 for 24 hours at 37°C. Cell-surface ∆F508-CFTR was assayed by a luminescence readout of HRP activity. (C) Luminescence readout of cell surface ΔF508-CFTR after 24-hour incubation with potentiators VX-770, P5, or genistein, together with 3 μM VX-809 (S.E.; n = 4). (D) Chemical structures of potentiators.
ΔF508-CFTR Potentiators Identified from Screens.
Sixty thousand drug-like, synthetic small molecules were screened for potentiator activity as well as 273 analogs of tetrahydrobenzothiophene P5. As summarized in Fig. 2A, 230 active compounds were identified from the 60,000-molecule screen and 38 active P5 analogs, with activity defined as >50% of the maximal iodide influx produced by forskolin (20 μM) + genistein (50 μM). The 50 most active compounds were counter-screened for effect on corrector efficacy in VX-809–treated ΔF508-HRP CFBE cells, which produced 41 compounds from the 60,000-molecule screen and 10 P5 analogs that did not significantly reduce the luminescence signal. We choose the 12 most active compounds and grouped them into nine compound classes (Fig. 2B). Among the active compounds was a thiophene (compound G01) with structural similarity to tetrahydrobenzothiophene P5. In addition, structurally similar compounds included two 2-thioxo-4-amino-thiazoles (compounds A01 and A02) and three pyrazole-pyrrole isoxazoles (compounds H01, H02, and H03). Supplemental Table 1 summarizes the concentration-dependence data for selected compounds in ΔF508-CFTR FRT cells, with the most potent compounds showing an EC50 of ∼0.03 μM for ∆F508-CFTR potentiation, without interference with VX-809 corrector action and with potentiator efficacy similar to maximal genistein.
Fig. 2.
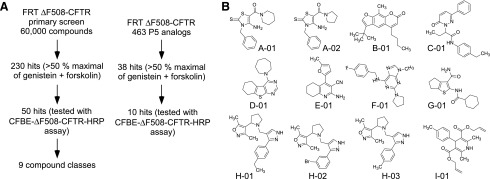
Screening results. (A) Summary of screening results. (B) Chemical structures of nine classes of potentiators identified from the screen.
Structure-Activity Analysis.
Based on potency data, compounds of classes A, H, and P5 were selected for further study. One hundred and eighty-nine commercially available class A and class H analogs were tested. Figure 3A shows the concentration-dependence data of selected compounds in ∆F508-CFTR FRT cells. Figure 3B shows that these compounds do not significantly impair VX-809 corrector action in ∆F508-HRP CFBE cells. Supplemental Tables 2–4 summarize the EC50 values for selective active compounds. Several class A analogs, including A03, A04, and A05, have a similar or better EC50 than compounds A01 and A02 identified in the primary screen. The most active compound, A04, has an EC50 of ∼18 nM. For class H, only a few analogs showed an EC50 similar to that of the original compounds H01 and H02. Several P5 analogs were more potent than P5, including P11 and P12.
Fig. 3.
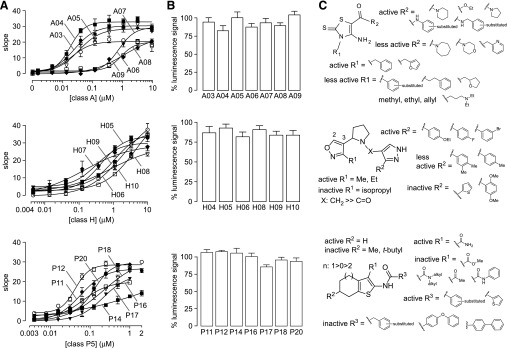
Structure-activity relationships of class A, H, and P5 potentiators. (A) Concentration-dependence data of class A, H, and P5 analogs in ΔF508-CFTR FRT cells (S.E.; n = 3). (B) Cell-surface ΔF508-CFTR expression in CFBE cells following incubation with 10 μM class A or H compounds or 2 μM P5 analogs, together with 3 μM VX-809 (S.E.; n = 4). A 100% luminescence signal is defined as the background-subtracted luminescence signal of VX-809–corrected ΔF508-HRP CFBE cells. (C) Structural determinants of potentiator activity of class A, H, and P5 compounds.
Structural determinants of activity for class A, H, and P5 compounds are summarized in Fig. 3C. Correctors of class A, 2-thioxo-4-aminothiazoles, with the best activity have R1 substituents of benzyl and 2-methyl-furan (Supplemental Table 1; A03 and A08). Reduced activity was seen with R1 as halide-substituted benzene, alkyl-substituted benzene, methyl, ethyl, phenethylene, and allyl moieties. Interestingly, a wide array of substituents, including piperdine, pyrrolidine, and substituted anilines, were tolerated at R2, with the most active being R2 as a simple ester (A04) and benzylamine (A03 and A05). Other rings, such as azepane, benzylpyridine, and morpholine, reduced activity. Class H compounds contain three linked heterocycles: pyrazole-pyrrole-isoxazole. Analogs containing only two rings, such as pyrrole-isoxazole and pyrazole-isoxazole, were inactive, indicating that all three heterocycles are needed for activity. Limited substituents on the isoxazole were studied, with small substituents, such as ethyl and methyl giving better activity, whereas bulky groups, such as isopropyl, giving reduced activity. The position at which the isoxazole linkage to pyrrole is crucial, as changing from the 3 to 2 position abolished activity. Changing the linker between the pyrazole and pyrrole rings from methylene (X:CH2) to carbonyl (X:C=O) resulted in loss of activity. The substitution (R2) on the pyrazole affected activity, with electron-poor rings, such as 4-fluorobenzene (H04) and 3-bromobenzene (H05), giving the best activity, whereas electron-neutral (H06) or electronic-rich (H10) rings giving reduced activity.
The benzothiophene P5, which contains a six-membered ring (n = 1), had greater potentiator activity than analogs with a five-membered ring (n = 0) (P18) or a seven-membered ring (n = 2). At the R1 position, the primary carboxamide (CONH2) is crucial for activity, as replacing R1 with cyano, ester, methyl-ketone, substituted anilines as well as substitutions on carboxamides resulted in loss of activity. Similarly, substitution on the 2 position (R2) of the tetrahydrobenzo ring with methyl and t-butyl also resulted in loss of activity. Alterations of R3 gave more potent P5 analogs, including replacement of the 2-chlorobenzene in P5 by 2-fluorobenzene (P12) or 2-thiophene (P11). Close structural analogs of P5 with chloro, methoxy, and nitro groups substituted at different positions showed a similar or better EC50 than P5. Changing R3 from benzene to other substituted benzyl, biphenyl, and phenoxy-phenyls reduced activity.
Biochemical Studies of Potentiator Effect on ΔF508-CFTR Expression.
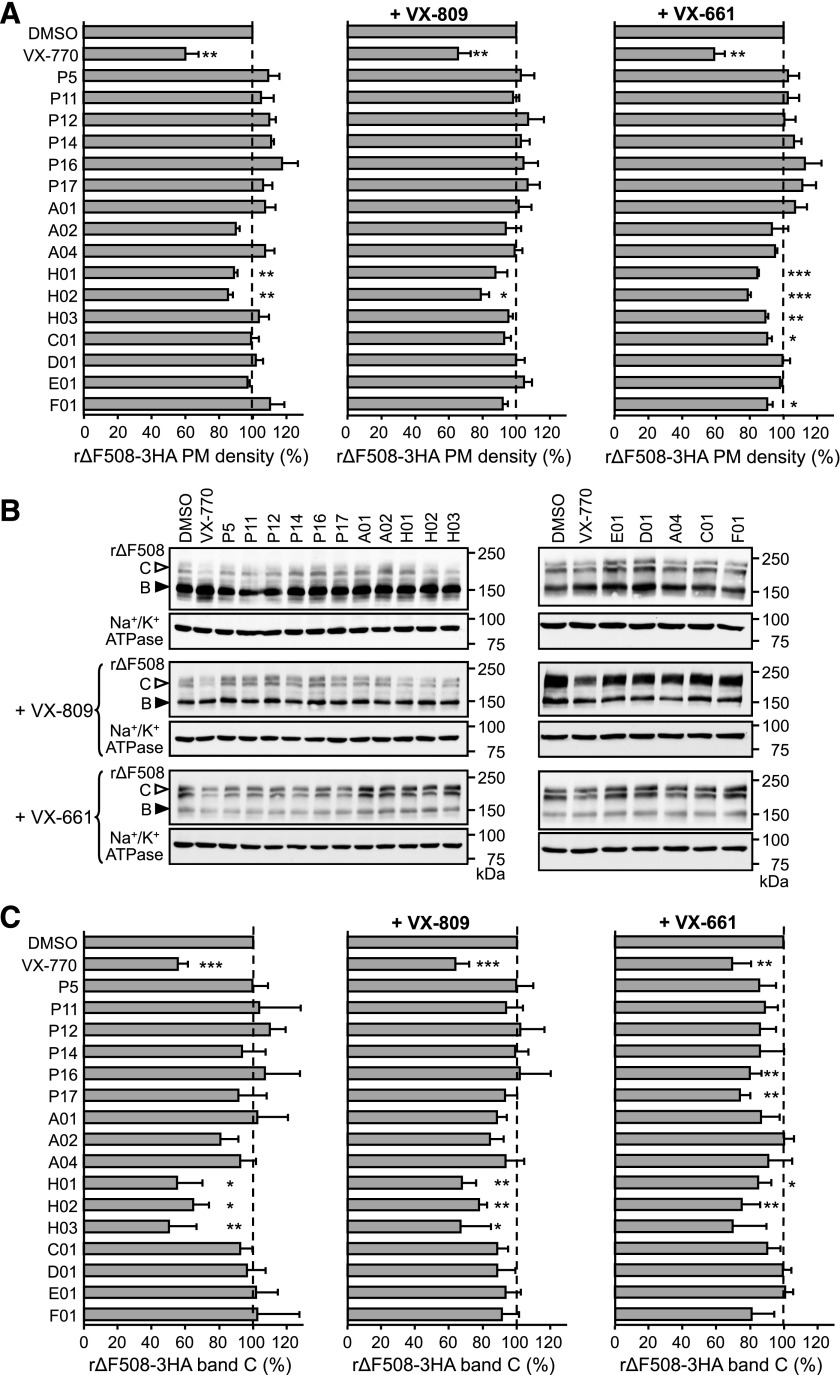
To biochemically confirm that chronic potentiator exposure does not interfere with ΔF508-CFTR plasma membrane expression, we used CFBE41o− cells stably expressing the 3HA-tagged ΔF508-CFTR (Sharma et al., 2004). 3HA-ΔF508-CFTR CFBE cells were treated with VX-770 and potentiators for 24 hours. Figure 4A (left) showed that while VX-770 reduced ΔF508-CFTR plasma membrane expression, most of the potentiators did not affect ΔF508-CFTR expression, with the exception of compounds H01 and H02. When the potentiators were incubated together with VX-809 (Fig. 4A, center) or VX-661 (Fig. 4A, right), most potentiators did not reduce ΔF508-CFTR PM expression. Compounds H01 and H02 reduced ΔF508-CFTR plasma membrane expression, although not as much as VX-770.
Fig. 4.
Potentiator effects on ΔF508-CFTR plasma membrane expression. (A) Plasma membrane expression of low-temperature rescued (26°C, 48 hours followed by 1-hour chase at 37°C) ΔF508-CFTR-3HA (rΔF508-3HA) in CFBE. Cells were treated with the indicated potentiators for 24 hours (P11–17, A1–2, and A4, 1 μM; H1–3, C1, D1, E1, and F1, 5 μM) alone (left) or in the presence of 3 μM VX-809 (center) or 3 μM VX-661 (right). Data are expressed as the percentage of dimethylsulfoxide-treated controls (S.E.; n = 3). (B) Representative immunoblots and (C) quantification of the potentiator effect on the expression pattern of rΔF508-3HA (26°C; 48 hours) in CFBE cells. Cells were treated with the indicated potentiators alone or in combination with 3 μM VX-809 or 3 μM VX-661 for 24 hours at 26°C. CFTR was visualized with anti-HA antibody, anti–Na+/K+-ATPase antibody served as the loading control. Densitometric analysis of complex-glycosylated (band C, empty arrowhead) ΔF508-CFTR is expressed as the percentage of dimethylsulfoxide control (S.E.; n = 3–9).
Potentiator effects on ΔF508-CFTR cellular expression were examined by immunoblot analysis. Potentiators were incubated alone or with 3 μM VX-809 or 3 μM VX-661 in rΔF508-3HA-CFBE cells for 48 hours at 26°C before lysis and immunoblot analysis (Fig. 4B). Densitometric analyses were done to quantify complex glycosylated ΔF508-CFTR (C-band) in cell lysates (Fig. 4C). Most of the potentiators did not significantly decrease the amount of the complex glycosylated ΔF508-CFTR (C-band), alone or in combination with VX-809 or VX-661. Several class H compounds (H01, H02, and H03) reduced the amount of the complex glycosylated ΔF508-CFTR, similar to data in Fig. 4A. Two P5 analogs, P15 and P17, appeared to decrease complex glycosylated ΔF508-CFTR in VX-661, but not VX-809 treated cells, suggesting that potentiator interference of corrector action might be corrector dependent.
Short-Circuit Measurements in Transfected FRT Cells and in Primary Cultures of ∆F508/∆F508 Human Bronchial Epithelial Cells.
Based on the results of the biochemical and structure-activity relationship studies, we chose the most potent class A and P5 analogs, A04 and P12, for further characterization by short-circuit current measurements. Apical membrane chloride current was measured in ΔF508-CFTR–expressing FRT cells after low-temperature rescue in response to A04 and P12, with data for VX-770 shown for comparison. Figure 5A shows the concentration-dependent increases in the apical membrane current seen in the presence of forskolin. Genistein produced a minimal increase in the chloride current after maximal A04, P12, and VX-770. CFTRinh-172 abolished all of the chloride current. As measured by a short-circuit current, the apparent EC50 for A04 and P12 potentiator activity was ∼100 nM. The EC50 for VX-770 was ∼30 nM, in agreement with published data (Van Goor et al., 2009). In side-by-side short-circuit current recordings (unpublished data), maximal potentiator concentrations produced open probabilities of ∼0.37 and ∼0.34 for A04 and P12, respectively, as referenced against the open probability of ∼0.40 produced by maximal VX-770 (Van Goor et al., 2009). A04 and P12 were also tested in FRT cells expressing wild-type CFTR (Fig. 5B). A low concentration of forskolin (0.5 μM) was used to prevent full activation of wild-type CFTR, which would mask potentiator action. After potentiator additions, 10 μM forskolin was added to fully activate wild-type CFTR, followed by 50 μM genistein, which had little effect, followed by 10 μM CFTRinh-172, which inhibited all of the chloride current. A04-activated wild-type CFTR when added after 0.5 μM forskolin, whereas P12 showed partial activation, with an apparent EC50 of ∼200 nM for both compounds. A04 and P12 were also tested in FRT cells expressing G551D-CFTR (Fig. 5C). G551D-CFTR produced little chloride current with maximal forskolin. A04 partially activated G551D-CFTR but only at a high concentration (10 μM), whereas P12 showed minimal activation even at a high concentration.
Fig. 5.
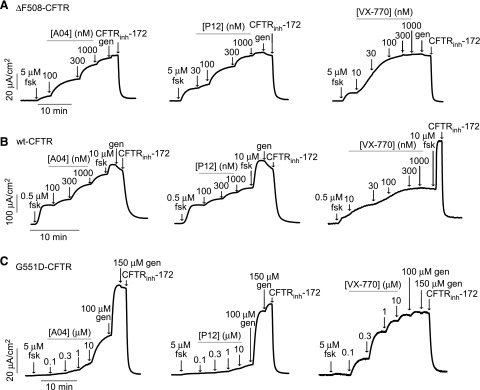
Functional assays in FRT cells expressing ΔF508, wild-type, and G551D CFTR. Representative short-circuit current measured in FRT cells expressing ΔF508-CFTR (A), wild-type CFTR (B), and G551D-CFTR (C) showing responses to indicated concentrations of forskolin (fsk), A04, P12, and VX-770. The ΔF508-CFTR–expressing cells were incubated at 27°C for 24 hours before measurement. Genistein (gen, 50 μM) and CFTRinh-172 (10 μM) were added where indicated.
Short-circuit current measurements were also done in well differentiated primary cultures of human bronchial epithelial cells from a ΔF508-homozygous subject. A short-circuit current was measured after chronic (18–24 hours) treatment with A04 or P12 together with VX-809. Cells incubated with 3 µM VX-809 alone showed an increased short-circuit current in response to forskolin and VX-770, which was inhibited by CFTRinh-172 (Fig. 6A, left). Little current was seen in the absence of VX-809. Cotreatment with 5 µM A04 or P12 together with 3 µM VX-809 did not reduce the chloride current when compared with VX-809 alone (Fig. 6A, center and right). Figure 6B summarizes the chloride current responses from the data, as in Fig. 6A, indicating that chronic treatment with A04 and P12 did not interfere with VX-809 corrector action. Finally, the potentiator action of A04 and P12 was tested in the primary cultures of ΔF508/ΔF508 human bronchial epithelial cells after VX-809 correction. Figure 6C shows the A04 and P12 concentration-dependent increases in the chloride current in the presence of forskolin. Genistein produced a minimal increase in the chloride current after maximal A04 or P12, and 10 μM CFTRinh-172 abolished all of the chloride current. The apparent EC50 for A04 and P12 potentiator activity was <300 nM in the ΔF508/ΔF508 human bronchial epithelial cells.
Fig. 6.
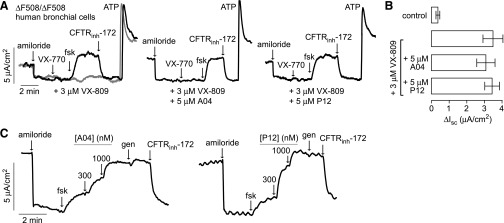
Functional assays in primary cultures of human bronchial epithelial cells from a homozygous ∆F508 CF patient. (A) Cultures were incubated at 37°C for 24 hours with 3 μM VX-809 (gray curve shows dimethylsulfoxide-vehicle treated cells) (left), 3 µM VX-809 + 5 µM A04 (center), or 3 µM VX-809 + 5 µM P12 (right). Amiloride (10 μM), VX-770 (10 μM), forskolin (fsk; 20 μM), and CFTRinh-172 (10 μM) were added where indicated. (B) Summary of changes in short-circuit current (ΔIsc) from experiments as in (A) (S.E.; n = 3 cultures each). (C) Short-circuit current measured in the ∆F508/∆F508 human bronchial epithelial cells showing responses to indicated A04 and P12 concentrations. Cells were incubated at 37°C for 24 hours with 3 μM VX-809 before measurement. Genistein (gen; 50 μM) and CFTRinh-172 (10 μM) were added where indicated.
Discussion
This study was done to identify new potentiators that do not interfere with the action of ∆F508-CFTR correctors. This study was motivated by recent studies showing that 1) the approved potentiator Ivacaftor (Kalydeco) reduced the correction efficacy of VX-809 and VX-661 (Cholon et al., 2014; Veit et al., 2014); 2) some, but not all, potentiators reduce correction efficacy (Veit et al., 2014); and 3) combination VX-770/VX-809 therapy showed limited clinical efficacy (Boyle et al., 2014; Vertex Pharmaceuticals press release, Cambridge, MA). VX-770 has been approved to treat CF patients with defective CFTR gating but not cellular processing, including G551D-CFTR. However, the majority of CF patients carry the ΔF508 mutation, in which the protein has defective gating as well as trafficking to the cell membrane. The limited clinical efficacy of combination VX-809/VX-770 therapy could perhaps be explained by in vitro data (Cholon et al., 2014; Veit et al., 2014), showing that VX-770 reduces the correction efficacy of VX-809 and VX-661 by increasing the turnover rate and destabilizing corrected ΔF508-CFTR. Thus, potentiators that do not interfere with corrector action or impair CFTR stability/turnover rate could have an improved clinical benefit over VX-770 in combination therapy of CF caused by the ∆F508 mutation and potentially other mutations.
Our strategy to identify ΔF508-CFTR potentiators that do not interfere with the corrector action used an initial, high-throughput functional, cell-based screen involving assay of iodide influx. Active compounds identified from the initial screen were counter-screened using a human lung epithelium-derived cell line expressing transfected HRP-tagged ∆F508-CFTR. The HRP-tag was engineered in the fourth extracellular loop for robust plate reader–based luminescence measurements of cell surface CFTR expression. A large number and variety of potentiators were identified, many of which did not interfere with corrector action. The most active potentiators, A04 and P12, were found to have nanomolar potency, as shown by short-circuit current measurements in low-temperature rescued ΔF508-CFTR FRT cells and VX-809–treated ΔF508/ΔF08 human bronchial epithelial cells. Interestingly, P12 and A04 only minimally activated G551D-CFTR at high concentrations. Computational modeling has suggested that increased stability of G551D-CFTR compared with wild-type CFTR renders the G551D-CFTR protein too rigid and inflexible for channel gating (Serohijos et al., 2008). VX-770–mediated destabilization of G551D-CFTR might increase G551D-CFTR flexibility and result in restored channel gating function (Cholon et al., 2014). Our results with P12 and A04 suggest that these classes of compound potentiate CFTR gating most likely by a different mechanism of action from VX-770.
In addition to the functional and cell surface enzyme-linked immunosorbent assay measurements of potentiator effect on corrector efficacy, which included studies on primary cultures of ∆F508/∆F508 human bronchial cells, biochemical analyses were done to quantify total cellular ΔF508-CFTR and the accumulation of complex-glycosylated ∆F508-CFTR. Most compounds identified from the screen did not reduce the level of complex glycosylated ΔF508-CFTR, except several class H compounds, which might preclude this class of compounds for further development. As noted, an interesting observation is that P16 and P17 appeared to decrease complex-glycosylated ΔF508-CFTR in VX-661, but not VX-809 or control cells. Potentiator interference of the corrector action thus might be corrector dependent. A specific potentiator-corrector combination might produce a different therapeutic effect in different CFTR mutations. Thorough in vitro studies of potentiator-corrector combinations using pertinent CF mutations might be crucial before clinical trials. The modular multiassay approach adopted in this study could facilitate the selection of potentiators for drug development.
Some 2-thioxo-4-aminothiazoles (class A) have reported antifungal (Chhabria et al., 2011) and antitubercular (Chhabria et al., 2014) activity. Reported biologic activities for tetrahydrobenzothiophenes include inhibition of tyrosine kinase receptor FLT3 (Patch et al., 2006) and influenza virus RNA polymerase assembly (Massari et al., 2013). Both thioxoaminothiazoles and tetrahydrobenzothiophenes can be prepared in three to five steps from commercially available starting materials, thus allowing facile and rapid diversification of these scaffolds for further medicinal chemistry and structure-activity relationship efforts to improve biologic activities and pharmacological properties. There are no biologic reports on the exact pyrazole-pyrrole-isoxazole ring system, as found in class H compounds. However, some pyrazoles with moderate structural similarity to class H have been reported to be 5-HT2A/5-HT2C receptor antagonists (Schadt et al., 2004), p38 kinase inhibitors (Naraian et al., 2005), and androgen receptor antagonists (Ito et al., 2009). To our knowledge, the channel-modulating effects of class A and H compounds have not been reported.
In summary, the results here provide a proof of concept for the identification of nanomolar-potency potentiators that do not interfere with the corrector action or CFTR stability.
Supplementary Material
Acknowledgments
The authors thank Dr. Paul Wolters (University of California, San Francisco, CA) for help in collecting human bronchi.
Abbreviations
- CF
cystic fibrosis
- CFBE
CFBE41o− TetON
- FRT
Fisher rat thyroid
- HA
human influenza hemagglutinin
- HRP
horseradish peroxidase
- PBS
phosphate-buffered saline
- VX-661
1-(2,2-difluoro-1,3-benzodioxol-5-yl)-N-(1-[(2R)-2,3-dihydroxypropyl]-6-fluoro-2-(2-hydroxy-1,1-dimethylethyl)-1H-indol-5-yl)
- VX-770
N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide
- VX-809
3-(6-[([1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl)amino]-3-methyl-2-pyridinyl)-benzoic acid
- YFP
yellow fluorescence protein
Authorship Contributions
Participated in research design: Phuan, Veit, Lukacs, Verkman.
Conducted experiments: Phuan, Veit, Tan.
Wrote or contributed to the writing of the manuscript: Phuan, Veit, Finkbeiner, Lukacs, Verkman.
Footnotes
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants R01-DK75302, P30-DK72517, and R01-DK35124]; National Institutes of Health National Institute of Biomedical Imaging and Bioengineering [Grant R37-EB00415]; National Institutes of Health National Eye Institute [Grant R01-EY135740]; the Cystic Fibrosis Foundation; the Canadian Institutes of Health Research; and the Canadian Cystic Fibrosis Foundation. G.L.L. is a recipient of a Canada Research Chair.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Ashlock MA, Olson ER. (2011) Therapeutics development for cystic fibrosis: a successful model for a multisystem genetic disease. Annu Rev Med 62:107–125. [DOI] [PubMed] [Google Scholar]
- Boucher RC. (2004) New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J 23:146–158. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Bell SC, Konstan MW, McColley SA, Rowe SM, Rietschel E, Huang X, Waltz D, Patel NR, Rodman D, VX09-809-102 study group (2014) A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med 2:527–538. [DOI] [PubMed] [Google Scholar]
- Chhabria M, Patel S, Dholakia S, Mistry H, Patel S. (2014) Synthesis and antitubercular activity of a series of thiazole derivatives. Anti-Infective Agents 12:149–158. [Google Scholar]
- Chhabria M, Rathod I, Vala K, Patel P. (2011) Identification of important structural features of thiazolo[4,5-d]pyrimidines required for potent antifungal activity. Med Chem Res 20:1450–1454. [Google Scholar]
- Cholon DM, Quinney NL, Fulcher ML, Esther CR, Jr, Das J, Dokholyan NV, Randell SH, Boucher RC, Gentzsch M. (2014) Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis. Sci Transl Med 6:246ra96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS. (1992) Cystic fibrosis: molecular biology and therapeutic implications. Science 256:774–779. [DOI] [PubMed] [Google Scholar]
- Davis PB. (2006) Cystic fibrosis since 1938. Am J Respir Crit Care Med 173:475–482. [DOI] [PubMed] [Google Scholar]
- Davis PB, Yasothan U, Kirkpatrick P. (2012) Ivacaftor. Nat Rev Drug Discov 11:349–350. [DOI] [PubMed] [Google Scholar]
- Ehrhardt C, Collnot EM, Baldes C, Becker U, Laue M, Kim KJ, Lehr CM. (2006) Towards an in vitro model of cystic fibrosis small airway epithelium: characterisation of the human bronchial epithelial cell line CFBE41o-. Cell Tissue Res 323:405–415. [DOI] [PubMed] [Google Scholar]
- Fulcher ML, Randell SH. (2013) Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol 945:109–121. [DOI] [PubMed] [Google Scholar]
- Galietta LJ, Haggie PM, Verkman AS. (2001) Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett 499:220–224. [DOI] [PubMed] [Google Scholar]
- Hanrahan JW, Sampson HM, Thomas DY. (2013) Novel pharmacological strategies to treat cystic fibrosis. Trends Pharmacol Sci 34:119–125. [DOI] [PubMed] [Google Scholar]
- Ito M, Suzaki T, Yamamoto S (2009) inventors, Ito M, Suzaki T, Takeda Pharmaceutical, Yamamoto S, assignee. Substituted pyrazole derivative. Patent WO2009028543 A1. 2009 Mar 5.
- Ma T, Vetrivel L, Yang H, Pedemonte N, Zegarra-Moran O, Galietta LJ, Verkman AS. (2002) High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J Biol Chem 277:37235–37241. [DOI] [PubMed] [Google Scholar]
- Massari S, Nannetti G, Goracci L, Sancineto L, Muratore G, Sabatini S, Manfroni G, Mercorelli B, Cecchetti V, Facchini M, et al. (2013) Structural investigation of cycloheptathiophene-3-carboxamide derivatives targeting influenza virus polymerase assembly. J Med Chem 56:10118–10131. [DOI] [PubMed] [Google Scholar]
- Naraian AS, et al. (2005) inventors, Pharmacia Corporation, assignee. Substituted pyrazoles as p38 kinase inhibitors. U.S. patent 6979686 B1. 2005 Dec 27.
- Okiyoneda T, Barrière H, Bagdány M, Rabeh WM, Du K, Höhfeld J, Young JC, Lukacs GL. (2010) Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly R, Elphick HE. (2013) Development, clinical utility, and place of ivacaftor in the treatment of cystic fibrosis. Drug Des Devel Ther 7:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SM, Verkman AS. (2013) Cystic fibrosis transmembrane regulator correctors and potentiators. Cold Spring Harb Perspect Med 3:a009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patch RJ, Baumann CA, Liu J, Gibbs AC, Ott H, Lattanze J, Player MR. (2006) Identification of 2-acylaminothiophene-3-carboxamides as potent inhibitors of FLT3. Bioorg Med Chem Lett 16:3282–3286. [DOI] [PubMed] [Google Scholar]
- Pedemonte N, Sonawane ND, Taddei A, Hu J, Zegarra-Moran O, Suen YF, Robins LI, Dicus CW, Willenbring D, Nantz MH, et al. (2005) Phenylglycine and sulfonamide correctors of defective delta F508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol Pharmacol 67:1797–1807. [DOI] [PubMed] [Google Scholar]
- Phuan PW, Veit G, Tan J, Roldan A, Finkbeiner WE, Lukacs GL, Verkman AS. (2014) Synergy-based small-molecule screen using a human lung epithelial cell line yields ΔF508-CFTR correctors that augment VX-809 maximal efficacy. Mol Pharmacol 86:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. VX08-770-102 Study Group (2011) A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 365:1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JR. (2008) CFTR function and prospects for therapy. Annu Rev Biochem 77:701–726. [DOI] [PubMed] [Google Scholar]
- van Amsterdam C, Arlt M, Bartoszyk G, Finsinger D, Schadt O, Schiemann K, Seyfried C (2004) inventors, Merck Patent Gmbh, assignee. Substituierte Pyrazolverbindungen. Patent DE10315569 A1. 2004 Oct 14.
- Serohijos AW, Hegedus T, Aleksandrov AA, He L, Cui L, Dokholyan NV, Riordan JR. (2008) Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc Natl Acad Sci USA 105:3256–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Pampinella F, Nemes C, Benharouga M, So J, Du K, Bache KG, Papsin B, Zerangue N, Stenmark H, et al. (2004) Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J Cell Biol 164:923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, et al. (2009) Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 106:18825–18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit G, Avramescu RG, Perdomo D, Phuan PW, Bagdany M, Apaja PM, Borot F, Szollosi D, Wu YS, Finkbeiner WE, et al. (2014) Some gating potentiators, including VX-770, diminish ΔF508-CFTR functional expression. Sci Transl Med 6:246ra97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit G, Bossard F, Goepp J, Verkman AS, Galietta LJ, Hanrahan JW, Lukacs GL. (2012) Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol Biol Cell 23:4188–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. (1992) Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol 262:L713–L724. [DOI] [PubMed] [Google Scholar]
- Yang H, Shelat AA, Guy RK, Gopinath VS, Ma T, Du K, Lukacs GL, Taddei A, Folli C, Pedemonte N, et al. (2003) Nanomolar affinity small molecule correctors of defective Delta F508-CFTR chloride channel gating. J Biol Chem 278:35079–35085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.