Abstract
Background
Approximately 15% of HIV-infected MSM engaged in HIV primary care have been diagnosed with an STI in the past year, yet STI testing frequency remains low.
Methods
We sought to quantify STI testing frequencies at a large, urban HIV care clinic, and to identify patient- and provider-related barriers to increased STI testing. We extracted laboratory data in aggregate from the electronic medical record to calculate STI testing frequencies (defined as the number of HIV-infected MSM engaged in care who were tested at least once over an 18-month period divided by the number of MSM engaged in care). We created anonymous surveys of patients and providers to elicit barriers.
Results
Extra-genital gonorrhea and chlamydia testing were low (29%–32%), but the frequency of syphilis testing was higher (72%). Patients frequently reported high-risk behaviors, including drug use (16.4%) and recent bacterial STI (25.5%), as well as substantial rates of recent testing (>60% in prior 6 months). Most (72%) reported testing for STI in HIV primary care, but one-third went elsewhere for “easier” (42%), anonymous (21%) or more frequent (16%) testing. HIV primary care providers lacked testing and treatment knowledge (25–32%), and cited lack of time (68%), discomfort with sexual history taking and genital exam (21%), and patient reluctance (39%) as barriers to increased STI testing.
Conclusion
STI testing in HIV care remains unacceptably low. Enhanced education of providers, along with strategies to decrease provider time and increase patient ease and frequency of STI testing, are needed.
Keywords: gonorrhea, chlamydia, syphilis, STI testing, HIV care, barriers to STI testing
INTRODUCTION
In the United States, bacterial sexually transmitted infections (STI), including gonorrhea, chlamydia and syphilis, are highly prevalent among men who have sex with men (MSM). Among MSM attending sexually transmitted disease (STD) clinics, the prevalence of infection with rectal and pharyngeal N. gonorrhoeae and of rectal C. trachomatis is as high as 10.2%, 9.2% and 14.1%, respectively,1–5 and asymptomatic syphilis occurs in 4.3%.6 Moreover, HIV-infected MSM are disproportionately affected by bacterial STI compared to their HIV-uninfected counterparts.7 In HIV primary care clinics, approximately 15% of MSM are diagnosed with an STI at baseline,8–10 and between 12.5% and 18% are diagnosed with an incident STI within the next year.8,9 STIs increase the risk of transmission of HIV,11 yet the majority of bacterial STIs are asymptomatic.1,12 Testing and treating STIs may help reduce the risk of HIV transmission, particularly in high-risk populations with a high prevalence of STI.13,14
The U.S. Centers for Disease Control and Prevention (CDC) recommends that all MSM undergo routine STI screening at all exposed sites (urethra, pharynx, and/or rectum) at least annually.15 MSM considered at highest risk (those reporting more than 10 sexual partners in the past year, a history of a bacterial STI in the past year, use of amphetamines or poppers, or recent unprotected anal intercourse) should be screened as frequently as every three months. Despite these guidelines, HIV-infected MSM engaged in primary care do not routinely undergo recommended STI screenings.16,17 In a study of 8 large HIV clinics between 2004 and 2006, STI screening rates varied significantly by clinic, infection and anatomic site. Overall, syphilis screening occurred most frequently (66% – 76% of 1,334 HIV-infected MSM screened at least once annually) whereas rectal chlamydia screening the least (2.3% – 4.3% screened at least once annually). In general, gonorrhea and chlamydia screening, particularly at extra-genital (i.e. pharyngeal and rectal) sites occurred infrequently.18
Given these data, we undertook an initiative to increase bacterial STI screening among HIV-infected MSM engaged in HIV primary care. As part of this initiative we sought to 1) quantify baseline STI testing rates at the largest HIV care clinic in the Pacific Northwest U.S., and 2) identify barriers to STI screening as described by patients attending this clinic, and by these patients’ primary care providers.
METHODS
In 2012, in response to a CDC-initiated effort to enhance technical assistance to clinics providing HIV care, the University of Washington (UW) Sexually Transmitted Disease Prevention Training Center (PTC) developed a strategic plan to assess and enhance STI screening among HIV-infected persons seen at the clinic which serves the majority of such persons in Seattle and King County, Washington. The UW PTC is one of eight regional CDC PTCs that aim to educate and train clinicians in HIV/STI. As part of our strategic plan, we outlined a stepwise approach to increase STI testing: 1) determine a baseline estimate of current STI testing frequency; 2) understand why STI testing was not occurring routinely; and 3) develop an intervention to increase screening. Because this project was designed as a clinical quality improvement project, human subjects approval was not required.
The Harborview Madison Clinic is the largest HIV primary care clinic in the Pacific Northwest region of the United States. Located in Seattle, King County, Washington, it is part of the county hospital, Harborview Medical Center, and staffed by University of Washington physicians. In addition to primary care services, Madison Clinic offers specialized pharmacy services, case management, psychiatric and psychological services, neurology, dermatology, oncology and acupuncture. In 2012, there were 2,570 HIV-infected patients engaged in primary care services at Madison Clinic; 81% of those were men and 66% (n=1,374) of these men were identified by their primary care provider as MSM. We chose this HIV primary care site for this effort, reasoning that we could reach the largest local patient population at risk for STI, and potentially impact rates of STI screening. Additionally, the close alliance between the UW PTC faculty and the Madison Clinic helped facilitate the project.
In order to quantify baseline STI testing frequency in this patient population, we extracted testing data from the clinic’s electronic medical record’s laboratory data in aggregate, evaluating all MSM engaged in care. The designation of MSM was determined through provider documentation, and engagement in care was defined as attending at least two clinic visits in an 18-month period (March 2011 – September 2012). We chose to quantify testing frequencies because we did not have information on symptom status, and were unable to assess screening rates. We calculated testing frequencies for each bacterial STI and corresponding anatomic site as the number of MSM who received at least one test divided by all MSM engaged in care, as follows:
Syphilis testing throughout the study period was accomplished either with an EIA or a RPR. Nucleic acid amplification test (NAAT), using the Aptima Combo 2 (GenProbe, San Diego, CA) was the only testing modality for urine-based gonorrhea and chlamydia testing throughout the time period. NAAT, also with the Aptima Combo 2, was introduced for extra-genital (i.e. pharyngeal and rectal) gonorrhea and chlamydia testing in September of 2011. Prior to that time, the clinic relied on culture for pharyngeal and rectal gonorrhea and chlamydia testing. Thus, we considered either culture or NAAT of pharyngeal and rectal specimens in our analysis.
In order to evaluate patient-related barriers to STI testing in HIV primary care, we created a short, anonymous paper-based survey which was offered to all men in the clinic waiting room during check-in over a three-week period in May 2012. We employed a rapid assessment survey style, using 11 multiple choice questions and a free-response section. The survey focused on assessing sexual risk behaviors, most recent STI testing and source of care, and reasons for not seeking STI care in the primary care setting.
We evaluated provider-related barriers to STI testing in HIV primary care using an anonymous online-survey which was sent via email to all clinic providers in June 2012. The 33 providers invited to participate were all HIV primary care providers in Madison Clinic who care for a panel of HIV-infected persons. We aimed to assess knowledge-related barriers with both directed multiple choice questions as well as clinical scenarios. We also evaluated provider perspective for both patient and provider-related barriers to STI testing.
RESULTS
Baseline Testing Frequencies
Between March 2011 and September 2012, 1,456 MSM attended at least 2 clinic visits. In that time, 1,048 (72%) men had at least one syphilis serology; 586 (40%) provided a urine sample for gonorrhea and chlamydia testing; 466 (32%) had oropharyngeal testing for N. gonorrhoeae (culture or NAAT), and 422 (29%) underwent rectal swab for gonorrhea and/or chlamydia testing.
Patient-Related Barriers to STI Testing
Demographics and Risk Behaviors
We received 110 surveys completed by HIV-infected MSM, approximately 7.6% of all MSM engaged in care. Most (69%) of the men reported being sexually active in the prior two months with a median of 1 sexual partner (range 1–20). The majority reported participating in both insertive (68%) and receptive (69%) anal intercourse, as well as performing (79%) and receiving (75%) fellatio; 57% of MSM reported participating in all behaviors (Figure 1). Patients reported high-risk sexual behaviors including using, or their sexual partners using, amphetamines or other injection drugs (16.4%) or having had a bacterial STI (25.5%) in the past 12 months.
Figure 1.
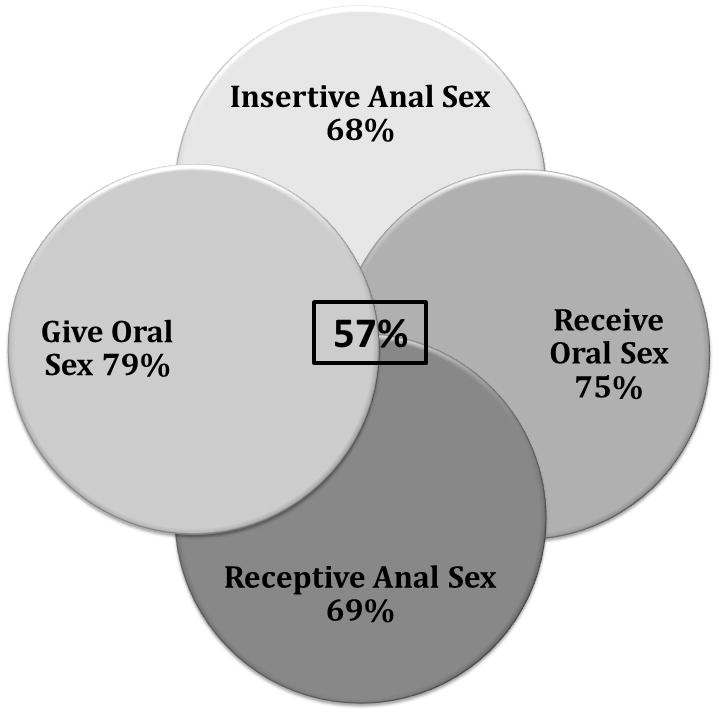
Reported sexual activities among HIV-infected MSM in an HIV Care Clinic (N=110)
Patient Self-reported STI Testing History
Patients reported their last STI screening as less than three months ago (44%), less than 6 months ago (20%), within the last 12 months (16%) or more than one year ago (19%). Over 83% reported being screened by a blood test, 56% with a urine test, 50% at the throat and 46% at the rectum. Among MSM who reported performing oral sex (n=87), 58% said they were screened at the throat at their last STI testing visit, and among the MSM who reported receptive anal sex (n=76), 53% said their rectum was tested at their last STI testing visit.
Usual place for STI testing
When asked where they usually go for STI testing, the majority of respondents reported Madison Clinic (i.e. their HIV primary care clinic) (72%). However, a substantial minority (28%) indicated that they attended the local STD clinic, while 3% stated they went to a local outreach site. Some (6.4%) reported that they did not routinely test for STI. The men who did not test at the HIV care clinic (n=31) were asked why they chose to seek STI care outside of their primary care clinic. The respondents wanted “easier” testing (42%), preferred anonymity (21%), wanted more frequent testing (16%), said they did not “need” the HIV care clinic (13%), forgot (5%), cited cost or convenience (5%), and reported not needing STI testing due to abstinence (5%) (answers not mutually exclusive) (Figure 2).
Figure 2.
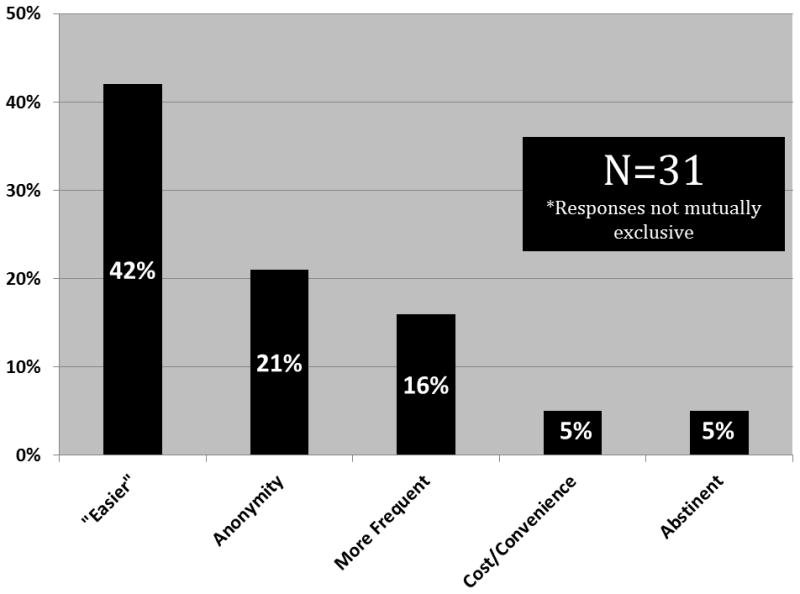
Reasons why HIV-infected MSM seek STI testing services outside of HIV primary care clinic
Provider-related Barriers to STI Testing
Twenty-eight (85%) of the clinic’s 33 care providers responded to the electronic survey. Respondents varied by level of training and consisted of ARNPs (n=1), medical students (n=1), residents (n=2), fellows (n=1), and attending physicians (n=23).
Knowledge-related Barriers
Some providers reported difficulties with both process and content knowledge: 25% were unaware of the availability of NAAT for extra-genital gonorrhea and chlamydia testing; 32% felt unsure about the current CDC STI testing guidelines; and 25% incorrectly identified the CDC-recommended two-drug therapy for pharyngeal gonorrhea.
Clinical Barriers
When asked about challenges related to complying with CDC guidelines for STI testing, providers responded that they lacked time (68%), patients seemed reluctant (39%), the provider was uncomfortable with discussing sexual practices and doing a genital exam (21%), they were afraid to appear judgmental (25%), and they lacked staff support and/or an interpreter (7%) (Figure 3). Providers reported their perception of why patients refused STI testing as patient reported not being “prepared” (55%), seeking testing elsewhere (i.e. the local STD Clinic) (82%), not believing they were at risk (64%), not having time (23%), or preferring a same-sex provider (27%) (responses not mutually exclusive).
Figure 3.
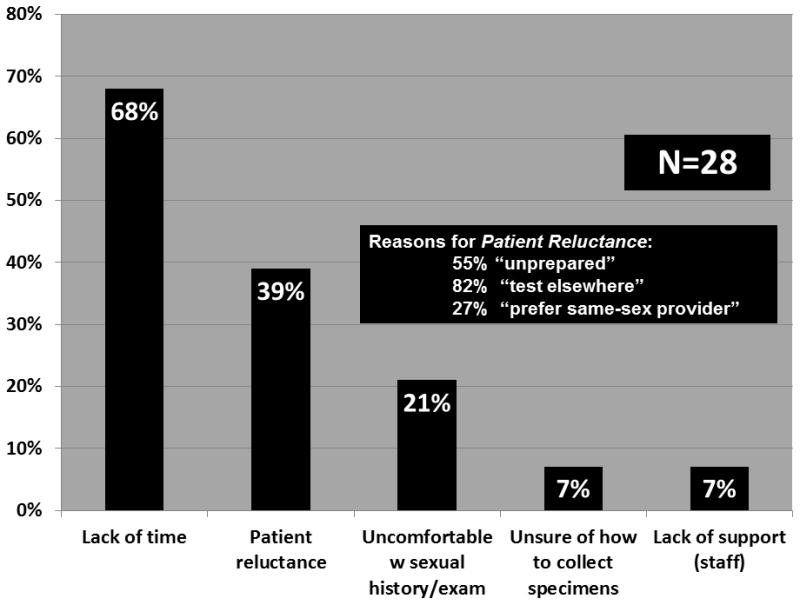
HIV care providers’ reported barriers to STI testing in HIV primary care
DISCUSSION
At the largest HIV care clinic in the Pacific Northwest U.S., we found that the rate of routine testing for bacterial STI among MSM was low. HIV-infected MSM engaged in care reported participating in high-risk sexual behaviors, believed that they were screening regularly, and for the most part sought STI testing at the HIV care clinic. However, nearly one-third sought care elsewhere because they wanted easier, more frequent testing, and some preferred anonymity. Equally important, our results indicate that many primary care providers in this setting would clearly benefit from enhanced STI and sexual health knowledge, as well as comfort with addressing sexual health. Moreover, most providers cited operational barriers to increased STI testing.
Our findings are similar to those of other studies that have shown that engagement in primary care does not decrease risk-taking behaviors. The patients who completed our survey, although engaged in HIV primary care, continue to participate in high-risk sexual behaviors, as evidenced by drug use (16%), self-reported bacterial STIs (25%) and reports of both insertive (68%) and receptive anal sex (69%). The participation in risky sexual behaviors among HIV-infected MSM engaged in care has been documented previously in a multi-site trial,8 suggesting this issue is not unique to the Pacific Northwest region. Evidence of these continued high-risk behaviors mandates an ongoing need for sexual risk assessment, risk reduction counseling, and STI testing and treatment in HIV care settings. Despite these findings, we found low levels of routine STI testing, similar to other published studies. A large medical record review of 8 HIV care clinics found that at least once annual extra-genital gonorrhea and chlamydia screening ranged from 2.3 – 18.3%.18 At the Johns Hopkins Hospital HIV Clinic between 1999 – 2007, only 39% of HIV-infected MSM were ever tested for gonorrhea and chlamydia, and only 10% of the enrollment visit STI testing included an extra-genital site.16 A 2008 – 2010 survey by the Medical Monitoring Project (MMP), which aims to generate a nationally representative sample of HIV-infected adults, estimated that among all MSM engaged in care, 54% were tested for syphilis, and 20% for gonorrhea and chlamydia in the last 12 months.17 Our baseline testing frequencies (29–32%) exceeded these numbers, but also occurred in the era of NAAT testing for extra-genital gonorrhea and chlamydia, an approach that is technically simpler than culture and considerably more sensitive. Interestingly, the frequency of syphilis testing (72%) in our analysis nearly matches the rate of syphilis testing (66–76%) in the eight-clinic review.18 The discrepancy between the frequency of extra-genital and syphilis testing is most likely related to the fact that syphilis testing relies on phlebotomy. Because HIV-infected patients routinely undergo blood draws to monitor HIV plasma viral loads and for anti-retroviral related toxicity, adding another test to the same sample is relatively simple. However, obtaining swabs of two anatomic sites – one of which necessitates the patient undressing – introduces additional barriers to testing.
Very few studies have examined patient-related barriers to STI testing in HIV primary care. One qualitative study that included both HIV-infected and -uninfected subjects asked about the features of an ideal STI/HIV testing environment. Similar to our findings, men in the that study wanted a testing environment that was “accessible… with walk-in hours”, offered anonymous testing, and was overall “community-based, friendly… and normalized… STD/HIV testing.”19 The patients who responded to our survey also indicated that they went outside of their primary care clinic for “easier”, anonymous, more frequent and convenient testing. Although “easier” was not defined in our survey, interpreted in the context of accessibility, it might also mean not needing an appointment, and being able to walk-in. However, “easier” may also refer to interactions with providers if related to the high proportion who preferred anonymity. Although anonymity is not possible in a primary care clinic, there may be ways to separate sexual health assessments and STI testing from the primary provider-patient relationship, allowing testing to occur within the medical home, yet not cause discomfort for either party. Additionally, as we move to less frequent routine HIV care appointment (i.e. every 6 months, as compared to every three months), high-risk MSM will need testing more frequently than can be offered during routine care. The availability of walk-in testing in between primary care visits may address this structural barrier.
Importantly, we found that a substantial proportion of health care providers lacked knowledge crucial for STI testing and treatment in terms of when and how to test, as well as how to treat a positive result. Ideally, this deficiency can be corrected through provider education. Similar to other studies, providers reported a lack of time and discomfort with taking a sexual history and performing genital exams as major barriers to STI testing. These have been consistent complaints from providers across the United States, both in studies specific to STI testing in HIV primary care20 and those on sexual behavior assessments in HIV/STD care settings.21 Those studies also identified competing medical priorities and cultural/language issues as a significant barriers to preventive services; our study did not directly assess those obstacles.
Our assessment has several limitations. Our testing coverage data is extracted in aggregate from the medical record. As such, it does not allow us to determine whether patients were asymptomatic at the time of testing (i.e. screening), their individualized risk status, anatomic sites of exposure, or frequency of testing. Thus, these testing rates may be either an under- or over-estimate of appropriate testing practice. Second, we used convenience sampling to recruit MSM to complete the patient survey; thus, these men may not be representative of the wider MSM clinic population at this clinic or in other parts of the United States. However, the self-reported history of recent bacterial STI (25%) is similar to directly assessed reports from other HIV care clinics.8 Additionally, we limited the questions to comply with a rapid assessment format because of the clinical setting of the survey and associated time constraints. Therefore, we were unable to assess STI testing knowledge as a potential barrier to more frequent testing. However, nearly half of the participants had tested in the last three months and over 60% within the last 6 months suggesting that on some level these men know regular STI testing is important. Finally, we are unable to correlate stage of training with the STI knowledge and comfort with sexual health evaluations as the provider survey was anonymous. However, even if all of the trainees reported deficits in knowledge and comfort questions, there were still several attending physicians who were unsure or uncomfortable as well.
Understanding the patient- and provider-related barriers to STI testing in HIV primary care is the first step to being able to implement an efficacious STI testing program. While increasing provider knowledge and comfort-level with sexual health needs of their HIV-infected patients must remain a priority, our data suggest that a convenient and “easy” STI testing program that relieves providers of the time burden while facilitating the assessment of sexual risk is needed. One such option is an STI self-testing program that allows for patients to self-assess risk, obtain their own swabs and utilize the laboratory for syphilis testing, virtually eliminating the need for the provider. In the context of an HIV care clinic, this program could function either with the provider recommending testing, or the patient seeking STI testing on his own, overcoming both patient and provider-related barriers. Current approaches to provide this option are in process.
SUMMARY.
The frequency of STI screening of MSM in HIV care remains low. Most patients seek STI testing through primary care, but some want easier, frequent, anonymous testing. Providers lack knowledge, comfort and time.
Acknowledgments
FUNDING SOURCES: This work was funded by the Centers for Disease Control and Prevention grants (University of Washington STD Training Center, PS1-1103), the National Institutes of Health (T32 AI07041 and K23 AI113185 to L.A.B.).
We appreciate the assistance of Amy Radford, University of Washington Seattle STD Prevention and Training Center Program Director, as well as the Madison Clinic staff, providers and patients, without whom this project would not be possible.
Footnotes
CONFLICTS OF INTERESTS: L.A.B. none; S.D. none; S.A.T. none; J.M.M. none
References
- 1.Morris SR, Klausner JD, Buchbinder SP, et al. Prevalence and incidence of pharyngeal gonorrhea in a longitudinal sample of men who have sex with men: the EXPLORE study. Clin Infect Dis. 2006 Nov 15;43(10):1284–1289. doi: 10.1086/508460. [DOI] [PubMed] [Google Scholar]
- 2.Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis. 2005 Jul 1;41(1):67–74. doi: 10.1086/430704. [DOI] [PubMed] [Google Scholar]
- 3.Gunn RA, O’Brien CJ, Lee MA, Gilchick RA. Gonorrhea screening among men who have sex with men: value of multiple anatomic site testing, San Diego, California, 1997–2003. Sex Transm Dis. 2008 Oct;35(10):845–848. doi: 10.1097/OLQ.0b013e318177ec70. [DOI] [PubMed] [Google Scholar]
- 4.Annan NT, Sullivan AK, Nori A, et al. Rectal chlamydia--a reservoir of undiagnosed infection in men who have sex with men. Sexually transmitted infections. 2009 Jun;85(3):176–179. doi: 10.1136/sti.2008.031773. [DOI] [PubMed] [Google Scholar]
- 5.Patton ME, Kidd S, Llata E, et al. Extragenital gonorrhea and chlamydia testing and infection among men who have sex with men--STD Surveillance Network, United States, 2010–2012. Clin Infect Dis. 2014 Jun;58(11):1564–1570. doi: 10.1093/cid/ciu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mimiaga MJ, Helms DJ, Reisner SL, et al. Gonococcal, chlamydia, and syphilis infection positivity among MSM attending a large primary care clinic, Boston, 2003 to 2004. Sex Transm Dis. 2009 Aug;36(8):507–511. doi: 10.1097/OLQ.0b013e3181a2ad98. [DOI] [PubMed] [Google Scholar]
- 7.CDC. Sexually Transmitted Disease Surveillance, 2011. Atlanta, GA: US Department of Health and Human Services; 2012. http://www.cdc.gov/std/stats11/Surv2011.pdf. [Google Scholar]
- 8.Mayer KH, Bush T, Henry K, et al. Ongoing sexually transmitted disease acquisition and risk-taking behavior among US HIV-infected patients in primary care: implications for prevention interventions. Sex Transm Dis. 2012 Jan;39(1):1–7. doi: 10.1097/OLQ.0b013e31823b1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rieg G, Lewis RJ, Miller LG, Witt MD, Guerrero M, Daar ES. Asymptomatic sexually transmitted infections in HIV-infected men who have sex with men: prevalence, incidence, predictors, and screening strategies. AIDS Patient Care STDS. 2008 Dec;22(12):947–954. doi: 10.1089/apc.2007.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keaveney S, Sadlier C, O’Dea S, Delamere S, Bergin C. High prevalence of asymptomatic sexually transmitted infections in HIV-infected men who have sex with men: a stimulus to improve screening. Int J STD AIDS. 2014 Sep;25(10):758–761. doi: 10.1177/0956462414521165. [DOI] [PubMed] [Google Scholar]
- 11.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999 Feb;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinghorn G. Pharyngeal gonorrhoea: a silent cause for concern. Sexually transmitted infections. 2010 Nov;86(6):413–414. doi: 10.1136/sti.2010.043349. [DOI] [PubMed] [Google Scholar]
- 13.HIV prevention through early detection and treatment of other sexually transmitted diseases--United States. Recommendations of the Advisory Committee for HIV and STD prevention. MMWR Recomm Rep. 1998 Jul;47(RR-12):1–24. [PubMed] [Google Scholar]
- 14.Korenromp EL, White RG, Orroth KK, et al. Determinants of the impact of sexually transmitted infection treatment on prevention of HIV infection: a synthesis of evidence from the Mwanza, Rakai, and Masaka intervention trials. J Infect Dis. 2005 Feb;191(Suppl 1):S168–178. doi: 10.1086/425274. [DOI] [PubMed] [Google Scholar]
- 15.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010 Dec 17;59(RR-12):1–110. [PubMed] [Google Scholar]
- 16.Berry SA, Ghanem KG, Page KR, Thio CL, Moore RD, Gebo KA. Gonorrhoea and chlamydia testing rates of HIV-infected men: low despite guidelines. Sex Transm Infect. 2010 Nov;86(6):481–484. doi: 10.1136/sti.2009.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flagg EW, Weinstock HS, Frazier EL, Valverde EE, Heffelfinger JD, Skarbinski J. Bacterial sexually transmitted infections among HIV-infected patients in the united states: estimates from the medical monitoring project. Sex Transm Dis. 2015 Apr;42(4):171–179. doi: 10.1097/OLQ.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoover KW, Butler M, Workowski K, et al. STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis. 2010 Dec;37(12):771–776. doi: 10.1097/OLQ.0b013e3181e50058. [DOI] [PubMed] [Google Scholar]
- 19.Mimiaga MJ, Goldhammer H, Belanoff C, Tetu AM, Mayer KH. Men who have sex with men: perceptions about sexual risk, HIV and sexually transmitted disease testing, and provider communication. Sex Transm Dis. 2007 Feb;34(2):113–119. doi: 10.1097/01.olq.0000225327.13214.bf. [DOI] [PubMed] [Google Scholar]
- 20.Carter JW, Hart-Cooper GD, Butler MO, Workowski KA, Hoover KW. Provider barriers prevent recommended sexually transmitted disease screening of HIV-infected men who have sex with men. Sex Transm Dis. 2014 Feb;41(2):137–142. doi: 10.1097/OLQ.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 21.Drainoni ML, Dekker D, Lee-Hood E, Boehmer U, Relf M. HIV medical care provider practices for reducing high-risk sexual behavior: results of a qualitative study. AIDS Patient Care STDS. 2009 May;23(5):347–356. doi: 10.1089/apc.2008.0063. [DOI] [PubMed] [Google Scholar]


