Abstract
Information on insulin resistance in human liver is limited. In mouse diet-induced obesity (DIO), hepatic insulin resistance initially involves: lipid + insulin-induced activation of atypical protein kinase C (aPKC); elevated Akt activity/activation but selective impairment of compartmentalized Akt-dependent FoxO1 phosphorylation; and increases in gluconeogenic and lipogenic enzymes. In advanced stages, e.g., in hepatocytes of type 2 diabetes (T2D) humans, insulin activation of insulin receptor substrate-1(IRS-1) and Akt fails, further increasing FoxO1-dependent gluconeogenic/lipogenic enzyme expression. Increases in hepatic PGC-1α also figure prominently, but uncertainly, in this scheme. Here, we examined signaling factors in liver samples harvested from human transplant donors with increasing BMI, 20→25→30→35→40→45. We found, relative to lean (BMI=20–25) humans, obese (BMI>30) humans had all abnormalities seen in early mouse DIO, but, surprisingly, at all elevated BMI levels, had decreased insulin receptor-1 (IRS-1) levels, decreased Akt activity, and increased expression/abundance of aPKC-ι and PGC-1α. Moreover, with increasing BMI, there were: progressive increases in aPKC activity and PKC-ι expression/abundance; progressive decreases in IRS-1 levels, Akt activity and FoxO1 phosphorylation; progressive increases in expression/abundance of PGC-1α; and progressive increases in gluconeogenic and lipogenic enzymes. Remarkably, all abnormalities reached T2D levels at higher BMI levels. Most importantly, both “early” and advanced abnormalities were largely reversed by 24-hour treatment of T2D hepatocytes with aPKC inhibitor. We conclude: hepatic insulin resistance in human obesity is: advanced; BMI-correlated; and sequentially involves increased aPKC-activating ceramide; increased aPKC levels and activity; decreases in IRS-1 levels, Akt activity, and FoxO1 phosphorylation; and increases in expression/abundance of PGC-1α and gluconeogenic and lipogenic genes.
Introduction
Obesity, the metabolic syndrome and type 2 diabetes mellitus (T2DM) have reached epidemic levels in Western/Westernized populations. Pathogenetic mechanisms underlying these disorders are obscure, particularly in humans, and particularly in poorly accessible, but critically important, liver. Nevertheless, a common pathogenetic factor in mouse models of obesity and humans with obesity or T2DM, is insulin resistance, which implies an impairment in glucose homeostasis and secondary increases in insulin secretion.
In mouse models, impairments in glucose homeostasis have been provoked by knocking out genes required for glucose uptake in muscle (1, 2), or using hypercaloric diets that impair insulin signaling factors that restrain hepatic gluconeogenesis. With either approach, compensatory hyperinsulinemia paradoxically activates insulin-sensitive factors in pathways that remain “open”: most notably, hyperinsulinemia-induced increases in hepatic lipogenic enzymes that follow either impaired glucose uptake in muscle (1, 2) or accelerated hepatic gluconeogenesis (3–6). Moreover, increases in hepatic lipogenesis contribute importantly to development of hepatosteatosis, abdominal obesity, hypertriglyceridemia and hypercholesterolemia (2–6).
In humans, insulin resistance in obese adolescents initially involves an increase in hepatic gluconeogenesis (7) that progresses (8). Similarly, in mouse diet-induced obesity (DIO), caloric excesses provoke initial increases in hepatic gluconeogenic enzymes that lead to systemic insulin resistance and liverdependent decreases in insulin signaling in muscle (3, 4). Assuming that dietary excesses are also important in human obesity, the startup of insulin resistance therein is likely to involve alterations in hepatic factors that promote gluconeogenesis.
Normally, insulin suppresses hepatic gluconeogenesis by diminishing mRNAs encoding phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase). This effect of insulin is mediated mainly through insulin receptor substrate(IRS)-1 (9), activation of phosphatidylinositol 3-kinase (PI3K), and generation of phosphatidylinositol-3,4,5-(PO4)3 (PIP3), which activates Akt. In turn, Akt phosphorylates/inactivates forkhead homeobox class O protein, FoxO1, which otherwise increases activity through a binding mechanism (10), and/or increases mRNA (11) encoding peroxisome proliferator-activated receptor-γ (PPARγ) coactivator-1α (PGC-1α), which, along with FoxO1 and hepatic nuclear factor-4 (HNF4), increases expression/abundance of PEPCK and G6Pase (10, 11). However, in liver, insulin, via IRS-2 (but not IRS-1) (9), PI3K and PIP3also activates atypical protein kinase C (aPKC), which, along with Akt, mediates stimulatory effects of insulin on hepatic lipogenesis (12–20).
Unfortunately, aPKCs are activated by lipids other than insulin-dependent PIP3and excessive aPKC can oppose certain effects of Akt. Thus, in liver, dietary excesses of fat (3) or carbohydrate (4) in mouse DIO lead to increases in ceramide, which, perhaps with other lipids [e.g., phosphatidic acid (PA)], directly activates aPKC. Combined activation of aPKC by ceramide and hyperinsulinemia-derived PIP3 causes excessive aPKC accumulation on scaffolding protein, WD40/ProF, and displacement of activated Akt from this platform, where Akt otherwise phosphorylates/inhibits FoxO1 (3, 4, 21–23). Accordingly, in early mouse DIO, insulin effects on gluconeogenic enzyme expression are impaired, hepatic glucose output increases, hyperinsulinemia ensues and activates hepatic IRS-1/Akt and IRS-2/aPKC, which, together, increase lipogenic enzyme expression and hepatic lipid production (3, 4, 12–20). In keeping with a key role of excessive hepatic aPKC activity in causing abnormalities in early mouse DIO, selective inhibition of hepatic aPKC reverses aberrant increases in expression of hepatic gluconeogenic and lipogenic enzymes, and this is followed improvements in insulin signaling in muscle and clinical abnormalities, viz., glucose intolerance, abdominal obesity, hepatosteatosis, hypertriglyceridemia, and hypercholesterolemia (3, 4, 20).
Although hepatic IRS-1/PI3K/Akt activation is hyperactivated in early phases of insulin resistance in mouse DIO (3, 4, 24), in more advanced states, hepatic IRS-1/PI3K/Akt activation is compromised, e.g., in livers of T2D rodents (16, 24) and hepatocytes of T2D humans (17). Additionally, in hepatocytes of T2D humans, increases in aPKC activity are intensified by excessive expression of mRNA encoding primate-specific PKC-ι through an insulin/aPKC-dependent, feed-forward/positive-feedback mechanism (17) [this auto-catalytic process is not seen with PKC-λ or PKC-ζ (17).
Although hepatic abnormalities in insulin-dependent aPKC and Akt signaling have been partially characterized in early phases of mouse DIO (3, 4) and advanced phases in T2D humans (17), information on hepatic insulin signaling in obese humans is lacking. Also note that human liver contains aPKC (17) and PGC-1α (11) isoforms that have overlapping, but distinctly different, properties, as compared to murine isoforms. We therefore performed a cross-sectional analysis of insulin signaling factors and expression/abundance of PGC-1α and gluconeogenic and lipogenic enzymes, and aPKC-ι itself, in livers harvested from nominally non-diabetic humans that had increasing degrees of obesity, and compared findings therein to lean non-diabetic and T2D humans. In short, we examined the progression of hepatic signaling abnormalities as a function of increases in body mass index (BMI) throughout the obesity/diabetes spectrum. Also, in primary cultures hepatocytes of T2D humans, we examined the dependence of “early” and advanced aberrations on aPKC.
Methods
Livers and Hepatocytes
Flash-frozen liver samples and cryo-preserved viable hepatocytes (Zen-Bio Corp, Research Triangle, NC, USA) were harvested from human transplant donors (livers rejected primarily for compatibility, or occasionally administrative, issues) in the following BMI groups: (#1) lean, 6 females (F) and 6 males (M), age=50.2±2.1, BMI=22.5±0.4; (#2) overweight 6F/3M, age=55.3±4.6, BMI=27.5±2.7; (#3) mildly obese, 4F/4M, age=54.4±2.2, BMI=33.0±0.6; (#4) moderately obese, 4F/5M, age=52.9±2.6, BMI=37.0±0.6; (# issues5) markedly obese, 4F/2M, age=48.2±3.8, BMI=44.1±1.0; and (#6) T2D, 4F/1M, age=53.4±3.9, BMI=29.7±3.6 (all values are mean±SEM). As transplant donors, subjects were on life-support systems and maintained on intravenous glucose-containing (presumably hypocaloric) fluids for variable periods of hospitalization prior to demise. Although the diagnosis of diabetes was based largely upon history, random blood glucose levels (mg/dL) in the above-described groups were in keeping: #1, 128±16; #2, 116±15; #3, 131±8; #4, 123±19; #5, 123±4; and #6/T2D 250±43. Other clinical data were not available, except that some T2D subjects received insulin treatment. Note that all subjects were 40–75 years of age, of variable sex, race and color, and, to avoid any biases, we used liver samples from all subjects available in each BMI group. Also note that non-diabetic subjects were historically free of, and not undergoing medical treatment for, any chronic disease or disorder prior to hospitalization, and did not receive medications that appeared to have influenced the findings. All studies were approved by the Human Experimentation Internal Review Board (IRB) of the University of South Florida College of Medicine, as well as IRBs at organ-origination sites.
Hepatocyte and Lysate Preparations and Assays
As described (17, 27), hepatocytes were incubated, livers and hepatocytes were homogenized, and aPKCs were immunoprecipitated from lysates with rabbit polyclonal antiserum (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) which recognizes both PKC-ζ and PKC-λ/ι, collected on Sepharose-AG beads and assayed. aPKC activation was also assessed by immunoblotting for phosphorylation of the auto(trans)phosphorylation site, thr-555/560 in PKC-ι/ζ, required for, and reflective of, activation (25). Akt2 enzyme activity was assayed in immunoprecipitates (Millipore kit), as described (3, 4, 16, 17, 19, 20). Akt activity was also assessed by immunoblotting for phosphorylation of ser-473-Akt.
Antibodies
Western analyses and immunoprecipitations were conducted as described (3, 4, 16, 17, 19, 20, 27) using: anti-phospho-serine-473-Akt, anti-glyceraldehyde-phosphate dehydrogenase (GAPDH), anti-WD40/ProF, anti-PEPCK, anti-G6Pase, anti-FAS, and anti-aPKC (Santa Cruz Biotechnologies, Santa Cruz, CA); antiphospho-threonine-560/555-PKC-ζ/λ/ι (Invitrogen, Carlsbad, CA); anti-p-serine-256-FoxO1, anti-FoxO1 (Abnova, Walnut, CA); anti-PKC-λ/ι (Transduction Antibodies, Bedford, MA); anti-phospho-serine-9-GSK3β, anti-GSK3β, anti-phospho-serine-2248-mTOR, anti-mTOR, anti-ACC and mouse anti-Akt Mab (Cell Signaling Technologies, Danvers, MA); anti-IRS-1, anti-IRS-2 (Millipore, Temecula, CA); anti-SREBP (Thermo Fisher Scientific, Freemont, CA); anti-PKC-ζ (Dr. Todd C. Sacktor, State University of New York, NY); and anti-PGC-1α (Genetex, Irvine, CA), a C-terminally-directed antiserum that measures 77kDa and 91kDa PGC-1α isoforms; however, we report only on the much more abundant 77kDa isoform, which, is specifically present in human liver, and strongly influenced by FoxO1 (11).
mRNA Measurements
As described (3, 4, 16, 17, 19, 20), tissues/hepatocytes were added to Trizol reagent (Invitrogen) and RNA was extracted and purified with RNA-Easy Mini-Kit and RNAase-free DNAase Set (Qiagen, Valencia, CA), and quantified by real-time reverse transcriptase-polymerase chain reaction (RT-PCR), using TaqMan reverse transcription reagent and SYBR Green kit (Applied Biosystems, Carlsbad, CA) with human nucleotide primers and horse radish peroxidase transferase as an internal recovery standard. Note, for PGC-1α, we measured mRNA encoding the shortened liver-specific human isoform in which exon 1 and 2 of the longer human isoform are replaced by a silent exon taken from the first intron of the longer isoform, as described (11).
Nuclear Preparations
Ceramide Species Quantitation
Ceramide species were measured by LC-MS/MS analysis of lipid extracts of liver lysates by Lipidomics Shared Resource, Medical University of South Carolina, Charleston, SC.
Liver Triglycerides
Statistical Evaluations
Data are expressed as mean±SEM, and P values were determined by one-way ANOVA and least-significant multiple comparison methods. Linear correlations and P values were determined by IBM-SPSS software.
Results
Alterations in Activities of aPKC and Akt, Recruitment of aPKC and Akt to the WD40/ProF Scaffolding Platform, and FoxO1 Phosphorylation in Livers of Obese and T2D Humans
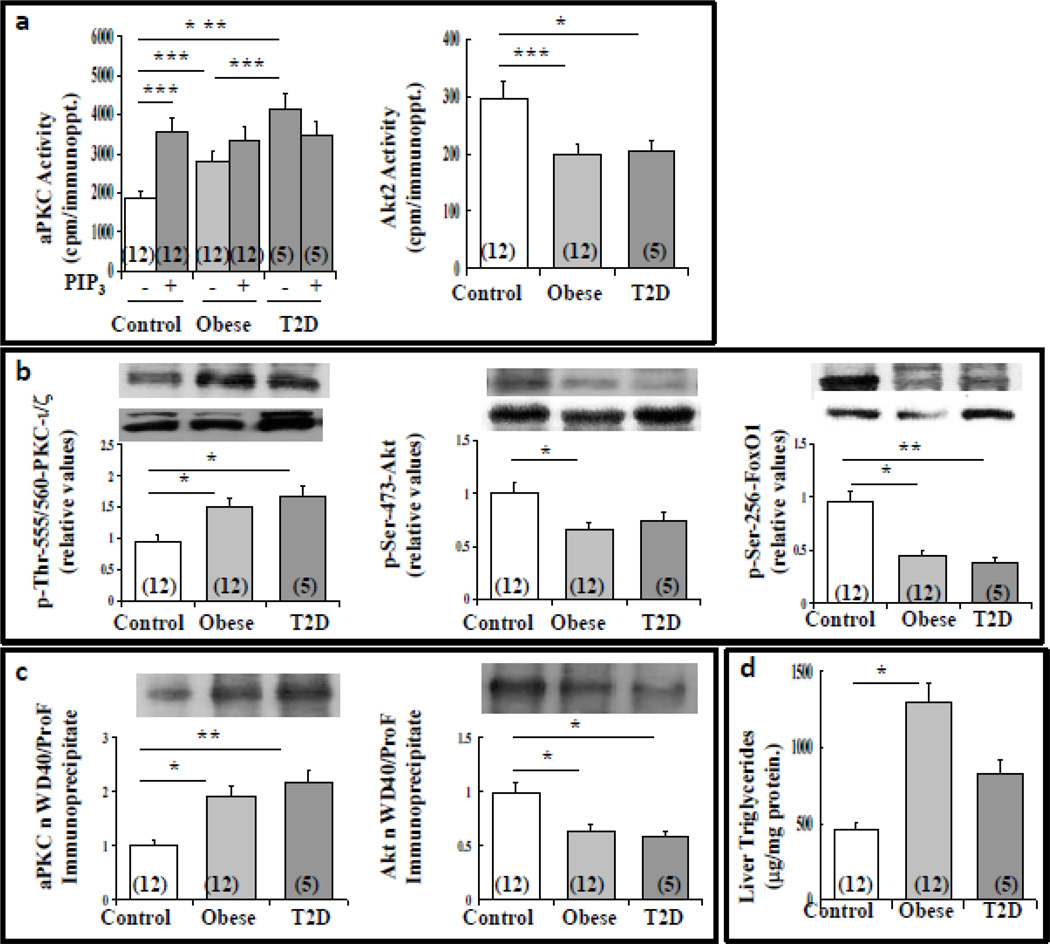
Initially, in livers of lean (BMI=20–25), obese (BMI=34–44), and T2D (BMI=23–44) humans, we found that aPKC enzyme activity (Fig 1a) and phosphorylation of the auto(trans)phosphorylation site, thr-555/560-PKC-ι/ζ (Fig 1b) were partially elevated in obese humans, and further activated in T2D humans. Accordingly, immunoprecipitable aPKC harvested from livers of lean controls was strongly activated in vitro by PIP3but not when harvested from T2D liver (Fig 1a), suggesting prior maximal activation by ceramide, phosphatidic acid (PA) (3, 25, 26), and/or insulin-stimulated PIP3.
Figure 1.
Alterations in enzyme activity of aPKC and Akt (panel a), phosphorylation of aPKC, Akt and FoxO1 (panel b), association of aPKC and Akt with immunoprecipitable WD40/ProF (panel c) and triglyceride levels (panel d) in livers of lean, obese and T2D humans. Values are mean ± SEM of (N) patients. Asterisks indicate: *, P<0.05; **, P<0.01; and ***, P<0.001 (ANOVA). Representative blots are shown in panels b and c (upper bands, phosphoproteins; lower bands, total proteins).
In contrast to aPKC, Akt2 activity, as per enzymatic assay (Fig 1a) and phosphorylation of ser-473-Akt (Fig 1b), was diminished in livers of obese and T2D humans, as was the phosphorylation of the Akt substrate, FoxO1 (Fig 1b). Note that insulin activation of both aPKC and Akt normally leads to increases in their association with scaffold protein, WD40/ProF; however, in livers of DIO mice, excess accumulation of aPKC diminishes Akt association with WD40/ProF (3, 4). As a result, Akt-dependent FoxO1 phosphorylation, which, occurs largely on this platform (3, 4, 21–23), is impaired, even when hepatic Akt activity is increased, as in early experimental DIO (3, 4). Similarly, aPKC association with WD40/ProF was increased, and Akt association with WD40/ProF was diminished, in livers of obese and T2D humans (Fig 1c); thus, decreases in FoxO1 phosphorylation probably reflected both impaired activation of Akt and its displacement from WD40/ProF.
Alterations in Hepatic Triglycerides in Obese and T2D Humans
Liver triglycerides were increased in obese non-diabetic humans, and trended upward, albeit to a lesser degree, in T2D humans (Fig 1d). Also note that labelled-acetate incorporation into neutral lipids is increased by an aPKC-dependent mechanism in hepatocytes of obese and T2D humans (4).
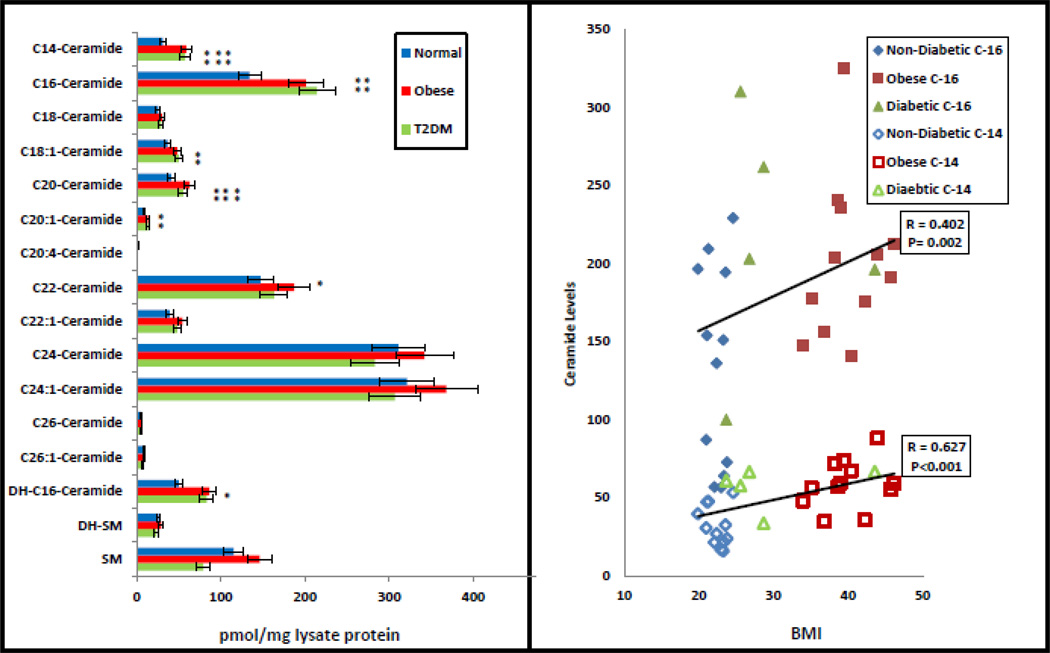
Alterations in Hepatic Ceramide Species in Obese and T2D Humans
As in experimental DIO models (3, 4), wherein ceramide was shown to directly activate hepatic aPKC (3), the content of ceramide, particularly species containing saturated C-14 and C-16 fatty acids, was comparably elevated in livers of obese and T2D humans, and these species were correlated with BMI levels (Fig 2).
Figure 2.
Contents of ceramide and sphingomyelin species in livers of lean, obese and type 2 diabetic (T2DM) humans. Values are mean ± SEM of 12 normal, 12 obese and 5 T2D humans. Asterisks indicate: *, P<0.05; **, P<0.01; and ***, P<0.001 (ANOVA). Shown at right are correlations between saturated C-14 (open symbols) and C-16 (closed symbols) ceramide species and BMI levels of individual subjects [note that values of T2D subjects are shown at the right irrespective oof BMI levels; also note that, owing to similar BMI values, some symbols are overlapping].
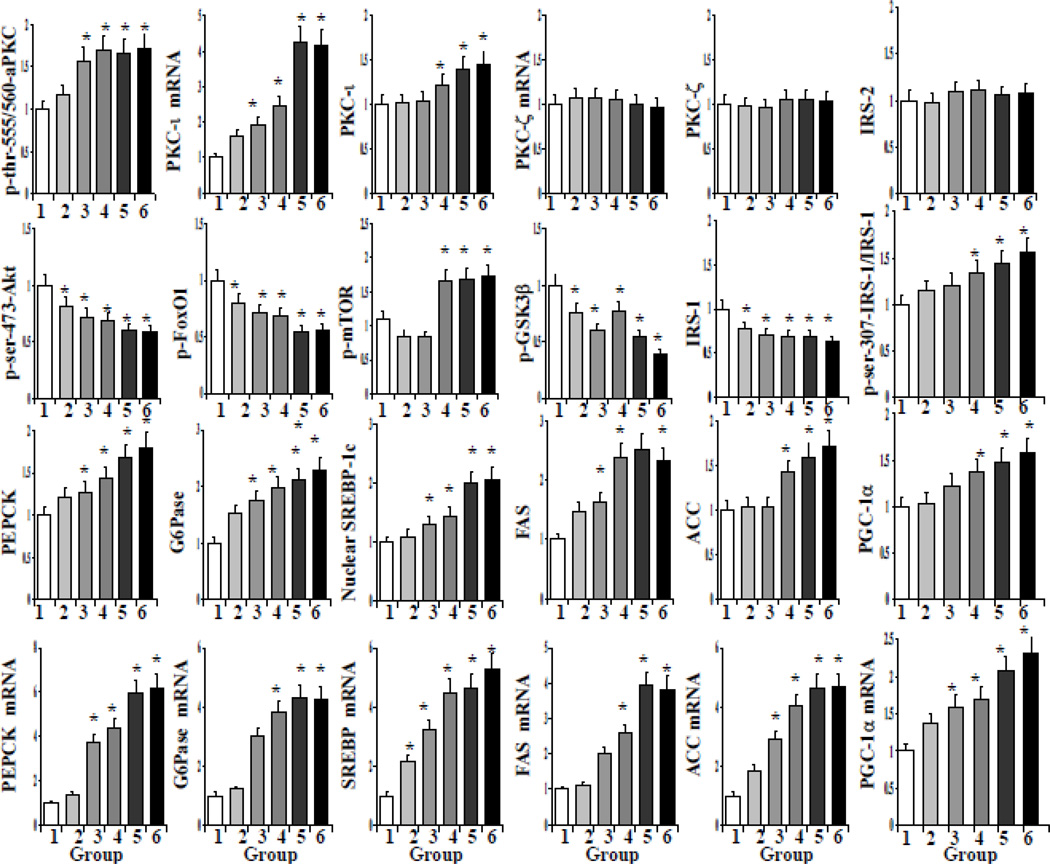
Graded Alterations in Signaling Factors and Gluconeogenic and Lipogenic Enzymes in Livers of Obese and T2D Humans
As initial findings did not reveal remarkable differences between moderately-to-markedly obese and T2D humans, we examined livers in greater detail to see if hepatic abnormalities progressed with BMI i, as follows: (group-1) lean controls, BMI=20→25; (group-2), BMI=25→30; (group-3), BMI=30→35; (group-4), BMI=35→40; (group-5), BMI=40→45; and (group-6), T2D (BMI=23–44). Relative to group-1, aPKC phosphorylation was elevated in all obesity groups, i.e., BMI>30, and increased to levels seen in T2D as BMI increased (Figs 3, 4). These increases in BMI and aPKC activity were accompanied by progressive decreases in: Akt phosphorylation; phosphorylation of Akt substrates, GSK3β and FoxO1; and levels of IRS-1, the major activator of hepatic Akt (9) (Figs 3, 4). In conjunction with decreases in FoxO1 phosphorylation, there were progressive increases in mRNA and protein levels of PGC-1α, gluconeogenic enzymes, PEPCK and G6Pase, lipogenic enzymes, SREBP-1c (i.e., the active nuclear SREBP-1c proteolytic fragment), FAS and ACC (Figs 3, 4). In addition, RNA and protein levels of PKC-ι, but not PKC-ζ, increased progressively along with BMI (Figs 3, 4). Despite decreases in Akt activity, mTOR phosphorylation increased in the more obese and T2D humans (Figs 3, 4). In contrast to IRS-1, IRS-2 levels were unchanged (Figs 3, 4). Also of interest, ser-307-IRS-1 phosphorylation, which negatively affects IRS-1, was increased (Figs 3, 4).
Figure 3.
BMI-related alterations in: (top row), phosphorylation of aPKC and expression and abundance of aPKCs and IRS-2; (2nd row), phosphorylation of Akt, FoxO1, mTOR and GSK3β, and IRS-1 levels; (3rd row), protein levels of gluconeogenic and lipogenic enzymes and PGC-1α; and (4th row) mRNA levels of gluconeogenic and lipogenic enzymes and PGC-1α in livers of lean group-1 [BMI=20-25, N=12], overweight group-2 [BMI=25-30, N=9], obese group-3 [BMI=30-35, N=8], obese group-4 [BMI=36-40, N=9]; obese group-5 [BMI=40-45, N=6], and T2D group-6 [BMI=23-44, N=5]. Relative values are mean ± SEM. Asterisk indicates: *, P<0.05 (ANOVA) versus lean control value. Representative blots are shown in Fig 4.
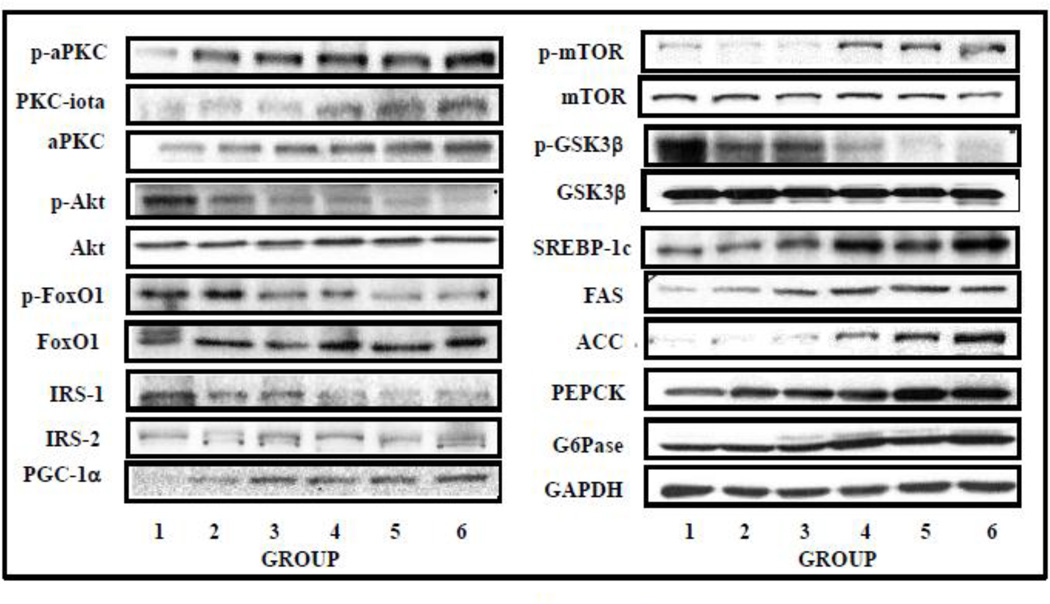
Figure 4.
BMI-related alterations in: phosphorylation status of aPKC, Akt, FoxO1, mTOR, and GSK3β; and levels of PKC-ι, aPKC, Akt, IRS-1, IRS-2, FoxO1, mTOR, GSK3β, PEPCK, G6Pase, active nuclear fragment of SREBP-1c, FAS, ACC, GAPDH and PGC-1α in livers of lean control, obese and T2D humans. Representative blots are shown here. See Fig 3 for group details and mean group values.
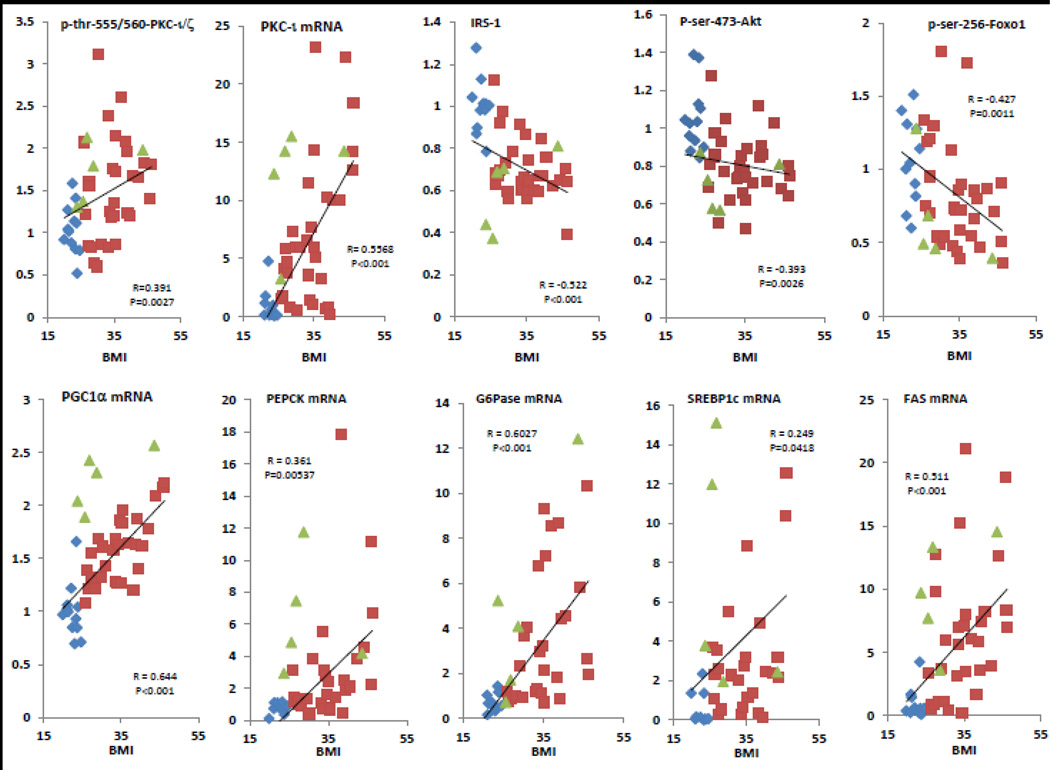
Shown in Figure 5 are correlation plots of the more relevant parameters as functions of BMI.
Figure 5.
Correlations between aPKC activity (p-thr-555/560-PKC-ι/ζ), PKC-ι mRNA, IRS-1 levels, Akt activity (p-ser-473-Akt), p-ser-256-FoxO1, PGC-1α mRNA, PEPCK mRNA, G6Pase mRNA, SREBP-1c mRNA and FAS mRNA versus BMI in livers of lean control (blue diamonds), obese (red squares) and type 2 diabetic (green triangles) humans.
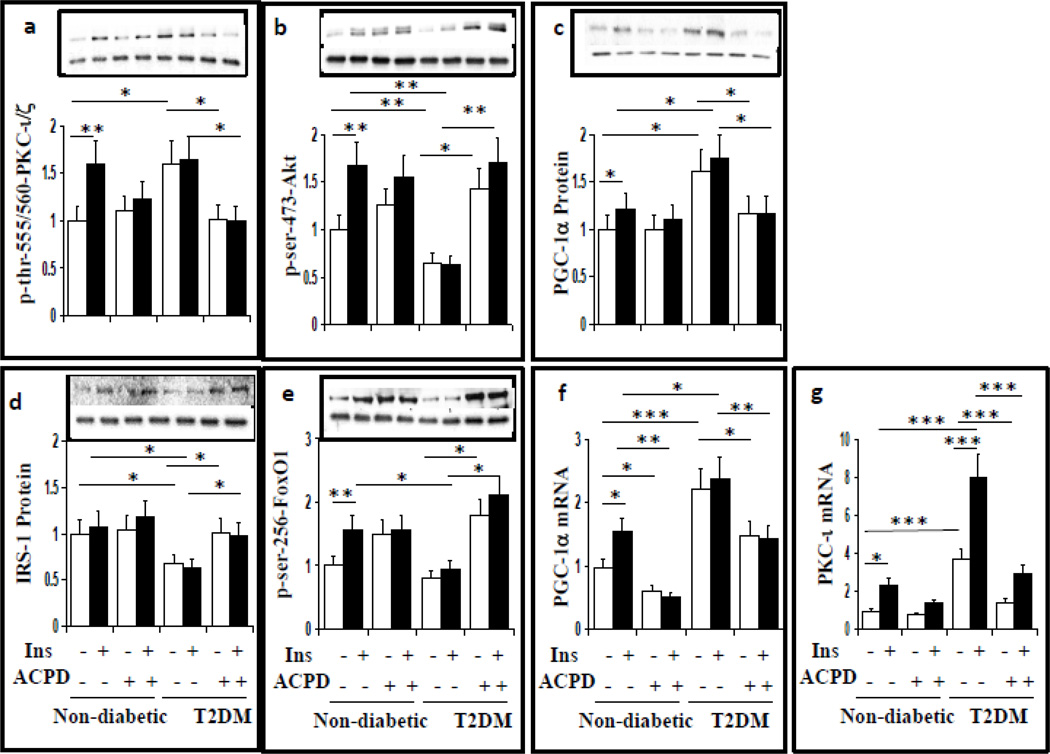
Effects of aPKC inhibition on IRS-1 Levels, Insulin Signaling to aPKC, Akt and FoxO1, Expression of PGC-1α and Gluconeogenic and Lipogenic Enzymes in Livers of T2D Humans
It was important to examine aPKC-dependence of early and advanced hepatic abnormalities. Here, we used 2-acetyl-cyclopentane-1,3-dione (ACPD) [which comparably inhibits recombinant PKC-ι and PKC-ζ (3, 4, 27)] in concentrations previously (3) and presently (Figs 6a, 7) found to inhibit insulin-stimulated aPKC activity in isolated hepatocytes of lean non-diabetic humans. Here again, ACPD inhibited T2D-induced and T2D-plus-insulin-induced increases in aPKC (Fig 6a), but not Akt, (Fig 6b) activity. Most importantly, ACPD concomitantly: (a) restored T2D-induced decreases in IRS-1 levels (Fig 6d), Akt activity (Fig 6b) and FoxO1 phosphorylation (Fig 6e); and (b) reversed insulin-stimulated and T2D-induced increases in expression (Fig 6f) and abundance of PGC-1α. (Fig 6c), and expression of PKC-ι (Fig 6g).
Figure 6.
Effects of aPKC inhibitor, ACPD, on phosphorylation/activation of aPKC (a) and Akt (b), PGC-1α protein levels (c), IRS-1 protein levels, (d), phosphorylation of FoxO1, (e) PGC-1α mRNA levels (f) and PKC-ι mRNA levels (g) in hepatocytes of lean control and T2D humans. Hepatocytes were incubated 24 hours ± 1µM insulin (Ins) ± 1µM ACPD, as described (17, 27) [note that, as reported (17) 1µM insulin was needed to maintain full activation of aPKC and Akt over the course of a 24-hour incubation]. Relative values are mean±SEM of 4 patients. Asterisks indicate P<0.05 (ANOVA). Representative blots of phosphoproteins are shown in upper bands, and loading controls in lower bands.
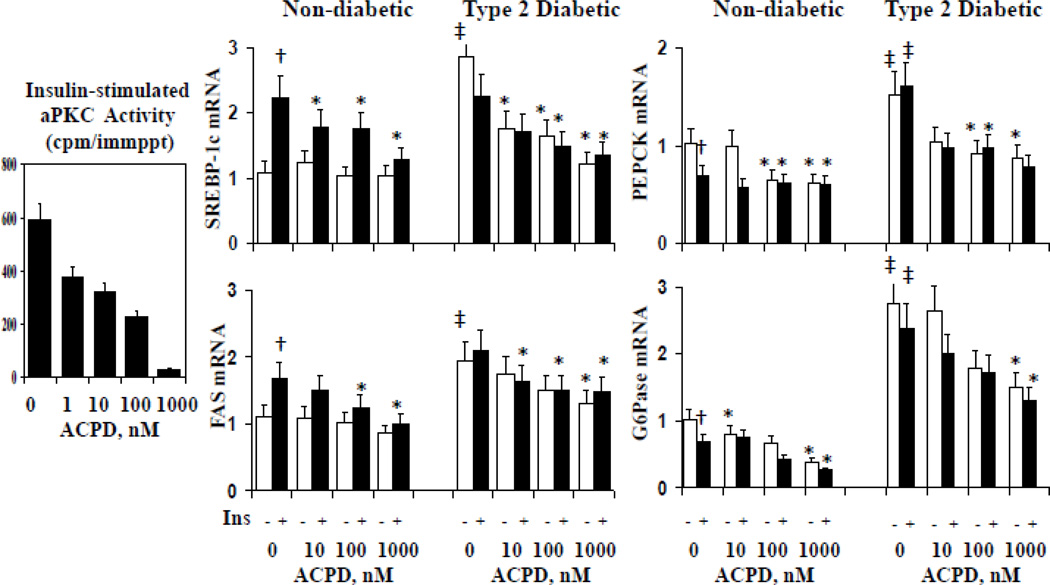
In addition to improving IRS-1 levels, Akt activity, FoxO1 phosphorylation, PGC-1α expression and PKC-ι expression, ACPD dose-dependently diminished: (a) insulin-stimulated aPKC activity in hepatocytes of lean non-diabetic humans; (b) stimulatory effects of insulin and T2D on expression of lipogenic enzymes, SREBP-1c and FAS, in hepatocytes of lean non-diabetic and T2D humans; and (c) stimulatory effects of T2D on gluconeogenic enzymes, PEPCK and G6Pase, in hepatocytes of T2D humans (Fig 7).
Figure 7.
Dose-related effects of aPKC inhibitor, ACPD, on insulin-stimulated aPKC activity in hepatocytes of lean non-diabetic humans (left panel), and expression (mRNA levels) of SREBP-1c, FAS, PEPCK and G6Pase in hepatocytes of lean and T2D humans. Hepatocytes were incubated 24 hours ± 1µM insulin (Ins) ± indicated concentrations of ACPD, as described (17, 27). Relative values are mean±SEM of 4 patients. Single crosses (insulin-stimulated value vs adjacent basal value), double crosses (basal or insulin-stimulated value in T2D versus corresponding control value), and asterisks (control or T2D value of samples incubated with ACPD versus corresponding value of samples incubated without ACPD) indicate P<0.05 (ANOVA).
It may be noted that ACPD increased basal as well as insulin-stimulated phosphorylation of Akt and FoxO1 in hepatocytes of non-diabetic and T2D humans (Fig 6), and that ACPD decreased basal as well as T2D-induced increases in gluconeogenic enzyme expression (Fig 7). These alterations most likely reflect a release of a tonic inhibition of Akt by aPKC, followed by increases in FoxO1 phosphorylation and decreases in expression of PGC-1α and gluconeogenic enzymes, as similar results were previously seen (17, 19, 20, 27, 28). Of further note, 24-hour insulin treatment provoked modest, but significant, increases in mRNA (Fig 6f) and protein (Fig 6c) levels of PGC-1α in hepatocytes of lean non-diabetic humans, and 24-hour inhibition of aPKC diminished basal and insulin-stimulated PGC-1α mRNA levels (Fig 6f).
Discussion
Similar to findings in initial phases of mouse DIO (3, 4), we found that hepatic ceramide levels and aPKC activity were increased, and Akt-dependent FoxO1 phosphorylation was decreased, in livers of obese humans. On the other hand, it was surprising to find decreases in IRS-1 levels and Akt activity, even at lesser BMI increases in human liver, as these abnormalities are initially absent in mouse DIO (3, 4). However, note: liver samples were obtained from humans with well-established, presumably long-standing, obesity, as compared to livers form mice with short-term (2–3-month) DIO (3, 4); and diminished IRS-1 levels and impaired Akt activation develop later, as glucose intolerance progresses in rodents (24, 29) and humans (17).
The present findings suggested important interrelationships between increases in: (a) hepatic aPKC activity; (b) hepatic signaling abnormalities, i.e., decreases in IRS-1 levels, Akt activity and FoxO1 phosphorylation; (c) expression/abundance of PGC-1α; and (d) expression/abundance of gluconeogenic and lipogenic enzymes. Thus, as in mouse DIO (3, 4), the present findings were in keeping with the idea that increases in hepatic ceramide (±PA) contributed importantly to activation of aPKC, which in turn provoked alterations in hepatic insulin signaling to IRS-1, Akt and FoxO1 that led to increases in expression of PGC-1α and gluconeogenic and lipogenic enzymes (Fig 8). However, whereas increases in hepatic ceramide (±PA) in mouse DIO may have reflected dietary excesses of lipid or carbohydrate (3, 4, 29), the present samples came from humans on life-support measures, and dietary contributions are uncertain. Nevertheless, there may well have been increases in fatty acids and glycerol released from expanded fat stores and converted to ceramide (±PA) in liver; alternatively, ceramide/PA may have been increased by other mechanisms. In any case, as in mouse DIO (3, 4), lipid-induced increases in hepatic aPKC activity would be expected to have contributed to presently-observed aPKC increases and Akt decreases on the WD40/ProF platform in livers of obese and T2D humans; in addition, presently-observed decreases in Akt activation would also diminish its presence on the WD40/ProF platform (21–23). Either way, the resultant impairment of FoxO1 phosphorylation would increase nuclear retention/import of FoxO1, and, as presently seen in livers of obese and T2D humans, elicit increases in transcription of PGC-1α and gluconeogenic and lipogenic genes.
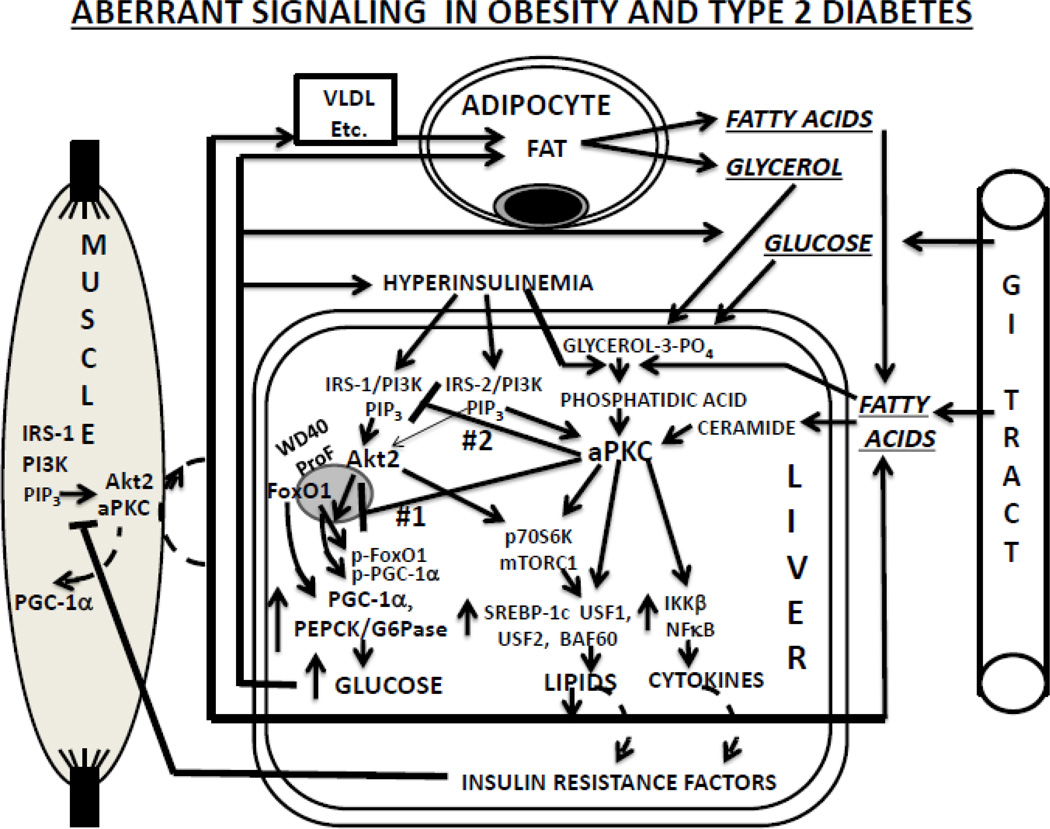
Figure 8.
Aberrations in insulin signaling and consequences thereof in human obesity and T2DM. Note that inhibition of Akt-dependent FoxO1 phosphorylation on the WD40/ProF platform (defect #1) presumably precedes IRS-1 loss and impairment in Akt activation (defect #2).
It is noteworthy, not only that hepatic IRS-1 levels, Akt activity and FoxO1 phosphorylation diminished as BMI levels increased, but, even more so that aPKC inhibition rapidly reversed IRS-1/AktFoxO1 abnormalities in T2D hepatocytes. These findings may reflect that aPKC directly phosphorylates IRS-1 and thereby impairs IRS-1/PI3K/Akt activation (30). However, aPKC also inhibits Akt by other mechanisms (31–35), and we also found BMI-related increases in phosphorylation of ser-307-IRS-1, which inhibits/destabilizes IRS-1.
Presently-observed increases in hepatic lipogenic enzymes in livers of obese and T2D humans were presumably provoked by combined increases in aPKC activity and phosphorylation/activation of mTOR. In this regard, note that both aPKC-phosphorylated/activated Brg1/Brm-associated factor-60C (BAF60c) and Akt- or other kinase-stimulated mTORC1 participate in SREBP-1c-dependent hepatic lipogenic enzyme expression (12–18). Also note that, although the elevation of mTOR phosphorylation in the face of diminished Akt activity may be surprising, it suggests that a modicum of Akt is sufficient, or factors other than Akt can increase mTOR phosphorylation in livers of obese and T2D humans, e.g., aPKC or other PKCs reportedly activate mTOR and/or p70/S6kinase (36, 37). In any case, presently-observed increases in SREBP-1c activity (nuclear levels of the active proteolytic fragment) and transcription of genes encoding SREBP-1c and SREBP-1c-dependent lipogenic enzymes, e.g., FAS and ACC, would be expected to provoke increases in hepatic and liver-derived circulating lipids, in particular, VLDL, that are cleared and stored in fat depots. In keeping with these ideas: expression of hepatic lipogenic enzymes and clinical lipid abnormalities, viz., abdominal obesity, hepatosteatosis, hypertriglyceridemia and hypercholesterolemia, are improved by selective inhibition of hepatic aPKC by various methods in mouse models of insulin resistance (3, 4, 16, 17, 19, 20, 27, 28); and inhibition of hepatic aPKC, shown here with pan-aPKC inhibitor, ACPD, and previously with other aPKC inhibitors (17, 27), largely reduced lipogenic enzyme expression in hepatocytes of T2D humans.
The progressive increases in hepatic PKC-ι mRNA and protein throughout the obesity/T2D spectrum are particularly noteworthy, as these increases are elicited by insulin via an aPKC-dependent mechanism (17). The present findings therefore suggest that, as in overt T2DM, a feed-forward/positive-feedback mechanism is operative in livers of obese humans and exacerbates the problem of excessive increases in aPKC activity; in turn, this would set up a vicious cycle in liver and heighten systemic insulin resistance. Furthermore, this auto-dependency of PKC-ι transcription suggests that increases in hepatic ceramide, PA and/or insulin-dependent PIP3 may initiate and/or exacerbate this abnormality in hepatic aPKC expression/abundance. Also note that, unlike liver, wherein IRS-2-dependent PI3K regulates aPKC activity during insulin action (9, 17) and PKC-ι levels are increased, the opposite situation exists in muscles of T2D humans (17), where IRS-1-dependent PI3K, which is downregulated in obesity and T2D, activates muscle aPKC (38–41). Thus, dependence of muscle aPKC activity on IRS-1/PI3K may explain why mRNA and protein levels of PKC-ι (the major aPKC component in human muscle) are diminished in muscles of T2D humans (17, 42, 43), and presumably contribute to the impairment in insulin-stimulated glucose disposal in T2D humans (42, 43).
It was interesting to find that mRNA and protein levels of hepatic PGC-1α increased progressively with BMI to reach T2D levels. Even more noteworthy, mRNA and protein levels of PGC-1α were reduced to normal by 24-hour incubation of hepatocytes of T2D humans with aPKC inhibitor, ACPD. Although a reduction in PGC-1α expression might be expected to occur with ACPD-induced increases in Akt activity and FoxO1 phosphorylation, it should be noted that 24-hour insulin treatment of hepatocytes of lean non-diabetic humans modestly increased PGC-1α expression by an aPKC-dependent mechanism. Accordingly, the decreases in PGC-1α expression seen in T2D hepatocytes following ACPD treatment may reflect a reversal of direct stimulatory effects of activated aPKC, as well as the restoration of Akt-dependent decreases in FoxO1 activity. As a corollary, these findings raise an interesting question of how insulin diminished PEPCK/G6Pase expression in the face of increased PGC-1α expression in normal hepatocytes. This paradox may be explained by Akt-dependent phosphorylation/inhibition of PGC-1α (46), and/or inhibition resulting from reduced availability of FoxO1 needed for direct binding and activation of PGC-1α (10); in either case, the inhibitory effects of insulin on PGC-1α activity would prevail over increases in PGC-1α expression during suppression of PEPCK/G6Pase expression. Further studies are needed to further elucidate how insulin increases PGC-1α expression by an aPKC-dependent mechanism, and how insulin diminishes gluconeogenic enzyme expression despite increases in PGC-1α expression.
As alluded to, although information on nutritional status during the hospitalization period prior to demise was not available, it may be questioned how hepatic ceramide levels might have been maintained in obese subjects who were most likely receiving hypocaloric intravenous fluids. As shown in Fig 8the expanded adipocyte mass in obese subjects could reasonably have provided the liver with substrates for production of ceramide and/or phosphatidic acid. This possibility is supported by a recent paper suggesting that increased ceramide metabolism in adipocytes can increase hepatic ceramides and activate hepatic aPKC (47).
Finally, it worth emphasizing that, in using post-mortem livers samples taken from rejected transplant donors in which relevant clinical information is lacking, there are some inherent caveats that must be kept in mind. First, the interval between death and tissue harvesting is uncertain and there may have been variable hypoxia. Second, as to factors directly affecting insulin sensitivity, patients in all groups were stressed to variable degrees, on the one hand, and most likely undernourished while receiving hypocaloric intravenous glucose-containing fluids, on the other hand, for variable periods prior to actual demise. Third, the diagnosis of type 2 diabetes was primarily based on clinical history, and there is a strong likelihood that some patients, particularly in the more obese groups, would have been classified as diabetic with more rigorous testing, thus contributing to the overlap of findings in the high-obese and diabetic groups. Fourth, glucose-containing intravenous solutions most likely increased blood insulin levels in most subjects who had responsive pancreatic islet β-cells. Despite these inherent caveats, the relationships between BMI and multiple insulin resistance parameters were internally-consistent, pathophysiologically logical, and, for the most part, surprisingly strong.
To summarize, the present findings show that an advanced form of hepatic insulin resistance is present throughout the obesity spectrum and progresses in intensity as BMI increases, ultimately reaching levels seen in overt T2DM. Although the present findings do not address the question of whether obesity initiates hepatic insulin resistance, or vice versa, findings in experimental models show that dietary excesses can provoke hepatic insulin resistance in mice, and epidemiological data suggest that dietary excesses are important determinants of hepatic insulin resistance in humans. Accordingly, we may surmise that many or most humans naturally possess the genetic apparatus that allows dietary excesses to initiate or abet development of hepatic insulin resistance, most likely through increases in ceramide or other aPKC-stimulatory lipids, and subsequent increases in activity and levels of aPKC that lead to impairments in IRS-1 and Akt, followed by activation of FoxO1 and PGC-1α and subsequent increases in PEPCK/G6Pase expression. Fortunately, improvements in all of these advanced hepatic abnormalities can be reversed by appropriate treatment modalities.
Acknowledgements
This work does not represent the views of the Department of Veteran Affairs or the United States government. Dr. Robert V. Farese is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was supported by funds from the Department of Veterans Affairs Merit Review Program and the National Institutes of Health Grants [7RO1DK 065969-09 to R.V. Farese].
List of abbreviations
- ACC
Acetyl-CoA carboxylase
- ACPD
2-Acetyl-1,3-cyclopentanedione
- aPKC
Atypical protein kinase C
- Akt
Protein kinase B
- BMI
Body Mass Index
- DIO
Diet-induced obesity
- FAS
Fatty acid synthase
- FoxO1
Forkhead box O1
- GAPDH
Glyceraldehyde-phosphate dehydrogenase
- G6Pase
Glucose-6-phosphatase
- GSK
Glycogen synthase kinase
- IRS-1
Insulin receptor-1
- PEPCK
Phosphoenolpyruvate carboxykinase
- PGC-1α
PPARγ coactivator-1-α
- PI3K
Phosphatidylinositol 3-kinase
- PIP3
Phosphatidylinositol-3,4,5-(PO4)3
- PPARγ
Peroxisome proliferator-activated receptor-γ
- SREBP-1c
Sterol receptor element binding protein-1c
- mTORC
Mammalian target of rapamycin-C
- T2D
Type 2 diabetes
- WD40/ProF
40KDa scaffolding protein, WD (tryp-asp-x-x)-repeat, propeller-like, FYVE-containing protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contribution Statement
RV Farese conceived, designed and directed the studies, analyzed data, and wrote the paper. MP Sajan and RA Ivey conducted studies, performed assays, assembled data, and assisted in interpretation of data.
Duality of Interest
There are no conflicts of interest amongst the authors.
References
- 1.Minokoshi Y, Kahn CR, Kahn BB. Tissue-specific ablation of the GLUT4 glucose transporter or the insulin receptor challenges assumptions about insulin action and glucose homeostasis. J. Biol. Chem. 2003;278:33609–33612. doi: 10.1074/jbc.R300019200. [DOI] [PubMed] [Google Scholar]
- 2.Farese RV, Sajan MP, Wang H, Li P, Mastorides S, Gower WR, Jr, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M. Muscle-specific knockout of PKC-λ impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 2007. 2007;117:2289–2301. doi: 10.1172/JCI31408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sajan MP, Acevedo-Duncan ME, Standaert ML, Ivey RA, III, Lee M, Farese RV. Akt-dependent phosphorylation of hepatic FoxO1 is compartmentalized on a WD40/Propeller/FYVE scaffold and is selectively inhibited atypical PKC in early phases of diet-induced obesity. Diabetes. 2014;63:2690–2701. doi: 10.2337/db13-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sajan MP, Acevedo-Duncan ME, Standaert ML, Ivey RA, III, Lee MC, Farese RV. Hepatic insulin resistance in ob/ob mice involves increases in ceramide, atypical PKC activity and selective impairment of Akt-dependent FoxO1 phosphorylation. J Lipid Res. 2015;56:70–80. doi: 10.1194/jlr.M052977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khamzina L, Vielleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinol. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 6.Liu H-Y, Hung T, Wen G-B, et al. Increased basal level of Akt-dependent insulin signaling may be responsible for the development of insulin resistance. Am. J. Physiol. Metab. 2009:E898–E906. doi: 10.1152/ajpendo.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sunehag AL, Toffolo G, Campioni M, Bier DM, Haymond MW. Effects of dietary macronutrient intake on insulin sensitivity and secretion and glucose and lipid metabolism in healthy obese adolescents. J Clin Endo Metab. 2006;90:4496–4502. doi: 10.1210/jc.2005-0626. [DOI] [PubMed] [Google Scholar]
- 8.Chung ST, Hsia DS, Chacko SK, Rodriguez LM, Haymond MW. Increased gluconeogenesis in youth with newly diagnosed type 2 diabetes. Diabetologia. 2015;58:596–603. doi: 10.1007/s00125-014-3455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo S, Copps KD, Dong X, Park S, Cheng Z, Pocai A, Rosetti L, Sajan M, Farese RV, White MF. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol. 2009;29:5070–5083. doi: 10.1128/MCB.00138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated gluconeogenesis through FOXO1-PGC-1α interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 11.Felder TK, Soyal SM, Oberkofler H, Hahne P, Auer S, Weiss R, Gadermaier G, Miller K, Krempler F, Esterbauer H, Patsch W. Characterization of novel peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) isoform in human liver. J Biol Chem. 2011;286:42923–42938. doi: 10.1074/jbc.M111.227496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleischmann M, Iynedjian PB. Regulation of sterol regulatory element binding protein 1 gene expression in liver: Role of insulin and protein kinase B/cAkt. Biochem. J. 2000;349:13–17. doi: 10.1042/0264-6021:3490013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. USA. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto M, Ogawa W, Akimoto K, Matsumoto M, Ogawa W, Akimoto K, Inoue H, Miyaki K, Furukawa K, Hayashi Y, et al. PKCλ in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest. 2003;112:935–944. doi: 10.1172/JCI18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taniguchi CM, Kondo T, Sajan MP, Luo J, Bronson R, Asano T, Farese RV, Cantley LC, Kahn CR. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKCλ/ζ. Cell Metab. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Sajan MP, Standaert ML, Rivas J, Miura A, Kanoh Y, Soto J, Taniguchi CM, Kahn CR, Farese RV. Role of atypical protein kinase C in activation of sterol regulatory element binding protein-1c and nuclear factor kappa B (NFκB) in liver of rodents used as model of diabetes, and relationships to hyperlipidaemia and insulin resistance. Diabetologia. 2009;52:1197–1207. doi: 10.1007/s00125-009-1336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sajan MP, Farese RV. Insulin Signalling in Hepatocytes of Type 2 Diabetic Humans. Excessive Expression and Activity of PKC-ι and Dependent Processes and Reversal by PKC-ι Inhibitors. Diabetologia. 2012;55:1446–1457. doi: 10.1007/s00125-012-2477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Wong RHF, Tang T, Hudak CS, Yang D, Duncan RE, Sul HS. Phosphorylation and recruitment of BAF60c in chromatin remodeling for lipogenesis in response to insulin. Mol Cell. 2014;49:293–297. doi: 10.1016/j.molcel.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sajan MP, Nimal S, Mastorides S, Acevedo-Duncan M, Kahn CR, Fields AP, Braun U, Leitges M, Farese RV. Correction of Metabolic Abnormalities in a Rodent Model of Obesity, Metabolic Syndrome and Type 2 Diabetes by Inhibitors of Hepatic Protein Kinase C-iota. Metabolism. 2012;61:459–469. doi: 10.1016/j.metabol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sajan MP, Standaert ML, Nimal S, Varanasi U, Pastoor T, Mastoroides S, Braun U, Leitges M, Farese RV. Critical role of atypical protein kinase C in activating hepatic SREBP-1c and NFκB in obesity. J Lipid Res. 2009;50:1133–1145. doi: 10.1194/jlr.M800520-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritzius T, Burkard G, Haas E, Heinrich J, Schweneke M, Bosse M, Zimmerman S, Frey AD, Caelers A, Bachmann AS, Moelling KA. WD-FYVE protein binds to the kinases Akt and PKCζ/λ. Biochem J. 2006;399:9–20. doi: 10.1042/BJ20060511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritzius T, Moelling K. Akt and Foxo1-interacting WD-repeat-FYVE protein promotes adipogenesis. The EMBO J. 2008;27:1399–1410. doi: 10.1038/emboj.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritzius T, Frey AD, Schweneker M, Mayer D, Moelling K. WD-repeat-propeller-FYVE protein, ProF, binds VAMP2 and protein kinase Cζ. FEBS J. 2007;274:1552–1566. doi: 10.1111/j.1742-4658.2007.05702.x. [DOI] [PubMed] [Google Scholar]
- 24.Standaert ML, Sajan MP, Miura A, Standaert ML, Sajan MP, Mirua A, Kanoh Y, Chen HC, Farese RV, Jr, Farese RV. Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabetic (ob/ob and Goto-Kakizaki) liver. Contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high-fat-induced insulin-resistant states. J Biol Chem. 2004;279:24929–24934. doi: 10.1074/jbc.M402440200. [DOI] [PubMed] [Google Scholar]
- 25.Farese RV, Sajan MP. Metabolic Functions of Atypical Protein Kinase C: “Good and Bad” as Defined by Nutritional Status. (Review) Am J Physiol Endocrinol Metab. 2010;298:E385–E394. doi: 10.1152/ajpendo.00608.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivey RA, III, Sajan MP, Farese RV. Pseudosubstrate arginine residues are required for auto-inhibition and are targeted by phosphatidylinositol-3.4.5-(PO4)3 during aPKC activation. Journal of Biological Chemistry. 2014;289:25021–25030. doi: 10.1074/jbc.M114.565671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sajan MP, Ivy RA, III, Farese RV. Meformin action in human hepatocytes: coactivation of atypical protein kinase C alters 5’-AMP-activated protein kinase effects on lipogenic and gluconeogenic enzyme expression. Diabetologia. 2013;56:2507–2516. doi: 10.1007/s00125-013-3010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sajan MP, Ivey RA, III, Lee MC, Mastorides S, Jurzak MJ, Samuels VT, Shulman GI, Braun U, Leitges M, Farese RV. PKCλ haplo-insufficiency prevents diabetes by a mechanism involving alterations in hepatic enzymes. Molecular Endocrinology. 2014;28:1097–1107. doi: 10.1210/me.2014-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang G, Badeaulou L, Bielawski J, et al. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Amer J. Physiol. Endocrinol. Metab. 2009;297:E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S, Lynn EG, Kim JA, Quon M. Protein kinase C-zeta phosphorylates insulin receptor substrate-1, -3 and -4 but not -2; isoform specific determinants of specificity in insulin signaling. Endocrinol. 2008;149:2451–2458. doi: 10.1210/en.2007-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konishi H, Kuroda S, Kikkawa U. The pleckstrin homology domain of RAC protein kinase associates with the regulatory domain of protein kinase Cζ. Biochem Biophys Res Comm. 1994;205:1770–1775. doi: 10.1006/bbrc.1994.2874. [DOI] [PubMed] [Google Scholar]
- 32.Doornbos RP, Theelen M, van der Hoeven PC, et al. Protein kinase Cζ is a negative regulator of protein kinase B activity. J Biol Chem. 1999;274:8589–8596. doi: 10.1074/jbc.274.13.8589. [DOI] [PubMed] [Google Scholar]
- 33.Weyrich P, Nuescheler D, Melzer M, et al. The Par6alpha/aPKC complex regulates Akt1 activity by phosphorylating Thr34 in the PH-domain. Mol Cell Endocrinol. 2007;268:30–36. doi: 10.1016/j.mce.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Mahfouz R, Khoury R, Blanchnio-Zabielska A, Turban S, Loiseauu N, Lipina C, Stretton C, Bourron O, Ferre P, Foufelle F, Hundal H, Hajduch E. Characterising the inhibitory action of ceramide upon insulin signaling in different skeletal muscle cell models; a mechanistic insight. PLOS One. 2014;9:1–9. doi: 10.1371/journal.pone.0101865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blouin CM, Prado C, Takane KK, Lasnier F, Garcia-Ocana A, Ferre P, Dugail I, Hajduch E. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes. 2010;59:600–610. doi: 10.2337/db09-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 37.Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005;280:25485–25490. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- 38.Sajan MP, Standaert ML, Miura A, Kahn RC, Farese RV. Tissue-specific differences in activation of atypical protein kinase C and protein kinase B in muscle, liver and adipocytes of insulin receptor substrate-1 knockout mice. Mol Endocrinol. 2004;18:2513–2521. doi: 10.1210/me.2004-0045. [DOI] [PubMed] [Google Scholar]
- 39.Ueki K, Yamauchi T, Tamemoto H, Tobe K, Yamamoto-Honda R, Akanuma Y, Kaburagi Y, Yazaki Y, Aizawa S, Nagai R, Kadowaki T. Restored insulin-sensitivity in IRS-1-deficient mice treated by adenovirus-mediated gene therapy. J Clin Invest. 2000;105:1437–1445. doi: 10.1172/JCI7656. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamouchi T, Tobe K, et al. Insulin signaling and insulin actions in the muscles and livers of insulin-resistant, insulin receptor substrate 1-deficient mice. Mol Cell Biol. 1996;16:3074–3084. doi: 10.1128/mcb.16.6.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valverde AM, Burks DJ, Fabregat I, Fisher TL, Carretero J, White MF, Benito M. Molecular mechanisms of insulin resistance in IRS-2-deficient hepatocytes. Diabetes. 2003;52:2239–2248. doi: 10.2337/diabetes.52.9.2239. [DOI] [PubMed] [Google Scholar]
- 42.Kim Y-B, Kotani K, Ciaraldi TP, et al. Insulin-stimulated protein kinase C-λ/ζ activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes; reversal with weight reduction. Diabetes. 2003;52:1935–1942. doi: 10.2337/diabetes.52.8.1935. [DOI] [PubMed] [Google Scholar]
- 43.Beeson M, Sajan MP, Dizon M, Grebenev G, Gomez-Daspet J, Miura A, Kanoh Y, Powe J, Bandyopadhyay G, Standaert ML, Farese RV. Activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance. Amelioration by rosiglitazone and exercise. Diabetes. 2003;52:1926–1934. doi: 10.2337/diabetes.52.8.1926. [DOI] [PubMed] [Google Scholar]
- 44.Standaert ML, Bandyopadhyay G, Perez L, Price D, Galloway L, Poklepovic A, Sajan MP, Cenni V, Sirri A, Moscat J, Toker A, Farese RV. Insulin activates PKC-ζ and PKC-λ by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J. Biol. Chem. 1999;274(36):25308–25316. doi: 10.1074/jbc.274.36.25308. [DOI] [PubMed] [Google Scholar]
- 45.Standaert ML, Bandyopadhyay G, Kanoh Y, Sajan MP, Farese RV. Insulin and PIP3 activate PKC-ζ by mechanisms that are both dependent and independent of phosphorylation of activation loop (T410) and Autophosphorylation (T560) Sites. Biochemistry. 2000;40:249–255. doi: 10.1021/bi0018234. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1a transcription activator. Nature. 2007;447:1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 47.Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, Pearson MJ, Sifuentes AJ, McDonald JG, Gordillo R, Scherer PE. Targeted induction of ceramide degradation leads tto improved systemic metabolism and reduced hepatic steatosis. Cell Metab. doi: 10.1016/j.cmet.2015.06.007. July 15, 20015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]