Abstract
Background.
Studies have shown an independent association between poor self-rated health (SRH) and increased mortality. Few studies, however, have investigated any possible impact on SRH of diagnostic labelling.
Objective.
To test whether SRH differed in persons with known and unknown hypothyroidism, diabetes mellitus (DM) or hypertension, opposed to persons without these conditions, after 11-year follow-up.
Methods.
Prospective population-based cohort study in North-Trøndelag County, Norway, HUNT2 (1995–97) to HUNT3 (2006–08). All inhabitants aged 20 years and older were invited. The response rate was 69.5% in HUNT2 and 54.1% in HUNT3. In total, 34144 persons aged 20–70 years were included in the study population. The outcome was poor SRH.
Results.
Persons with known disease had an increased odds ratio (OR) to report poor SRH at follow-up; figures ranging from 1.11 (0.68–1.79) to 2.52 (1.46–4.34) (men with hypothyroidism kept out owing to too few numbers). However, in persons not reporting, but having laboratory results indicating these diseases (unknown disease), no corresponding associations with SRH were found. Contrary, the OR for poor SRH in women with unknown hypothyroidism and unknown hypertension was 0.64 (0.38–1.06) and 0.89 (0.79–1.01), respectively.
Conclusions.
Awareness opposed to ignorance of hypothyroidism, DM and hypertension seemed to be associated with poor perceived health, suggesting that diagnostic labelling could have a negative effect on SRH. This relationship needs to be tested more thoroughly in future research but should be kept in mind regarding the benefits of early diagnosing of diseases.
Key words: Cohort, diabetes mellitus, hypertension, hypothyroidism, longitudinal, self-rated health.
Introduction
A person’s perception of own health is a valuable health measure (1,2). Although not uniquely conceptualized, self-rated health (SRH) is found to be associated with several aspects of life (3); functional disability, psychological factors, various socioeconomic factors as well as morbidity and mortality (4–7). However, whether SRH is affected by focus on elevated disease risk or by awareness of asymptomatic disease has been sparsely investigated.
Lowering of diagnostic cut-offs are regularly discussed by ‘Task forces’ for many chronic conditions, and for risk factors gradual lowering of cut-offs already has been implemented. As a consequence, the European guidelines for handling risk for fatal cardiovascular disease label almost the total population as ‘at risk’ (8).
Prevention of disease implies early behavioural and medical assessments, but it can also imply unnecessary risk detection and early diagnostics without necessarily improving disease prognosis. In academic medicine, themes such as ‘too much medicine’ and ‘overdiagnosis’ are increasingly discussed (9,10). However, among health care politicians, people’s reluctance to consult health care seems to be of greater concern than the potential problems of overdiagnosis.
Except for arterial hypertension (11–14), adverse effects of labelling of disease have been sparsely explored. Interestingly, one study showed increased mortality among participants made aware of their chronic kidney disease, relative to the unaware participants, also after adjustments for disease severity (15).
In a cross-sectional study, we recently reported known hypothyroidism, diabetes mellitus (DM) and hypertension to be independently associated with poor SRH, without corresponding association between unknown or probable disease and poor SRH (16). As cross-sectional design hinders evaluation of causation, in this study, we aimed to study if SRH was influenced by awareness versus ignorance of disease in persons with probable hypothyroidism, DM or hypertension at 11-year follow-up.
Methods
Study population
All adult inhabitants aged 20 years and older have been invited to three surveys of the Nord-Trøndelag Health Study (HUNT); this study includes data from HUNT2 (1995–97) and HUNT3 (2006–08) (17,18). All together 65237 participants (69.5% of the invited) and 50807 (54.1%) completed health-related questionnaires, inter alia on SRH, thyroid diseases, DM and hypertension in HUNT2 and HUNT3, respectively. According to the HUNT2 study protocol, thyroid-stimulating hormone (TSH) was measured in all women and in a 50% random sample of men aged 40 years and older, whilst in HUNT3, TSH was measured in all participants. Blood pressure (BP), non-fasting serum glucose, height and weight were measured in all participants.
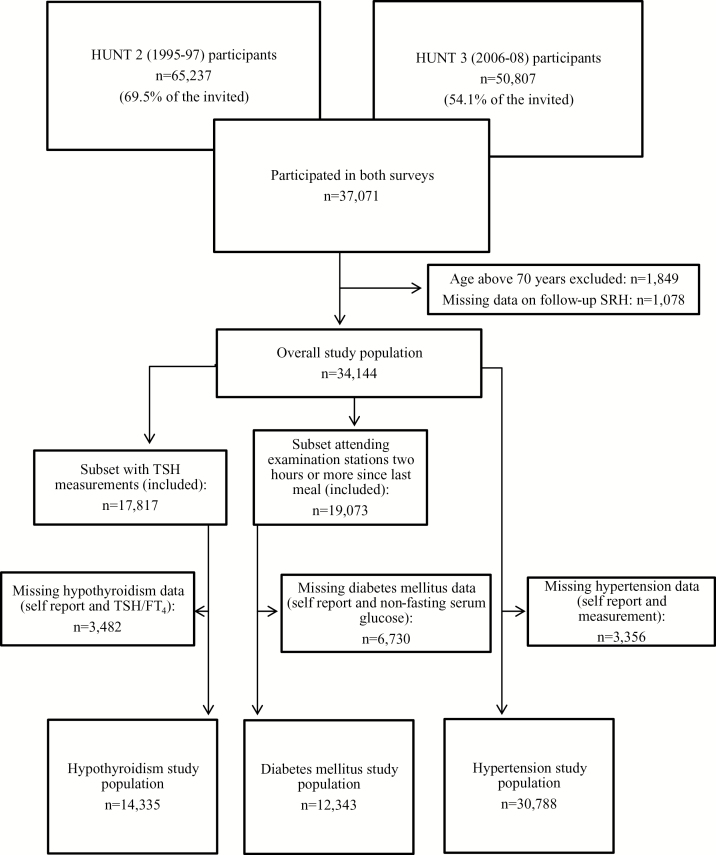
Among 37071 persons having participated in both surveys, we excluded persons with baseline age >70 years (1849), owing to loss to follow-up, and persons with missing data on SRH at follow-up (1078), leaving 34144 persons aged 20–70 years in the overall study population (Fig. 1). Median follow-up time was 11.1 years (range 10.8–11.7).
Figure 1.
Flow chart of inclusion and exclusion in the hypothyroidism, DM and hypertension study parts
Self-rated health
In the main questionnaires (both at baseline and follow-up), the first question answered before attending the examination stations was ‘How is your health at the moment?’. The question with four answer alternatives used in the HUNT Study is widely used internationally, in order to get answers either in positive or negative direction: ‘poor’, ‘not so good’, ‘good’ and ‘very good’ (19). We dichotomized the answer alternatives into poor or good.
Diseases
Diseases under study were categorized according to self-report and measurements at baseline and follow-up into disease category A, B, C, D and E (Table 1).
Table 1.
Categorization of hypothyroidism, DM and hypertension into disease statuses and categories. The HUNT Study, 1995–97 (baseline) and 2006–08 (follow-up)
| Disease statuses | Self-reported disease | Abnormal measurement | Disease categories | ||
|---|---|---|---|---|---|
| Baseline | follow-up | Baseline | follow-up | ||
| No disease, baseline and follow-up | No | No | No | No | Category A |
| Unknown, baseline and/or follow-up | No | No | Yes/no | Yes | Category B |
| Unknown baseline, known follow-up | No | Yes | Yes | – | Category C |
| No disease baseline, known follow-up | No | Yes | Yes | – | Category D |
| Known baseline | Yes | – | – | – | Category E |
Hypothyroidism
At baseline, the laboratory reference range for TSH was 0.2–4.5 mU/l and for free T4 8.0–20.0 pmol/l. At follow-up, the reference range for TSH was 0.2–4.5 mU/l and for free T4 9.0–19.0 pmol/l. Different laboratories were used at baseline and follow-up (20).
At both surveys, the participants answered questions on history of hypothyroidism and hyperthyroidism.
Of the overall study population, 14335 persons had valid measurements and self-reported data on thyroid function (Fig. 1).
No hypothyroidism (A) included participants with negative answers on the thyroid-related questions, and having TSH and FT4 within reference range at baseline and follow-up. Unknown hypothyroidism (B) included participants with negative answers on the thyroid-related questions at baseline and follow-up, but with TSH above and FT4 either below or within (subclinical hypothyroidism) the reference range either at baseline and follow-up or at follow-up only. Participants with self-reported history of hypothyroidism at baseline or follow-up were assigned to the corresponding known hypothyroidism categories (C–E), see Table 1. Our definitions of subclinical hypothyroidism and hypothyroidism are commonly accepted in clinical practice (http://bestpractice.bmj.com).
Diabetes mellitus
The participants answered questions on history of DM and had non-fasting serum glucose measured between 10 a.m. and 6 p.m., at baseline and follow-up (17).
Few well-designed studies have validated the usefulness of non-fasting serum glucose as a test for DM. However, Engelgau et al. (21) found sensitivity between 68% and 74% and specificity between 66% and 77% when setting the non-fasting serum glucose cut-off at 5.6 mmol/l, depending on age. Further, small differences in serum glucose levels have been found between fasting and non-fasting individuals (22). In our study population, 40% had measured serum glucose 2 hours or more after their last meal, whereas only 4% had measured serum glucose 4 hours or more after the last meal. To balance higher specificity of the DM classification on the expense of statistical power, we chose to include participants fasting 2 hours or more. Of these, 12343 had valid DM self-reported data, thus were eligible for inclusion (Fig. 1). Self-reported DM has been validated previously in this population (23).
No DM (A) included participants denying having DM and having glucose below the cut-off level at baseline and follow-up. Unknown DM (B) included those denying DM but having glucose on or above cut-off at either baseline and follow-up or follow-up only. Participants with affirmative answer of DM at baseline or follow-up were assigned to the corresponding known DM categories (C–E), see Table 1.
Hypertension
At baseline, participants were asked about the doctor’s clinical advice after the latest BP measurement prior to participation in HUNT. The answer categories were ‘no follow-up and no medication necessary’, ‘recommended follow-up examination but not to take medicine’, ‘start or continue taking medicine for high BP’ or ‘never measured’. Standardized BP measurements were performed and mean systolic and mean diastolic arterial BP of measurement two and three were used as BP measures. Cut-off values defining hypertension were made according to the European society of hypertension’s definitions; systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg.
At follow-up, the participants were not asked about the doctor’s advice, rather; whether they had started on BP-lowering medication or not. The measurements were similar. Of the overall study population, 30788 had valid data on BP self-reports and measurements and included in the hypertension part of the study (Fig. 1).
No hypertension (A) included participants reporting ‘no follow-up- or never measured’ on baseline BP questions, with normal systolic and diastolic BP both at baseline and follow-up, and not being on BP-lowering medication at follow-up. Similar self-reports, but elevated BP at both surveys or at follow-up only, were categorized as unknown hypertension (B), whereas participants reporting otherwise were included in known hypertension categories (C–E), see Table 1.
Statistical analyses
Descriptive analyses of the baseline characteristics were stratified by gender and by baseline to follow-up disease categories (A–E). We used gender-stratified, logistic regression models to estimate age and multiple adjusted odds ratios (OR) with 95% confidence intervals for poor SRH at follow-up, by categories of hypothyroidism, DM and hypertension. A priori selected confounders identified by directed acyclic graphs were baseline SRH, age, body mass index (BMI), smoking habits, educational status, self-esteem and limiting long-term illness or injury. Owing to a non-linear relationship with SRH, age was categorized as 20–36 years, 37–53 years and 54–70 years. BMI (kg/m2) was calculated of measured height and weight and categorized according to the World Health Organization’s (WHO) definition; underweight (18.5kg/m2), normal weight (18.5–24.9kg/m2), overweight (25.0–29.9kg/m2) and obese (>30.0kg/m2). Smoking status was dichotomized as daily smokers (current smoker at follow-up) and nondaily smokers (never smokers, former smokers and occasional smokers). We categorized educational level into higher education (>12 years) or not. The four-item version of the Rosenberg Self-esteem scale (consisting of question number 10, 2, 5 and 7 in the full version) used in the HUNT surveys has been validated by Tambs (24). The sum score was categorized into high (7–12) and low (0–6) self-esteem. Participants reporting limiting long-term illness or injury (question: ‘Do you suffer from any long-term illness or injury (at least 1 year) of a physical or psychological nature that impairs your functioning in your everyday life?’) were categorized as ‘prevalent’ otherwise ‘absent’.
We tested for statistical interaction between independent variables on the outcome by Wald tests.
The statistical significance level was set at P < 0.05, except for interaction analyses: P < 0.10.
All analyses were performed with IBM SPSS Statistics version 21 for windows.
Results
Persons without disease tended to be younger and fewer reported limiting long-term illness or injury, compared to persons with known disease at any time (Table 2). We found the overall proportion of women reporting poor SRH at follow-up to be slightly higher (5.4%) than in men (Table 3). The proportion reporting poor SRH increased by age and was higher among persons with lower education, among daily smokers and among persons with low self-esteem. Also, poor SRH was more frequent among underweight, overweight and obese than among normal weight participants. In persons with long-term illness, the proportion with poor SRH was more than 2-fold higher than among persons without long-term illness/injury. SRH at baseline and follow-up was consistent for the majority, but more women than men reported deteriorated subjective health.
Table 2.
Baseline demographic, health and lifestyle characteristics of the study population by hypothyroidism, DM and hypertension categories. The HUNT Study, 1995–97 (baseline) and 2006–08 (follow-up)
| N | Age (years, iqr) | BMI (kg/m2, iqr) | Daily smoke (%) | Higher education (%) | Low self-esteem (%) | Long-term illness (%) | Baseline SRH, good (%) | |
|---|---|---|---|---|---|---|---|---|
| Women | 18659 | |||||||
| Hypothyroidism | ||||||||
| A. No disease, baseline and follow-up | 8265 | 52 (46–61) | 26 (23–29) | 26.4 | 18.8 | 4.3 | 32.9 | 72.8 |
| B. Unknown, baseline and/or follow-up | 128 | 53 (45–64) | 26 (23–28) | 20.8 | 24.8 | 0 | 31.2 | 82.5 |
| C. Unknown baseline, known follow-up | 250 | 55 (47–63) | 26 (24–30) | 14.9 | 18.3 | 5.3 | 34.2 | 72.6 |
| D. No disease baseline, known follow-up | 419 | 53 (47–61) | 26 (24–29) | 29.1 | 16.5 | 3.9 | 42.2 | 61.2 |
| E. Known baseline | 831 | 54 (46–63) | 27 (24–30) | 24.2 | 17.5 | 8.2 | 54.7 | 48.6 |
| DM | ||||||||
| A. No disease, baseline and follow-up | 6462 | 44 (34–54) | 25 (23–28) | 29.1 | 24.9 | 2.3 | 26 | 78.4 |
| B. Unknown, baseline and/or follow-up | 3141 | 50 (41–58) | 26 (24–29) | 30.1 | 19.1 | 3.2 | 30.8 | 74.6 |
| C. Unknown baseline, known follow-up | 187 | 56 (49–62) | 31 (28–34) | 25.6 | 8.1 | 1.2 | 38.4 | 67.4 |
| D. No disease baseline, known follow-up | 174 | 52 (46–61) | 31 (28–34) | 29.5 | 10.5 | 4.2 | 32.6 | 67.4 |
| E. Known baseline | 126 | 57 (49–63) | 30 (27–34) | 19.1 | 17.7 | 7.4 | 54.4 | 47.1 |
| Hypertension | ||||||||
| A. No disease, baseline and follow-up | 9241 | 48 (44–54) | 25 (23–27) | 30.6 | 29.2 | 2.2 | 21.7 | 81.9 |
| B. Unknown, baseline and/or follow-up | 2682 | 53 (48–62) | 26 (24–29) | 27.3 | 17.3 | 4.4 | 31.5 | 76.6 |
| C. Unknown baseline, known follow-up | 1572 | 58 (50–66) | 27 (25–30) | 22.8 | 12.2 | 4.6 | 39.7 | 68.8 |
| D. No disease baseline, known follow-up | 983 | 52 (46–59) | 26 (24–29) | 36.9 | 17.2 | 3.3 | 37.7 | 65.5 |
| E. Known baseline | 2299 | 59 (52–66) | 28 (25–31) | 18.9 | 9 | 12.7 | 55.5 | 50.4 |
| Men | 15485 | |||||||
|---|---|---|---|---|---|---|---|---|
| Hypothyroidism | ||||||||
| A. No disease, baseline and follow-up | 4197 | 53 (46–61) | 26 (25–29) | 25 | 23.4 | 1.2 | 33 | 76.6 |
| B. Unknown, baseline and/or follow-up | 50 | 61 (54–69) | 26 (25–28) | 21 | 17.2 | 1.8 | 44.3 | 82 |
| C. Unknown baseline, known follow-up | 45 | 55 (48–64) | 28 (25–29) | 7.8 | 15.7 | 2.3 | 35.4 | 72.5 |
| D. No disease baseline, known follow-up | 74 | 53 (48–62) | 27 (25–29) | 24.1 | 26 | 1.7 | 35.6 | 69.2 |
| E. Known baseline | 95 | 55 (46–67) | 28 (26–30) | 24.2 | 20 | 6.7 | 55.8 | 58.4 |
| DM | ||||||||
| A. No disease, baseline and follow-up | 4554 | 45 (35–54) | 26 (24–28) | 25.3 | 23.9 | 1.3 | 26.8 | 82.1 |
| B. Unknown, baseline and/or follow-up | 3468 | 49 (40–57) | 26 (25–29) | 26.4 | 21.4 | 1.5 | 29 | 79 |
| C. Unknown baseline, known follow-up | 199 | 55 (46–61) | 30 (27–33) | 24.5 | 17 | 1.1 | 42.6 | 63.8 |
| D. No disease baseline, known follow-up | 157 | 53 (44–59) | 29 (27–33) | 32.9 | 22 | 1.2 | 43.9 | 65.9 |
| E. Known baseline | 127 | 55 (49–60) | 27 (25–30) | 23.9 | 23.9 | 1.5 | 43.3 | 56.7 |
| Hypertension | ||||||||
| A. No disease, baseline and follow-up | 6559 | 49 (44–56) | 26 (24–28) | 25.7 | 24.9 | 0.8 | 23.5 | 80.8 |
| B. Unknown, baseline and/or follow-up | 2904 | 53 (47–60) | 26 (24–28) | 25.5 | 22.8 | 1.6 | 27.2 | 78.7 |
| C. Unknown baseline, known follow-up | 1523 | 57 (50–64) | 28 (26–30) | 26 | 18.2 | 1.6 | 36 | 64.7 |
| D. No disease baseline, known follow-up | 662 | 52 (46–59) | 27 (25–29) | 31.4 | 20.7 | 1.4 | 35.1 | 60.8 |
| E. Known baseline | 2390 | 56 (50–64) | 28 (26–30) | 24.6 | 15 | 5 | 51.1 | 61.9 |
Within each disease category, median age in years and BMI in kilograms per metre square with iqr and proportions in percentage of daily smokers, participants with higher education, low self-esteem, long-term limiting illness or injury and baseline SRH good are reported. iqr, interquartile range.
Table 3.
Total study population, proportion reporting poor SRH at follow-up by sex and baseline covariates. The HUNT Study, 1995–97 (baseline) and 2006–08 (follow-up)
| Women | Men | |||
|---|---|---|---|---|
| n | Poor SRH (%) | N | Poor SRH (%) | |
| Overall study population | 18659 | 30 | 15485 | 24.6 |
| Age | ||||
| 20–36 years | 5281 | 18.9 | 3878 | 13 |
| 37–53 years | 8570 | 31.1 | 7401 | 25 |
| 54–70 years | 4808 | 40.3 | 4206 | 34.5 |
| (0% missing) | ||||
| BMI (kg/m2) | ||||
| <18.5 | 152 | 35.5 | 31 | 22.6 |
| 18.5–24.9 | 8691 | 23.7 | 5286 | 21 |
| 25.0–29.9 | 6920 | 32.1 | 8095 | 24.2 |
| >30 | 2819 | 35.5 | 2023 | 35.3 |
| (0.4% missing) | ||||
| Daily smoker | ||||
| No | 13150 | 27.6 | 11520 | 22.2 |
| Yes | 5428 | 35.7 | 3901 | 31.2 |
| (0.4% missing) | ||||
| Higher education | ||||
| Yes | 4290 | 19.3 | 3535 | 15.7 |
| No | 14001 | 32.8 | 11713 | 26.9 |
| (1.8% missing) | ||||
| Self-esteem | ||||
| High | 15853 | 28.7 | 12754 | 23.9 |
| Low | 479 | 48.9 | 153 | 37.9 |
| (14.4% missing) | ||||
| Long-term illness/impairment | ||||
| No | 13184 | 20.7 | 10945 | 17 |
| Yes | 4859 | 53.4 | 4230 | 43.2 |
| (2.7% missing) | ||||
| SRH, baseline | ||||
| Good | 14228 | 19.3 | 12432 | 16.5 |
| Poor | 4264 | 65.5 | 2956 | 58.4 |
| (0.8% missing) | ||||
Hypothyroidism
Poor SRH was less frequently reported by both men and women with unknown hypothyroidism compared with other classification categories, including the euthyroid persons (category A/reference) (Table 4). On the other side, among women we found a strong and positive association between categories C–E and poor SRH, compared with euthyroid women, also after adjustments including baseline SRH. In men, the associations were similarly positive for categories B–D, however, not at a statistically significant level. Men with known hypothyroidism at baseline had a lower OR to report poor SRH at follow-up.
Table 4.
The association of hypothyroidism, DM and hypertension statuses with poor SRH at follow-up. The HUNT study 1995–97 (baseline) and 2006–08 (follow-up)
| Women | OR (95% CI) | |||
|---|---|---|---|---|
| N | Model 1 | Model 2 | Model 3 | |
| Hypothyroidism | ||||
| A. No disease, baseline and follow-up | 6731 | 1.00 | 1.00 | 1.00 |
| B. Unknown, baseline and/or follow-up | 103 | 0.51 (0.33–0.79) | 0.59 (0.36–0.96) | 0.64 (0.38–1.06) |
| C. Unknown baseline, known follow-up | 197 | 1.44 (1.11–1.87) | 1.62 (1.20–2.19) | 1.83 (1.33–2.52) |
| D. No disease baseline, known follow-up | 338 | 1.81 (1.48–2.22) | 1.72 (1.36–2.17) | 1.56 (1.22–2.00) |
| E. Known baseline | 545 | 1.77 (1.51–2.07) | 1.66 (1.37–2.00) | 1.39 (1.13–1.70) |
| DM | ||||
| A. No disease, baseline and follow-up | 3750 | 1.00 | 1.00 | 1.00 |
| B. Unknown, baseline and/or follow-up | 1378 | 1.08 (0.96–1.22) | 0.99 (0.85–1.14) | 1.00 (0.86–1.17) |
| C. Unknown baseline, known follow-up | 86 | 1.74 (1.19–2.54) | 1.31 (0.83–2.09) | 1.46 (0.89–2.38) |
| D. No disease baseline, known follow-up | 95 | 1.67 (1.14–2.43) | 1.12 (0.72–1.76) | 1.11 (0.68–1.79) |
| E. Known baseline | 68 | 2.23 (1.45–3.44) | 1.53 (0.89–2.61) | 1.21 (0.67–2.16) |
| Hypertension | ||||
| A. No disease, baseline and follow-up | 7799 | 1.00 | 1.00 | 1.00 |
| B. Unknown, baseline and/or follow-up | 2173 | 1.01 (0.91–1.11) | 0.86 (0.76–0.97) | 0.89 (0.79–1.01) |
| C. Unknown baseline, known follow-up | 1259 | 1.44 (1.28–1.62) | 1.19 (1.04–1.37) | 1.21 (1.04–1.41) |
| D. No disease baseline, known follow-up | 802 | 2.02 (1.76–2.31) | 1.60 (1.36–1.88) | 1.52 (1.28–1.81) |
| E. Known baseline | 1841 | 1.76 (1.59–1.94) | 1.44 (1.28–1.63) | 1.33 (1.17–1.52) |
| Men | ||||
|---|---|---|---|---|
| Hypothyroidism | ||||
| A. No disease, baseline and follow-up | 3383 | 1.00 | 1.00 | 1.00 |
| B. Unknown, baseline and/or follow-up | 41 | 0.72 (0.37–1.38) | 0.61 (0.28–1.34) | 0.63 (0.12–3.34) |
| C. Unknown baseline, known follow-up | 35 | 1.23 (0.66–2.30) | 1.36 (0.65–2.84) | 1.10 (0.51–2.39) |
| D. No disease baseline, known follow-up | 51 | 1.91 (1.19–3.07) | 2.22 (1.23–4.00) | 1.26 (0.81–1.95) |
| E. Known baseline | 70 | 1.04 (0.65–1.68) | 0.78 (0.45–1.35) | 0.41 (0.19–0.90) |
| DM | ||||
| A. No disease, baseline and follow-up | 2753 | 1.00 | 1.00 | 1.00 |
| B. Unknown, baseline and/or follow-up | 1653 | 1.06 (0.93–1.21) | 1.07 (0.92–1.25) | 1.07 (0.91–1.25) |
| C. Unknown baseline, known follow-up | 94 | 1.89 (1.30–2.74) | 1.49 (0.94–2.34) | 1.29 (0.80–2.10) |
| D. No disease baseline, known follow-up | 82 | 2.04 (1.37–3.03) | 1.63 (1.01–2.64) | 1.55 (0.93–2.57) |
| E. Known baseline | 67 | 2.93 (1.84–4.66) | 2.99 (1.76–5.06) | 2.52 (1.46–4.34) |
| Hypertension | ||||
| A. No disease, baseline and follow-up | 5271 | 1.00 | 1.00 | 1.00 |
| B. Unknown, baseline and/or follow-up | 2327 | 0.92 (0.82–1.03) | 0.93 (0.81–1.06) | 0.95 (0.83–1.09) |
| C. Unknown baseline, known follow-up | 1199 | 1.58 (1.38–1.79) | 1.56 (1.34–1.82) | 1.60 (1.36–1.87) |
| D. No disease baseline, known follow-up | 526 | 2.22 (1.87–2.64) | 2.01 (1.64–2.46) | 2.04 (1.65–2.52) |
| E. Known baseline | 1934 | 1.90 (1.70–2.12) | 1.72 (1.51–1.96) | 1.54 (1.34–1.77) |
OR for poor SRH with 95% CIs. Model 1: adjusted for age only. Model 2: model 1 + smoking status, BMI, education level, self-esteem and long-term illness or injury. Model 3: model 2 + baseline SRH. N represents numbers included in the fully adjusted analyses, cases with missing data excluded. CI, confidence interval.
Diabetes mellitus
Adjusted for age and other factors according to Table 4, there was virtually no difference in the frequency of reporting poor SRH between persons with unknown DM at follow-up (category B) and persons without the disease (category A). Persons with known DM (categories C–E) were however more likely to report poor SRH at follow-up, compared with persons without DM (Table 4).
Hypertension
Women with unknown hypertension were less likely to report poor SRH compared to normotensive women, whereas no difference was found in men between these categories (Table 4). Compared to the same reference group, persons with known hypertension (categories C–E) were more likely to report poor SRH at follow-up.
Discussion
In this large-scale, prospective, population-based study, we found that persons with known hypothyroidism, DM or hypertension were more likely to report poor SRH at 11-year follow-up, compared to healthy persons. The exception was men with known hypothyroidism at baseline. Contrary, persons with unknown hypothyroidism, DM and hypertension throughout follow-up did not report poor SRH more frequently; in fact, those with unknown hypothyroidism and women with unknown hypertension reported poor SRH less frequently than healthy persons.
Cross-sectional studies have shown an association between disease labelling and the outcomes sense of well-being, psychological distress and poor SRH, all in accordance with our findings (12–14,16). Such associations could have been influenced by residual confounding by different personality traits in groups being compared (25). In the present study, however, persons with previously unknown disease were more likely to report poor SRH when they had become diagnosed with hypothyroidism, DM or hypertension, indicating that the disease labelling was the main factor.
To our knowledge, no prior studies have analysed the association between disease awareness/unawareness and SRH in a longitudinal design. Latham and Peek showed a predictive effect of SRH on incident self-reported morbidity; healthy persons with fair or poor SRH at baseline had an increased risk of incident self-reported morbidity at subsequent 2-year interval in 16 years of follow-up (7). Notably, they did not include unknown disease in their investigations.
The associations we found between baseline covariates and baseline SRH are in accordance with previous research (3,26). As expected, baseline poor SRH and prevalent long-term illness showed a strong association with poor SRH at follow-up. However, these covariates did not have a substantial impact on the relationship between disease statuses and follow-up SRH when included in the regression models.
The distribution of SRH at follow-up seems comparable to what has been observed in other European studies (27–29). The prevalence of hypothyroidism, DM and hypertension, based on self-report, varies somewhat between studies and is found to underestimate the measured prevalence of DM and hypertension (30–32). In our study, the prevalence of baseline known DM was low; however, by inclusion of participants >70 years, the overall self-reported prevalence was 3.2% (data not shown). This is comparable with other studies and with figures in the WHO Health for All database, and indicating a strong correlation between DM and age. The prevalence at follow-up (5.2%, not shown) is further supporting this correlation.
We expect poor SRH to be related to increased health care utilization (2), which in turn should be related to the risk of getting a diagnosis of hypothyroidism, DM or hypertension, by opportunistic screening mechanisms. By such, poor SRH at follow-up could be explained, at least partly, by personality profile, other chronic diseases and baseline SRH even in persons with known hypothyroidism, DM or hypertension. However, adjusting for self-esteem, long-term illness and baseline SRH did not substantially change our estimates.
Theoretically, confounding by disease severity could influence our results. However, hypothyroidism, DM (mainly type 2 in this age group) and hypertension are easily treated and most often considered non-severe and non-symptomatic or low symptomatic conditions since they tend to be diagnosed in a presymptomatic stage nowadays.
We could expect treatment of disease to increase SRH; however, the evidence is conflicting for DM and hypertension (33–35). The relationship is not straightforward; medically treated persons are likely to have more severe disease and to be exposed for side effects of medication, counteracting any positive effect on SRH. Any impact of treatment of disease on SRH is therefore difficult to predict. Not treating hypothyroidism with thyroxin supplement would be considered unethical, and we expect it to increase SRH in hypothyroid persons.
The definitions of DM and hypertension used in the present study could be questioned. As only non-fasting serum glucose was measured, some persons with false DM might be included as unknown DM. The relatively high numbers in DM category B could be indicative of this problem. This should weaken any association between poor SRH and unknown DM. Some persons categorized as having unknown hypertension could have knowledge about their hypertension, even though they have not yet been prescribed antihypertensive medication. As hypertension labelling has been found to be associated with poor SRH (14), this misclassification should also weaken potential associations. Further, according to our definition, persons in the known hypertension categories are the only ones exposed for possible side effects of BP-lowering medication, which could contribute to poor SRH, as reported.
Lastly, residual confounding by disease severity could explain differences between categories, but control of this would demand randomized controlled trials; in this setting, an unethical study design.
In our view, the strengths of our study were its population-based prospective cohort design with a large number of participants. As part of a broad health survey, the participants were not aware of the specific research hypotheses, which should limit reporting bias.
Despite our findings, the clinical implications should not include less focus on diagnosing persons that could benefit from being diagnosed with thyroid disease, DM and hypertension at an early stage. It seems, however, reasonable that physicians emphasize a salutary strategy when communicating risks or diagnoses, with the aim not to reduce a persons’ health perception. Wennberg et al. (36) found SRH to be independently associated with mortality in persons with DM and advocated a more detailed consultation and intensified support in such patients. Others have found a similar relation with hypertension and emphasize the importance of taking patients health rating into account (37,38). Exploration of what lies behind any poor SRH in the individual patient should be encouraged among physicians.
In conclusion, our data indicate that diagnostic labelling could harm perceived health. This possible relationship needs to be empirically demonstrated by future research, but as perceived health is related to morbidity, and even mortality, we should emphasize a salutary attitude also in early diagnostics.
Declaration
Ethical approval: the study was approved by the Regional Committee for Medical Research. All participants signed written informed consent.
Funding: no external funding. PJ received a PhD grant, funded by the Norwegian University of Science and Technology, NTNU.
Conflict of interest: all authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Author contributions
All authors meet the four criteria for authorship recommended by the International Committee of Medical Journal Editors. SF, AL and SK have been active supervisors in study conception, design, conduct, interpretation and reporting. PJ analysed the data and drafted the manuscript. Critical revisions were done by all supervisors, and all authors approved the final version of the manuscript.
Acknowledgements
The Nord-Trøndelag Health Study (HUNT Study) is a collaboration between HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology NTNU), Nord-Trøndelag County Council, Central Norway Health Authority and the Norwegian Institute of Public Health.
References
- 1. Meurer LN, Layde PM, Guse CE. Self-rated health status: a new vital sign for primary care? WMJ 2001; 100: 35–9. [PubMed] [Google Scholar]
- 2. Miilunpalo S, Vuori I, Oja P, Pasanen M, Urponen H. Self-rated health status as a health measure: the predictive value of self-reported health status on the use of physician services and on mortality in the working-age population. J Clin Epidemiol 1997; 50: 517–28. [DOI] [PubMed] [Google Scholar]
- 3. Smith PM, Glazier RH, Sibley LM. The predictors of self-rated health and the relationship between self-rated health and health service needs are similar across socioeconomic groups in Canada. J Clin Epidemiol 2010; 63: 412–21. [DOI] [PubMed] [Google Scholar]
- 4. Idler EL, Kasl S. Health perceptions and survival: do global evaluations of health status really predict mortality? J Gerontol 1991; 46: S55–65. [DOI] [PubMed] [Google Scholar]
- 5. Møller L, Kristensen TS, Hollnagel H. Self rated health as a predictor of coronary heart disease in Copenhagen, Denmark. J Epidemiol Community Health 1996; 50: 423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schou MB, Krokstad S, Westin S. How is self-rated health associated with mortality? Tidsskr Nor Laegeforen 2006; 126: 2644–7. [PubMed] [Google Scholar]
- 7. Latham K, Peek CW. Self-rated health and morbidity onset among late midlife U.S. adults. J Gerontol B Psychol Sci Soc Sci 2013; 68: 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Getz L, Sigurdsson JA, Hetlevik I, et al. Estimating the high risk group for cardiovascular disease in the Norwegian HUNT 2 population according to the 2003 European guidelines: modelling study. BMJ 2005; 331: 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heath I. Overdiagnosis: when good intentions meet vested interests—an essay by Iona Heath. BMJ 2013; 347: f6361. [DOI] [PubMed] [Google Scholar]
- 10. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ 2012; 344: e3502. [DOI] [PubMed] [Google Scholar]
- 11. Haynes RB, Sackett DL, Taylor DW, Gibson ES, Johnson AL. Increased absenteeism from work after detection and labeling of hypertensive patients. N Engl J Med 1978; 299: 741–4. [DOI] [PubMed] [Google Scholar]
- 12. Bloom JR, Monterossa S. Hypertension labeling and sense of well-being. Am J Public Health 1981; 71: 1228–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamer M, Batty GD, Stamatakis E, Kivimaki M. Hypertension awareness and psychological distress. Hypertension 2010; 56: 547–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barger SD, Muldoon MF. Hypertension labelling was associated with poorer self-rated health in the Third US National Health and Nutrition Examination Survey. J Hum Hypertens 2006; 20: 117–23. [DOI] [PubMed] [Google Scholar]
- 15. Whaley-Connell A, Shlipak MG, Inker LA, et al. Awareness of kidney disease and relationship to end-stage renal disease and mortality. Am J Med 2012; 125: 661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jørgensen P, Langhammer A, Krokstad S, Forsmo S. Is there an association between disease ignorance and self-rated health? The HUNT Study, a cross-sectional survey. BMJ Open 2014; 4: e004962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holmen J, Midthjell K, Krüger Ø, et al. The Nord-Trøndelag Health Study 1995–97 (HUNT 2): objectives, methods, and participation. Norsk Epidemiol 2003; 13: 19–32. [Google Scholar]
- 18. Krokstad S, Langhammer A, Hveem K, et al. Cohort profile: the HUNT Study, Norway. Int J Epidemiol 2012; 42: 968–77. [DOI] [PubMed] [Google Scholar]
- 19. Bowling A. Just one question: if one question works, why ask several? J Epidemiol Community Health 2005; 59: 342–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Åsvold BO, Vatten LJ, Midthjell K, Bjøro T. Serum TSH within the reference range as a predictor of future hypothyroidism and hyperthyroidism: 11-year follow-up of the HUNT Study in Norway. J Clin Endocrinol Metab 2012; 97: 93–9. [DOI] [PubMed] [Google Scholar]
- 21. Engelgau MM, Thompson TJ, Smith PJ, et al. Screening for diabetes mellitus in adults. The utility of random capillary blood glucose measurements. Diabetes Care 1995; 18: 463–6. [DOI] [PubMed] [Google Scholar]
- 22. Moebus S, Göres L, Lösch C, Jöckel KH. Impact of time since last caloric intake on blood glucose levels. Eur J Epidemiol 2011; 26: 719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Midthjell K, Holmen J, Bjørndal A, Lund-Larsen G. Is questionnaire information valid in the study of a chronic disease such as diabetes? The Nord-Trøndelag diabetes study. J Epidemiol Community Health 1992; 46: 537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tambs K. Moderate effects of hearing loss on mental health and subjective well-being: results from the Nord-Trøndelag Hearing Loss Study. Psychosom Med 2004; 66: 776–82. [DOI] [PubMed] [Google Scholar]
- 25. Kinnunen ML, Metsäpelto RL, Feldt T, et al. Personality profiles and health: longitudinal evidence among Finnish adults. Scand J Psychol 2012; 53: 512–22. [DOI] [PubMed] [Google Scholar]
- 26. Bopp M, Braun J, Gutzwiller F, Faeh D; Swiss National Cohort Study Group. Health risk or resource? Gradual and independent association between self-rated health and mortality persists over 30 years. PLoS One 2012; 7: e30795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olsen KM, Dahl SA. Health differences between European countries. Soc Sci Med 2007; 64: 1665–78. [DOI] [PubMed] [Google Scholar]
- 28. Young H, Grundy E, O’Reilly D, Boyle P. Self-rated health and mortality in the UK: results from the first comparative analysis of the England and Wales, Scotland, and Northern Ireland Longitudinal Studies. Popul Trends 2010; 139: 11–36. [DOI] [PubMed] [Google Scholar]
- 29. Cummins S, Stafford M, Macintyre S, Marmot M, Ellaway A. Neighbourhood environment and its association with self rated health: evidence from Scotland and England. J Epidemiol Community Health 2005; 59: 207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leese GP, Flynn RV, Jung RT, et al. Increasing prevalence and incidence of thyroid disease in Tayside, Scotland: the Thyroid Epidemiology Audit and Research Study (TEARS). Clin Endocrinol (Oxf) 2008; 68: 311–6. [DOI] [PubMed] [Google Scholar]
- 31. Fleming DM, Schellevis FG, Van Casteren V. The prevalence of known diabetes in eight European countries. Eur J Public Health 2004; 14: 10–4. [DOI] [PubMed] [Google Scholar]
- 32. Tolonen H, Koponen P, Mindell JS, et al. Under-estimation of obesity, hypertension and high cholesterol by self-reported data: comparison of self-reported information and objective measures from health examination surveys. Eur J Public Health 2014; 24: 941–8. [DOI] [PubMed] [Google Scholar]
- 33. Nielsen ABS, Jensen P, Gannik D, et al. Change in self-rated general health is associated with perceived illness burden: a 1-year follow up of patients newly diagnosed with type 2 diabetes. BMC Public Health. 2015; 15: 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oparil S. Antihypertensive therapy—efficacy and quality of life. N Engl J Med 1993; 328: 959–61. [DOI] [PubMed] [Google Scholar]
- 35. Muller M, Jochemsen HM, Visseren FL, et al. Low blood pressure and antihypertensive treatment are independently associated with physical and mental health status in patients with arterial disease: the SMART study. J Intern Med 2013; 274: 241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wennberg P, Rolandsson O, Jerdén L, et al. Self-rated health and mortality in individuals with diabetes mellitus: prospective cohort study. BMJ Open 2012; 2: e000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Engström G, Hedblad B, Janzon L. Subjective well-being associated with improved survival in smoking and hypertensive men. J Cardiovasc Risk 1999; 6: 257–61. [DOI] [PubMed] [Google Scholar]
- 38. Nielsen AB, Siersma V, Hiort LC, et al. Self-rated general health among 40-year-old Danes and its association with all-cause mortality at 10-, 20-, and 29 years’ follow-up. Scand J Public Health 2008; 36: 3–11. [DOI] [PubMed] [Google Scholar]