Abstract
Mycobacterium tuberculosis (Mtb) infection remains one of the world’s major causes of illness and mortality. A clear understanding of the host defense against Mtb is imperatively needed forthe control of this epidemic. When tuberculosis (TB) infection occurs, a variety of pro and anti-inflammatory cytokines play a vital role in the pathogenesis of this disease. Interleukin-10 (IL-10) is one of the most important anti-inflammatory cytokines reported to suppress the protective immune response against tuberculosis.
Aim
The aim of the present study was to evaluate the association of plasma IL-10 levels with various disease stages of TB and the possible effects of treatment on these levels.
Materials and methods
A group of 30 patients with active pulmonary TB and a control group of 21 healthy individuals were enrolled in this study. The levels of IL-10 were measured before, during, and after treatment using commercially available enzyme-linked immune-sorbent assay (ELISA). Data were analyzed using GraphPad Prism version 5.0.
Results
The results showed that the levels of IL-10 had significant differences between the TB and control groups (p<0.05). The patients with abnormal chest X-Ray findings had higher IL-10 levels when compared to patients with normal X-Rays (p=0.03). A subgroup of 18 patients were followed during the treatment and the mean plasma concentration of IL-10 in patients before therapy was higher than in patients at 3 months of therapy and in patients after 6 months of therapy (p=0.01). However, the IL-10 level remained significantly higher in patients at the end of treatment compared with controls.
These findings could be used in follow-up as clinical biomarker of the success of tuberculosis therapy.
Keywords: Tuberculosis, chest X-Ray, Cytokine, Interleukin-10, tuberculosis treatment
Introduction
Tuberculosis (TB) is a preventable and curable infectious disease, and yet it remains a significant global public health challenge. Each year, tuberculosis infects millions of people and it is the second leading cause of death by infectious diseases worldwide [1]. A better understanding of the host immune response against M. tuberculosis is crucial to elucidate this fatal disease. Several studies have shown that when tuberculosis infection occurs, a variety of pro and anti-inflammatory cytokines are produced at disease sites and then released into circulation [2,3]. Interleukin-10 (IL-10) is one of the most important anti-inflammatory cytokine reported to affect multiple cell types, including macrophages, monocytes, dendritic cells, CD4 T cells and CD8 T cells [4]. The dominant function of IL-10 is to down-regulate the immune response and limit tissue injury. However, the excessive production of this cytokine directly inhibits CD4+ T cell responses which may result in a failure to control the infection [5].
The main objective of this study was to investigate the levels of IL-10 in plasma of patients with different clinical manifestations of tuberculosis and to evaluate the possible effects of treatment on these levels.
Material and methods
Study subjects
The study was conducted at “Leon Daniello” Pneumology Hospital in Cluj Napoca, Romania. Twenty eight patients with active pulmonary TB attending the hospital were included. Study participants were evaluated by medical history, physical examination, chest X-ray, sputum acid-fast bacilli smear, mycobacterial culture and drug susceptibility testing. Patients with human immunodeficiency virus and diabetes mellitus were excluded. A control group of 21 healthy individuals (with no history of TB and no evidence of disease by clinical examination) were recruited. The protocol for this study was approved by the Ethics Committee of Iuliu Hatieganu University of Medicine and written informed consent was signed by each patient.
Blood sampling
Venous blood samples were collected from patients before starting anti-tuberculosis treatment (ATT), after 3 months of ATT and after 6 months of ATT. Blood samples were directly centrifuged at 1000 × g for 15 minutes and then plasma were collected and stored at −20 °C until use.
Measurement of IL-10 cytokine levels
The plasma levels of IL-10 were quantified using a sandwich enzyme immunoassay kit according to the manufacturer’s protocol (R&D Systems, Inc., Minneapolis, U.S.A.). The minimum detectable dose of IL-10 was typically less than 3.9 pg/mL.
Statistical analyses
All data were analyzed using GraphPad Prism version 5.0 (GraphPad Software Inc., La Jolla, CA, USA). Differences in cytokine levels among groups were evaluated by the Kruskal-Wallis and Mann-Whitney U tests. A p-value of <0.05 was considered statistically significant.
Results
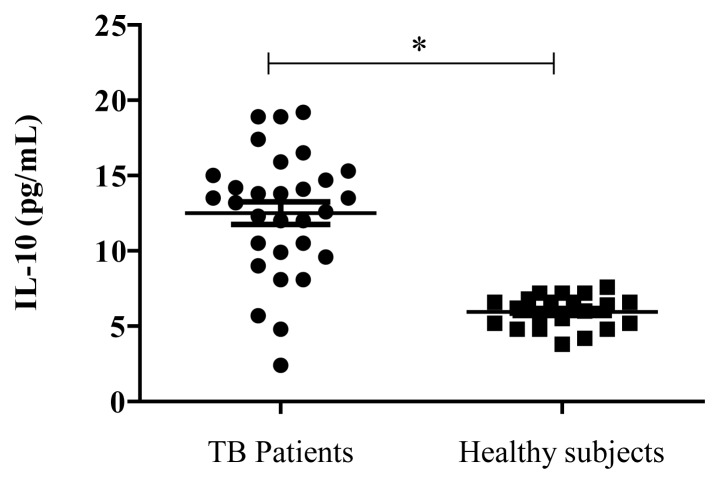
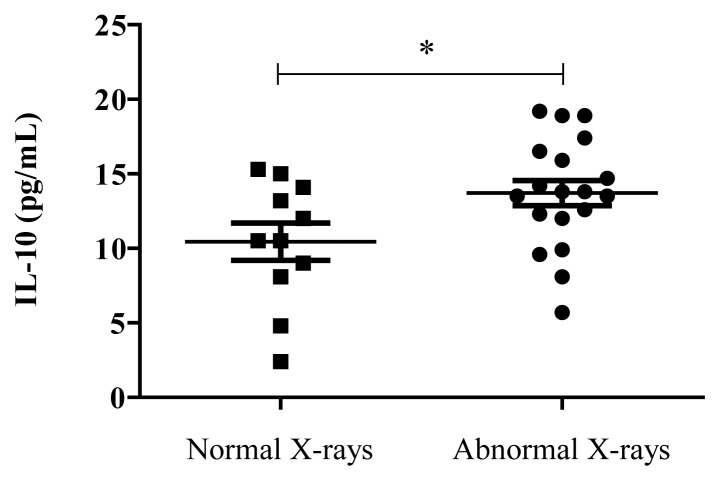
There were no statistically significant age or gender differences among the groups. Body temperature at admission was significantly higher in TB patients than in healthy individuals. Of 30 TB patients enrolled in the study, 22 cases had positive smears for AFB and 8 cases had negative smears. The results showed that the levels of IL-10 were higher in TB patients compared to healthy subjects (Fig. 1). The clinical severity of pulmonary TB in patients was determined on the basis of chest radiography results. Patients with abnormal chest X-Ray findings had higher IL-10 levels when compared to patients with normal X-Rays (Fig. 2).
Figure 1.
Distribution of Interleukin-10 levels in the plasma of patients with tuberculosis (n=30) and healthy controls (n=21), * p <0.0001.
Figure 2.
Distribution of plasma Interleukin-10 levels in patients with normal X-rays (n=11) and abnormal X-rays (n=19), *p<0.05.
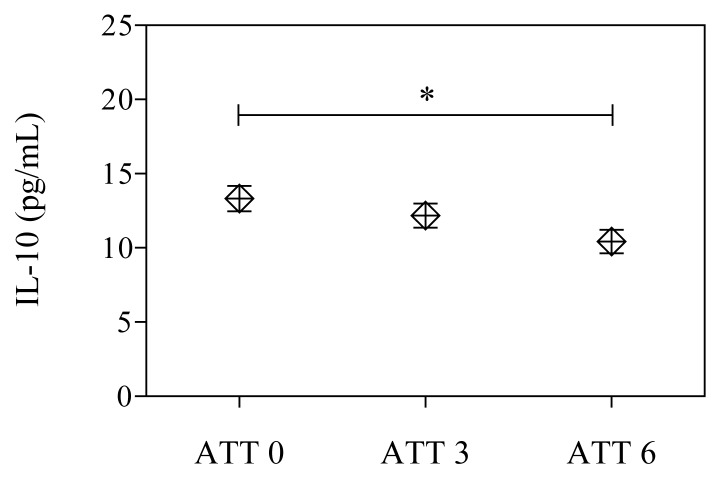
In our study, a subgroup of 18 patients was followed during and after anti-tuberculous therapy. The mean IL-10 level in TB patients before ATT was higher than in patients during therapy and in patients after treatment (Fig. 3).
Figure 3.
Changes of IL-10 levels in plasma of TB patients (n=18) before anti-tuberculous therapy (ATT0), after three months (ATT3) and at the end of treatment (ATT6), * p<0.05.
Discussion
Interleukin-10 is one of the most important anti-inflammatory cytokine reported to inhibit CD4+ T cell responses by inhibiting APC function of cells infected with mycobacteria [6]. To evaluate the association of IL-10 cytokine with various disease stages of TB, plasma levels of IL-10 were measured in active TB patients before treatment, after three months of treatment and at the end of treatment.
The results showed that the levels of IL-10 were significantly higher in TB patients compared to healthy subjects (p<0.05). After determining the clinical severity of TB in patients on the basis of chest radiography results, we observed that patients with abnormal chest X-Ray findings had higher IL-10 levels when compared to patients with normal X-Rays (p=0.03).
Our findings are similar with previous studies that have shown higher levels of IL-10 in the active TB group than in the control group, reporting even a correlation between IL-10 and susceptibility for TB [7,8,9]. IL-10 can be found in the serum, plasma and bronchoalveolar lavage fluid of active TB patients and may contribute to the anergy and failure of lymphocytes to proliferate in response to Mtb [10,11]. It is the balance between the inflammatory and protective immune response that determines the outcome of tuberculosis infection [5].
In our study, the possible effect of treatment on plasma IL-10 levels in TB patients was evaluated. All the patients included in the study received the first standard antituberculous treatment under direct observed treatment strategy which consisted of isoniazid, rifampin, pyrazinamide and ethambutol (2HRZE/4HR) according to the national TB program. To our knowledge, this is the first study to evaluate the effects of antituberculous treatment on plasma IL-10 levels in Romanian TB patients. IL-10 levels were determined in 18 patients after three months of treatment and at the end of treatment. We observed a statistically significant decreasing of IL-10 level during treatment. The mean IL-10 level in patients with active TB was higher than in patients during therapy (P not significant) and in patients after treatment (P<0.01).
Another study has recently shown a consistent decrease in IL-10 levels in active TB patients at all-time points of therapy, suggesting that patients who maintain high IL-10 levels at the end of treatment are exposed to TB recurrence [12]. Sahiratmadja et al. also observed reduced IL-10 production during TB therapy, suggesting that this cytokine may be a useful biomarker signature to assess the disease progression [13]. Studies on peripheral blood mononuclear cells obtained from TB patients have shown that neutralization of endogenous IL-10 increased T-cell proliferation and IFN- production [14,15]. Collectively, these studies concluded that IL-10 was functioning to limit the immune response to Mtb and may contribute to TB pathogenesis [15,16].
Previous studies suggest that in tuberculosis, over expressed IL-10 during the chronic phase of the infection showed evidence of reactivation of tuberculosis with a highly significant increase in bacterial numbers within the lungs. This reactivation was shown to be associated with the formation of macrophage-dominated lesions. In addition, IL-10 plays a pivotal role during the chronic/latent stage of pulmonary tuberculosis, with increased production playing a potentially central role in promoting reactivation tuberculosis [17].
In the present study, no significant association was found regarding the plasma level of IL-10 and BCG vaccination, positive smear or weight lost (data not shown).
The limitation of the current study is that the specific cellular source of IL-10 in plasma of our patients has not been assessed. Further studies are necessary to identify the exact source of IL-10 in response to Mtb infection and to see if it could be used in the follow-up as a clinical biomarker of disease progression.
Acknowledgement
We thank all study participants and the staff of the Leon Daniello Hospital. Thanks also to Victor CRISTEAN and Nicolae MIRON for their significant laboratory assistance. This work was supported by the Romanian Government and “Agence Universitaire de la Francophonie” through “Eugen Ionescu” Scholarship program.
References
- 1.World Health Organization. Global tuberculosis report 2013. 2013 [Google Scholar]
- 2.Munk ME, Emoto M. Functions of T-cell subsets and cytokines in mycobacterial infections. Eur Respir J Suppl. 1995;20:668s–675s. [PubMed] [Google Scholar]
- 3.Deveci F, Akbulut HH, Turgut T, Muz MH. Changes in serum cytokine levels in active tuberculosis with treatment. Mediators Inflamm. 2005;5:256–262. doi: 10.1155/MI.2005.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Bose M. Role of cytokines in immune response to pulmonary tuberculosis. Asian Pac J Allergy Immunol. 2001;19(3):213–219. [PubMed] [Google Scholar]
- 6.Rojas M, Olivier M, Gros P, Barrera LF, García LF. TNF-alpha and IL-10 modulate the induction of apoptosis by virulent Mycobacterium tuberculosis in murine macrophages. J Immunol. 1999;162(10):6122–6131. [PubMed] [Google Scholar]
- 7.Olobo JO, Geletu M, Demissie A, Eguale T, Hiwot K, Aderaye G, et al. Circulating TNF-alpha, TGF-beta, and IL-10 in tuberculosis patients and healthy contacts. Scand J Immunol. 2001;53(1):85–91. doi: 10.1046/j.1365-3083.2001.00844.x. [DOI] [PubMed] [Google Scholar]
- 8.Bonecini-Almeida MG, Ho JL, Boéchat N, Huard RC, Chitale S, Doo H, et al. Down-modulation of lung immune responses by interleukin-10 and transforming growth factor β (TGF-β) and analysis of TGF-β receptors I and II in active tuberculosis. Infect Immun. 2004;72(5):2628–2634. doi: 10.1128/IAI.72.5.2628-2634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung YJ, Ryan L, LaCourse R, North RJ. Increased interleukin-10 expression is not responsible for failure of T helper 1 immunity to resolve airborne Mycobacterium tuberculosis infection in mice. Immunology. 2003;109(2):295–299. doi: 10.1046/j.1365-2567.2003.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huard RC, Chitale S, Leung M, Lazzarini LCO, Zhu H, Shashkina E, et al. The Mycobacterium tuberculosis complex-restricted gene cfp32 encodes an expressed protein that is detectable in tuberculosis patients and is positively correlated with pulmonary interleukin-10. Infect Immun. 2003;71(12):6871–6883. doi: 10.1128/IAI.71.12.6871-6883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redford PS, Murray PJ, O’Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 2011;4(3):261–270. doi: 10.1038/mi.2011.7. [DOI] [PubMed] [Google Scholar]
- 12.Lago PM, Boéchat N, Migueis DP, Almeida AS, Lazzarini LC, Saldanha MM, et al. Interleukin-10 and interferon-gamma patterns during tuberculosis treatment: possible association with recurrence. Int J Tuberc Lung Dis. 2012;16(5):656–659. doi: 10.5588/ijtld.11.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahiratmadja E, Alisjahbana B, de Boer T, Adnan I, Maya A, Danusantoso H, et al. Dynamic changes in pro- and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease activity and response to curative treatment. Infect Immun. 2007;75(2):820–829. doi: 10.1128/IAI.00602-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94(6):2435–2442. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong JH, Zhang M, Modlin RL, Linsley PS, Iyer D, Lin Y, et al. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. 1996;64(3):913–918. doi: 10.1128/iai.64.3.913-918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, et al. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest. 2000;105(9):1317–1325. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner J, Gonzalez-Juarrero M, Ellis DL, Basaraba RJ, Kipnis A, Orme IM, et al. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169(11):6343–6351. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]