Abstract
The presence of skin metastasis in ovarian cancer patients is uncommon and related with poor prognosis. We report a 49-year-old patient with recurrent ovarian cancer presented with extensive skin metastasis on the anterior chest (including bilateral breast skin), lower abdomen, vulva and the upper part of the lower limbs at 21 months after initial diagnosis of ovarian cancer. The skin biopsy revealed metastasis of adenocarcinoma in the dermis. The patient underwent palliative chemotherapy and she died after 2 months of the diagnosis of the skin metastasis. It is the first case of skin metastasis from a micropapillary serous ovarian carcinoma published in Romania.
Keywords: ovarian cancer, skin metastasis, chemotherapy
Introduction
Ovarian cancer is the second most frequent gynecological cancer. It has the highest mortality rate in developed countries [1]. Similar trends have been observed in Romania, where ovarian cancer is second only to cervical cancer, according to the North Western Regional Cancer Registry [2].
The majority of patients with epithelial ovarian cancer are diagnosed in advanced stages of the disease based on intraperitoneal dissemination, which is the most common way of metastasis. The disease can also spread by the well-known lymphatic and hematogenous routes. Regarding distant metastasis, the usual sites are the pleura, the liver, the lungs, extra-abdominal lymph nodes and extremely rarely, the skin.
We report a case of a patient with FIGO stage IIIC micropapillary serous ovarian carcinoma, which relapsed as metastatic skin lesions, located on the anterior chest, lower abdomen, vulva and lower limbs, at 21 months post-primary surgery.
Case report
The patient was a 49-year-old woman, gravida 6, para 1, who was admitted to the “Ion Chiricuta” Institute of Oncology, Cluj-Napoca, with suspicion of ovarian cancer, in March 2013.
The preoperative value of cancer antigen 125 was 3178 U/mL. On 26 March 2013, we performed exploratory laparotomy.
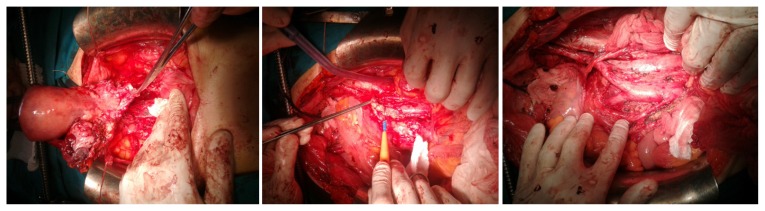
Intraoperatively, the following were found: moderate ascites (approximately 1.5L); the right adnexa presented as a tumor mass of 9/5/4 cm, with heterogeneous cystic and parenchymal appearance; the left adnexa presented as a tumor mass of 7/4/3 cm, with heterogeneous cystic and parenchymal appearance, which formed a tumoral block with the uterus; there were multiple metastases in the vesical and cul-de-sac peritoneum; the great omentum had multiple tumor lesions measuring maximum 3 cm in diameter; there were also multiple enlarged bilateral iliac and para-aortic lymph nodes (up to the emergence of renal blood vessels); multiple metastases, measuring maximum 1 cm in diameter, were present in the diaphragmatic peritoneum, close to the suprahepatic veins; there was no sign of liver metastasis at palpation. The surgical stage of the patient was FIGO stage IIIC. We performed a total abdominal hysterectomy with bilateral salpingo-oophorectomy, pelvic peritonectomy, total omentectomy, pelvic, para-aortic and interiliac lymphadenectomy (Fig. 1), concluding by suboptimal debulking with residual masses up to 1 cm on the right diaphragm, near the suprahepatic veins.
Figure 1.
Hysterectomy with bilateral salpingo-oophorectomy, pelvic peritonectomy, pelvic and paraaortic lymphadenectomy.
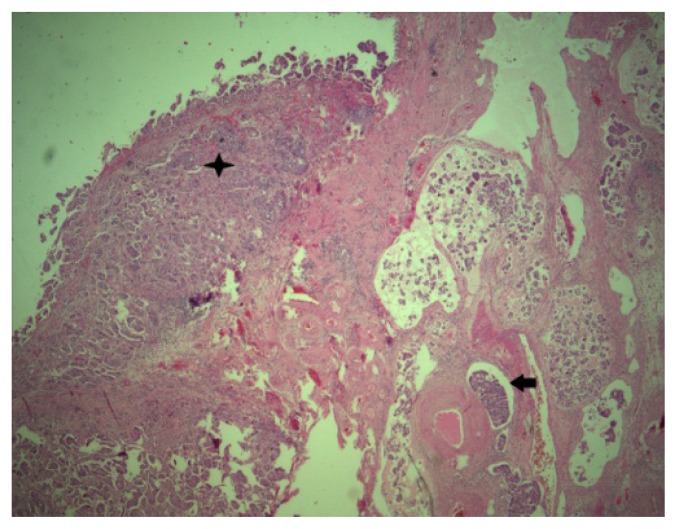
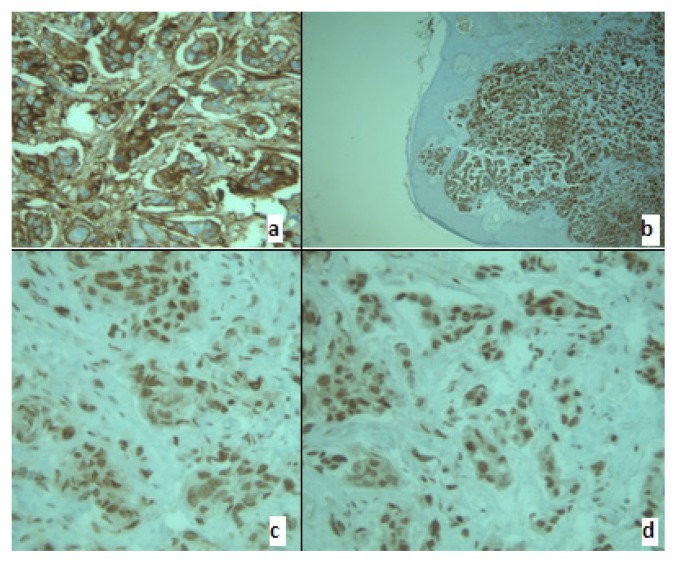
The patient’s postoperative evolution was favorable. Histological examination revealed a micropapillary serous ovarian carcinoma, Silverberg grade 3 (Fig. 2, Fig. 3a,b,c,d).
Figure 2.
Primary ovarian tumor 25X HE.
Star - Tumoral deposit on the ovarian surface.
Arrow- Tumoral lymphatic embolus.
Figure 3.
Membrane and cytoplasmic positive CA 125(a) and CK7 (b). Nuclear positive p53 (c0 and WT1 (d).
Between 10 April 2013 and 18 October 2013, the patient received 9 cycles of adjuvant chemotherapy consisting of paclitaxel (total dose=250mg) and carboplatin 5AUC (total dose=500mg) every three weeks. On 7 November 2013, the level of cancer antigen 125 was increased to 117 U/mL (compared to 72 U/mL in August 2013), which was interpreted as recurrence of the disease, and the patient started second-line adjuvant chemotherapy consisting of topotecan (1.2mg/m2, total dose=1.5mg), 9 cycles, every three weeks, until 26 May 2014. On 16 June 2014, due to the fact that the level of cancer antigen 125 continued to increase, the chemotherapy regimen was changed to gemcitabine (1250mg/m2, total dose=1775mg), 1 cycle. On 9 July 2014, positron emission tomography-computerized tomography evaluation revealed a significant disease progression in the right axillary, supra- and infra-diaphragmatic lymph nodes, and also in the peritoneum. Between 18 September 2014 and 17 November 2014, due to the fact that the level of cancer antigen 125 continued to increase, the chemotherapy regimen was changed to include paclitaxel and carboplatin, 2 cycles, as fourth-line chemotherapy, and Caelyx (50mg/m2, total dose=70mg), 2 cycles, as fifth-line chemotherapy.
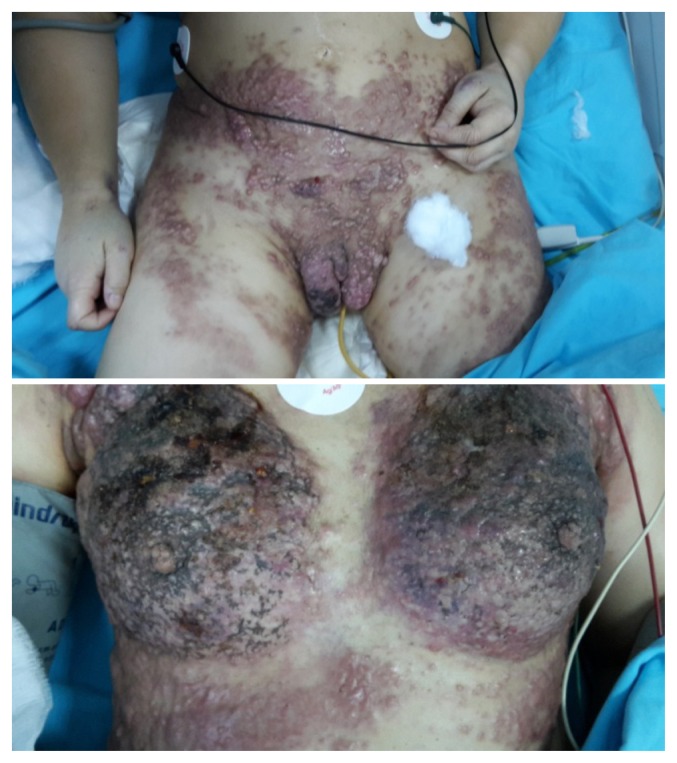
In November 2014, 21 months after the initial diagnosis, the patient presented multiple skin lesions located on the anterior chest (including bilateral breast skin), lower abdomen, vulva and the upper part of the lower limbs (Fig. 4).
Figure 4.
Multiple skin metastasis on the anterior chest (including bilateral breast skin), lower abdomen, vulva and the upper part of the lower limbs.
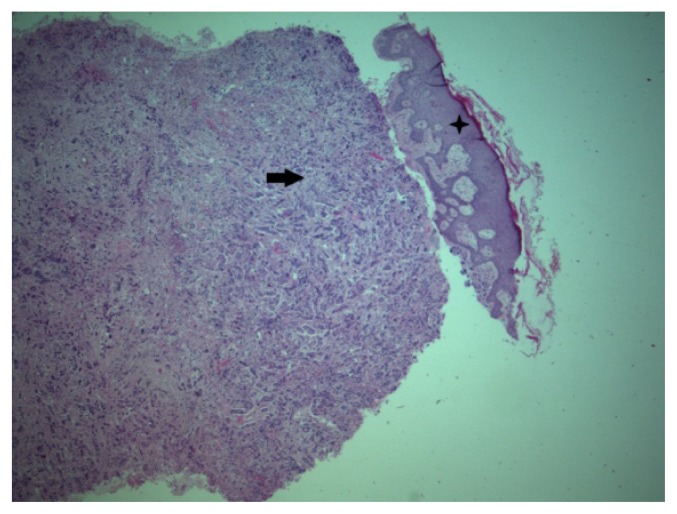
We performed anterior chest skin biopsy, and the histological examination, including immunohistochemistry, revealed a metastatic serous adenocarcinoma lesion (Fig. 5).
Figure 5.
Skin metastasis 25X HE.
Star – Epiderma.
Arrow – Dermis metastasis.
On 4 December 2014, the blood test results permitted the administration of sixth-line chemotherapy consisting of cyclophosphamide (600mg/m2, total dose=850mg) and carboplatin 5AUC (total dose=510mg).
On 19 December 2014, the patient was referred to the Intensive Care Unit of the “Ion Chiricuta” Institute of Oncology, Cluj-Napoca, for acute restrictive respiratory insufficiency due to bilateral pleurisy.
Despite the fact that the patient underwent bilateral thoracentesis with evacuation of about 1,800 ml of serous citrine fluid, evolution was unfavorable, with progressive worsening of dyspnea, with desaturation, tachypnea, requiring endotracheal intubation and finally, mechanical ventilation. On 5 January 2015, the patient died from cardiopulmonary arrest.
Discussion
Even though most patients with epithelial ovarian cancer demonstrate advanced stages at presentation, commonly the disease is restrained to the peritoneal cavity. The primary mode of dissemination in ovarian cancer is by neoplastic peritoneal involvement [3]. Other dissemination pathways are via lymphatic and hematogenous vessels [4]. Regarding distant metastasis, the usually involved sites are, in order, the pleura, the liver, the lungs, the central nervous system, the skin, extra-abdominal lymph nodes, the spleen, bones and breasts [5].
Skin metastases from ovarian carcinoma are highly uncommon and their proportion ranges between 1.9 and 5.1% in different studies [5,6,7]. The literature describes several sites of skin metastasis in ovarian cancer, of which the abdominal wall is the most frequently involved [4,5,8]. Other locations for skin metastases are the chest wall and the breast. Kim et al. presented an upper leg skin metastasis of a stage IIIC papillary serous ovarian cancer [9]. In another article, Matsui et al. described a scalp metastasis of a serous ovarian cancer [10].
Skin metastases present various clinico-pathological forms: small nodules, herpetiform erythematous lesions, and cicatricial plaques [11]. In our case, the metastases initially evolved as lymphangitis and cellulitis located on the anterior chest wall, breasts, lower abdominal wall, vulva and the upper part of the lower limbs.
Several theories regarding skin metastasis in ovarian cancer have been elaborated. The most plausible ones are: direct invasion from the underlying growth, contiguous extension of the tumor cells through lymphatics, and accidental neoplastic implantation during surgical procedures [5,7,11–13]. In some cases, skin metastasis may occur in surgical scars (laparotomy scar, trocar port site, drainage scar, and port and catheter scar) [5].
In their study, Cormio et al. concluded that the factors correlated with the survival of patients with distant ovarian carcinoma metastasis were performance status, the interval of time between diagnosis and the onset of distant metastasis, and the existence of other neoplastic sites [12]. In two previous studies, the interval of time between the diagnosis and the appearance of skin metastasis was 42 and 44 months [9,13]. In Dauplat’s series, the median of this interval was 31.9 months (range 4–77) [4]. In our case, this interval was 21 months, similar to the case published by Yilmaz Z et al., 24 months [8].
In most cases, cutaneous metastases occur late in the disease progression and their presence is almost invariably correlated with intraperitoneal extension and with a poor prognosis [7,14,15]. In our case, the patient also had other sites of the disease (abdominal, pleural, right axillary, supra- and infra-diaphragmatic lymph nodes) and she died of progressive pleural disease.
Currently, few recommendations regarding the treatment of cutaneous metastases from ovarian cancer are available. The type of treatment depends on the patient’s general status, subjective symptoms and the extension and location of the lesions. First of all, palliative chemotherapy should be considered in treating systemic disease. Surgery may be applied for local skin metastases. For extensive cutaneous metastases, electrocoagulation and external radiotherapy have shown good results for the local control of pain, hemorrhage, and infection, but not for the treatment of the systemic disease [16,17].
In conclusion, we presented the first case of skin metastasis from a micropapillary serous ovarian carcinoma published in Romania. Skin metastasis rarely occurs in ovarian cancer patients and is associated with a poor prognosis of the patient. Treatment is multimodal, combining chemotherapy and surgical excision (in selected patients), but there are no published protocols for the treatment of ovarian skin metastasis.
Acknowledgement
This paper was published under the frame of European Social Fund, Human Resources Development Operational Programme 2007–2013, project no. POSDRU/159/1.5/S/138776.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Coza D, Suteu O, Neamtiu L, Nicula FA, Achimas-Cadariu P, Irimie A. Cancer in North-Western Region of Romania 2009. Cancer incidence, mortality and prevalence. [Cancerul in regiunea de nord-vest a Romaniei in anul 2009. Incidenta, mortalitatea si prevalenta cancerului]. Cluj-Napoca: Casa Cartii de Stiinta; 2012. [Google Scholar]
- 3.Prat J FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Dauplat J, Hacker NF, Nieberg RK, Berek JS, Rose TP, Sagae S. Distant metastases in epithelial ovarian carcinoma. Cancer. 1987;60:1561–1566. doi: 10.1002/1097-0142(19871001)60:7<1561::aid-cncr2820600725>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Cormio G, Capotorto M, Vagno GD, Cazzola A, Carriero G, Selvaggi L. Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol Oncol. 2003;90:682–685. doi: 10.1016/s0090-8258(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 6.Dauplat J, Hacker NF, Nieberg RK, Berek JS, Rose TP, Sagae S. Distant metastasis in epithelial ovarian carcinoma. Cancer. 1987;60:1561–6. doi: 10.1002/1097-0142(19871001)60:7<1561::aid-cncr2820600725>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.White JW, Spencer PS, Helm TN. Skin metastases in cancer patients. Cutis. 1987;39:119–121. [PubMed] [Google Scholar]
- 8.Yilmaz Z, Bese T, Demirkiran F, Ilvan S, Sanioglu C, Arvas M, et al. Skin metastasis in ovarian carcinoma. Int J Gynecol Cancer. 2006;16( Suppl 1):414–418. doi: 10.1111/j.1525-1438.2006.00486.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim MK, Kim SH, Lee YY, Choi CH, Kim TJ, Lee JW, et al. Metastatic skin lesions on lower extremities in a patient with recurrent serous papillary ovarian carcinoma: a case report and literature review. Cancer Res Treat. 2012;44(2):142–145. doi: 10.4143/crt.2012.44.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsui H, Suzuka K, Yamazawa K, Tanaka N, Mitsuhashi A, Seki K, et al. Scalp metastasis of a serous ovarian cancer. Acta Obstet Gynecol Scand. 2002;81:577–578. doi: 10.1034/j.1600-0412.2002.810621.x. [DOI] [PubMed] [Google Scholar]
- 11.Brownstein MH, Helwig EB. Patterns of cutaneous metastasis. Arch Dermatol. 1972;105:862–868. [PubMed] [Google Scholar]
- 12.Schonmann R, Altaras M, Biron T, Bernheim J, Fishman A. Inflammatory skin metastases from ovarian carcinoma-a case report and review of the literature. Gynecol Oncol. 2003;90:670–672. doi: 10.1016/s0090-8258(03)00366-4. [DOI] [PubMed] [Google Scholar]
- 13.Cormio G, Rossi C, Cazzolla A, Resta L, Loverro G, Greco P, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. 2003;13(2):125–129. doi: 10.1046/j.1525-1438.2003.13054.x. [DOI] [PubMed] [Google Scholar]
- 14.Tharakaram S. Metastases to the skin. Int J Dermatol. 1988;27:240–242. doi: 10.1111/j.1365-4362.1988.tb03216.x. [DOI] [PubMed] [Google Scholar]
- 15.Krumerman M, Garret R. Carcinoma metastatic to skin. N Y State J Med. 1977;77:1900–1903. [PubMed] [Google Scholar]
- 16.Reinhold RG, Lokich JJ. Electrocoagulation: palliative surgery to control metastatic cutaneous malignancy. J Surg Oncol. 1979;11:207–211. doi: 10.1002/jso.2930110304. [DOI] [PubMed] [Google Scholar]
- 17.Tapley ND. Radiation therapy with electron beam for skin metastases. Sem Oncol. 1981;8:49–58. [PubMed] [Google Scholar]