Abstract
Melanoma, a cancer that arises from melanocytes, is one of the most unresponsive cancers to known therapies and has a tendency to produce early metastases. Several studies showed encouraging results of the efficacy of photodynamic therapy (PDT) in melanoma, in different experimental settings in vitro and in vivo, as well as several clinical reports.
Aims
Our study focuses on testing the antimelanoma efficacy of several new, synthetic photosensitisers (PS), from two different chemical classes, respectively four porphyrins and six phthalocyanines.
Methods
These PS were tested in terms of cell toxicity and phototoxicity against a radial growth phase melanoma cell line (WM35), in vitro. Cells were exposed to different concentrations of the PS for 24h, washed, then irradiatied with red light (630 nm) 75 mJ/cm2 for the porphyrins and 1 J/cm2 for the phthalocyanines. Viability was measured using the MTS method.
Results
Two of the synthetic porphyrins, TTP and THNP, were active photosensitizers against WM35 melanoma in vitro. Phthalocyanines were effective in producing a dose dependent PDT-induced decrease in viability in a dose-dependent manner. The most efficient was Indium (III) Phthalocyanine chloride, a metal substituted phthalocyanine.
Conclusions
The most efficient photosensitizers for PDT in melanoma cells were the phthalocyanines in terms of tumor cell photokilling and decreased dark toxicity.
Keywords: melanoma, photodyamic therapy, porphyrins, phthalocyanines, cell photokilling
Introduction
Melanoma, a cancer that arises from melanocytes, is one of the most unresponsive cancers to known therapies and has a tendency to produce early metastases [1,2]. Early detection, surgery, and adjuvant therapy enable improved outcomes; nonetheless, the prognosis of metastatic melanoma remains poor [3].
There are studies that show encouraging results of the efficacy of photodynamic therapy (PDT) in melanoma, in different experimental settings in vitro and in vivo as well as several clinical reports. In vitro and in vivo, on human and mice melanoma cell lines, PDT induced significant cell death [4–11], tumor size decrease, delay of tumor growth and increase of life span [12–20]. Several clinical reports showed that PDT, using verteporfin, a porphynic PS, was well tolerated and effective on skin melanoma metastases [21] and induced complete remission [22,23] or were partially effective in choroidal melanoma [24,25].
PDT is a simple procedure that requires the administration of a PS, followed by irradiation. PS activation generates singlet oxygen (O2−) and other reactive oxygen species (ROS) [26–28]. The antitumor effects result from direct tumor photodamage, destruction of tumor vasculature and activation of an immune response [11].
The ideal PS criteria are: chemical purity, preferential and fast tumor accumulation and rapid clearance, high light absorption coefficient, no dark toxicity, minimal or absent remaining skin photosensitivity [29]. There are several classes of PS: porphyrins, chlorines, phthalocyanines, texapyrins, porphycens, antracens, chlorophyll derivatives, purpurins, hypocrellins and hypericin. More than 30 different PS are used in preclinical studies [29], but only ALA (5-aminolevulinic acid, Levulan) and its methyl ester (Metvix), m-THCP (meta-tetrahydroxyphenylchlorin, Foscan), porfimer sodium (Photofrin) have been approved for use in clinical oncology [11].
However, classical PDT has shown some limitations in clinical application [3]. The most important challenge is to find improved sensitizers, able to overcome melanoma resistance, due to melanosomal trapping, pigmentation, oxidative stress defense, immune evasion [25]. Our study focuses on testing the antimelanoma efficacy of several new, synthetic PS, from two different chemical classes, respectively porphyrins and phthalocyanines. These PS were tested in terms of cell toxicity and phototoxicity against a radial growth phase melanoma cell line (WM35), in vitro.
Materials and methods
1. Synthesis and characterization of the photosensitizers
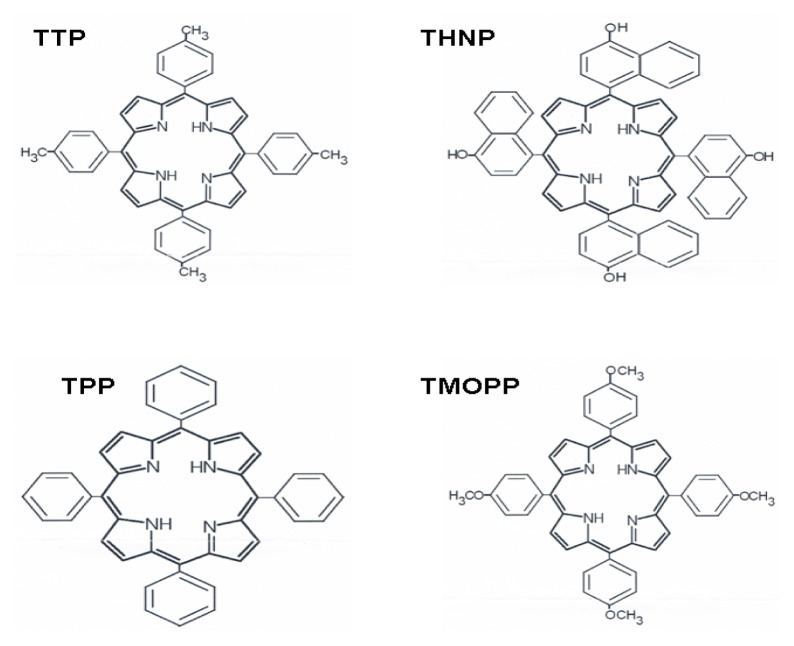
The porphyrins 5,10,15,20-tetra-p-tolyl porphyrin (TTP), 5,10,15,20-tetra-p-naphthyl-porphyrin (THNP), 5,10,15,20-tetra-p-phenyl orphyrin (TPP), 5,10,15,20-tetra-p-methoxy-phenyl porphyrin (TMOPP) (Fig. 1) were obtained in the laboratory by using the Lindsey method [30]. Mass spectrum (FAB) Found (MW): TTP = 672, THNP=882, TPP=616, TMOPP=736 (Fig. 1).
Figure 1.
Chemical structures of the porphyrins tested as PS in PDT against melanoma.
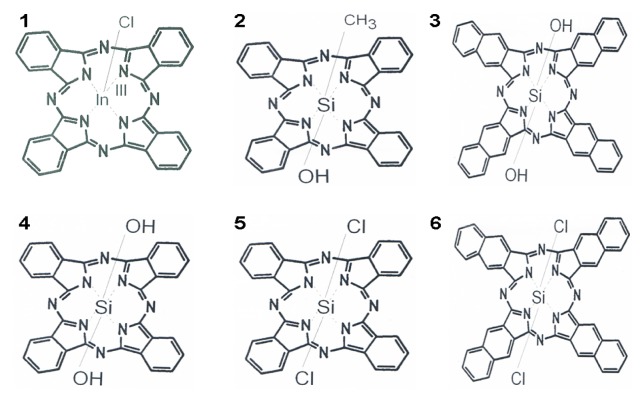
The phthalocyanines (Pc) used were: 1: chloride indium (III) phthalocyanine [ClIn (III)Pc], 2: dihydroxide -silicon 2,3 naphtalocyanine - [(OH)2SiNc], 3: hydroxide methylsilicon (IV) phthalocyanine (OH)CH3Si(IV)Pc, 4: dihydroxide silicon phthalocyanine [OH)SiPc], 5: dichloride silicon phthalocyanine [Cl2SiPc], 6: dichloride silicon 2,3-naphtalocyanine -[Cl2SiNc] (Fig. 2). All these compounds have been provided by Sigma Aldrich, and used without any purification.
Figure 2.
Chemical structures of the six phthalocyanines tested as PS in PDT against melanoma.
Mass spectrum (FAB) Found (MW): ClIn(III)Pc =662.79, (OH)2SiNc=774.86, (OH)CH3Si(IV)Pc =572.65, (OH)SiPc =574.63, Cl2SiPc =611.51, Cl2SiNc =811.86.
The phthalocyanines are as follows: 1: chloride indium (III) phthalocyanine [ClIn (III)Pc], 2: dihydroxide -silicon 2,3 naphtalocyanine - [(OH)2SiNc], 3: hydroxide methylsilicon (IV) phthalocyanine [(OH)CH3Si(IV)Pc], 4: dihydroxide silicon phthalocyanine [(OH)SiPc, 5: dichloride silicon phthalocyanine [Cl2SiPc], 6: dichloride silicon 2,3-naphtalocyanine -[Cl2SiNc], Pc1 contains a metal core, represented by Indium, while the others have silicon in the active center; they are either chlorinated (1, 5, 6), or hydroxilated compounds (2, 3, 4) to allow better tissue penetration (a combination of hydro/lipophylic properties).
2. Melanoma bioassays
2. 1. Cell culture
The assessment was performed on a human radial growth phase (RGP) melanoma cell line (WM35). Melanoma cells (Wistar Institute, Philadelphia, PA, USA) were maintained in RPMI medium supplemented with 5% fetal calf serum, 50 μg/ml gentamicyn and 5 ng/ml amphotericin (Biochrom). Cultures were fed twice weekly and incubated in a humid atmosphere at 37°C and 5% CO2. All experiments were conducted in subdued light, in triplicate.
2.2. Light source
irradiation was done with red light (wave length 630 nm, lamp power 2.5 mW/cm2) provided by a Philips LED red light system, with doses of 4.5 J/cm2 for porphyrins and 6 J/cm2 for phthalocyanines respectively.
2. 3. Photosensitiser exposure
Cells were exposed to PS for 24h prior to irradiation. Synthetic PS were solubilized in DMSO to obtain a stock solution of 10 mg/ml. Dilutions of this solution in fresh medium were made immediately before use. The DMSO final concentration in the medium was <0.05%, not harmful to the cells [31].
2.4. Cytotoxicity assay
The cells were seeded at a density of 104/well in ELISA 96 wells micro titration flat bottom plaques (TPP, Switzerland) and settled for 24h. Then the cells were exposed to each photosensitiser, prepared as described above, in concentrations ranging from: 1–2000 μg/ml for porphyrins, 2.5–250 μg/ml for phthalocyanines respectively in medium for 24h. Cells were then washed, irradiated and further incubated for 24h with fresh medium. Viability was measured by colorimetric measurement of formazan, a coloured compound generated by mitochondrial reductase activity in viable cells using CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (Promega, USA). Untreated cultures exposed to medium were used as controls. Briefly, WM35 cultures were exposed to 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetra-zolium, inner salt (MTS) /phenazine metosulphate (PMS) mixture for 2h, then the optical density values were tested at absorbance of 490 nm (as indicated by the producer) by an ELISA plate reader (Tecan, Switzerland). Cytotoxicity was evaluated as OD 490 and % of untreated controls [32].
2.5. Statistical method
The statistical significance of the difference between treated and control groups was evaluated by paired Student TTEST, results were considered significant for p≤0.05. Statistical package Prism version 4.00 for Windows, GraphPad Software, San Diego, California, USA, www.graphpad.com was used for data analyses.
Results
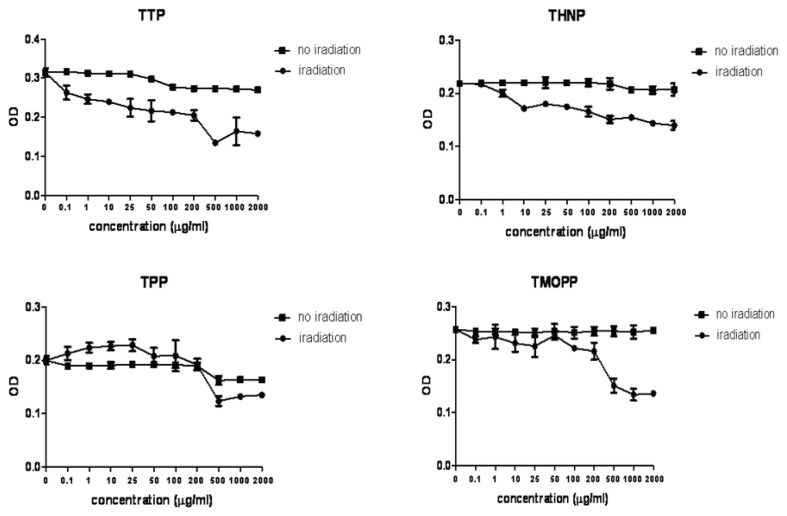
The effectiveness of the porphyrins: TTP, THNP, TPP, TMOPP (Fig. 1) and the phthalocyanines (1–6): ClIn(III)Pc, (OH)2SiNc, (OH)CH3Si(IV)Pc, (OH)Si2,3Pc, Cl2Si2,3Pc, Cl2Si2,3Nc (Fig. 2) was tested against the radial growth phase human melanoma cell line WM35. Porphyrins, especially TTP exhibited a slight cytotoxicity at high doses. PDT induced different rates of viability decrease (Fig. 3). The order of efficacy was: TTP, THNP, TPP and TMOPP. TTP induced photokilling at concentrations as low as 0.1 μg/ml (p≤ 0.003, compared to irradiated controls) and was dose dependent, while the cytotoxic effect, also dose dependent, appeared at concentrations above 100 μg/ml (p≤0.015, compared to controls). The same effect was seen in the case of THNP (p≤0.02, for concentrations above 1μg/ml, compared to irradiated controls), with cytotoxicity at concentrations above 500 μg/ml (p≤0.011, compared to controls). Despite the lack of cytotoxicity, the other porphyrins showed decreased PDT efficacy.
Figure 3.
Cell viability testing following PDT mediated by the four porphyrins. Viability data are presented as OD490, TTP and THNP (upper panels) proved to be good PS against WM 35 melanoma cells.
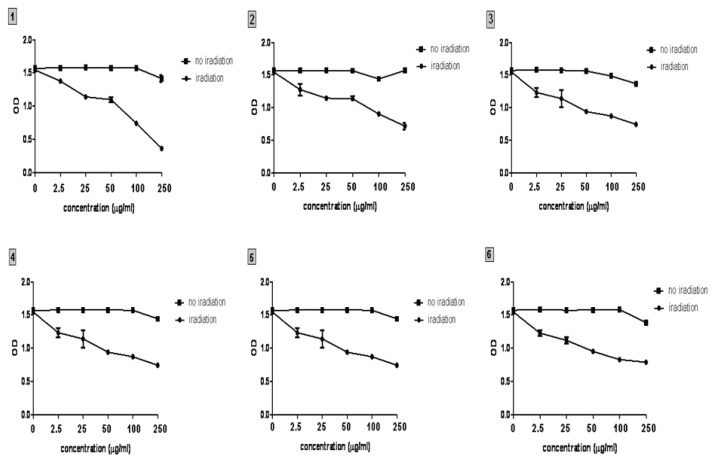
Phthalocyanines were effective in producing a dose dependent PDT-induced loss of mitochondrial activity in a dose-dependent manner (Fig. 4). The cell viability decrease was significant for all concentrations (p≤ 0.001 for Pc1, p≤0.006 for Pc2, p≤0.002 for Pc3, p≤0.014 for Pc4, p≤0.041 for Pc5, and p≤0.006 for Pc6, compared to irradiated controls). The order of efficacy was: Pc1, 3, 2, 4, 6 and 5. Pc’s did not exhibit dark toxicity after 24h incubation in any of the cases case.
Figure 4.
Cell viability testing following PDT mediated by the six phthalocyanines. Viability data are presented as OD490, all Pc induced photokilling with no dark toxicity, Pc1 showed the best phototoxic efficacy against WM 35 melanoma cells.
Discussion
This report has demonstrated that two of the synthetic porphyrins, TTP and THNP are active photosensitizers against WM35 melanoma in vitro. However, the safety profile of the compounds needs to be improved to meet the requirements [29], namely high phototoxicity with minimal citotoxicity.
The other class of the compounds tested, the Pc, yielded more promising results, regarding the safety profile. However, the decrease in viability was not as high as expected. This may be due to a very low dose of irradiation, of only 6 J/cm2. Other reports on G361 human melanoma cells with a disulfonated chloroaluminum phthalocyanine (ClAlPcS2) showed PDT experiments using light doses of 25 J/cm2 [33], others used PDT regimens with Pc’s and light doses of 10 mJ/cm2 or 20 mJ/cm2 [9,34]. Since our research is a preliminary comparative PDT viability study, we aimed to find the best suited PS against the WM35 melanoma cell line. PDT efficacy directly depends on the PS properties and the light dose [25]. The PDT irradiation doses were intentionally kept lower, in order to differentiate among the PS’s efficacies.
Other studies also reported similar PDT results by using various Pc compounds as PS against different melanoma cell lines and in vivo melanoma models [33–38, 41]. A comparative study with Photophrin, a porphyrinic PS and a newly synthesized tetrabenzamido-substituted Zn(II) phthalocyanine (ZnNcA) against B16 melanoma mouse model showed better results for ZnNcA [33].
Porphyrins were the first substances used as PS. However, in melanoma, porphyrins like aminolevulinic acid, it’s methyl ester, Metvix, and Photofrin lacked the efficiency showed in non-melanoma skin cancers [5, 39,40]. This was probably due to the presence of the melanin pigment which acts as a defense mechanism. First, melanin is able to absorb the wavelengths of light necessary to activate the porphyrins and secondly, it can behave as an intracellular ROS scavenger, neutralizing the PDT induced ROS [25].
Phthalocyanines are macrocyle compounds, similar to porphyrins. They are activated by the same wavelengths of light as porphyrins. There are two advantages of the Pc’s over porphyrins, as potential PDT agents: higher ROS generation and better spectroscopic properties [41]. These make them more suitable as anti melanoma agents since the ROS production is higher and can potentially overcome the melanin and other enzymatic antioxidant defenses of the melanoma cells.
A major problem of the Pc’s is the lack of tumor specificity that porphyrins possess. Thus, multiple synthetic compounds were synthesized in order to find ways to improve the tumor penetration of the Pc’s [41].
Also, aggregation is a common problem of the macrocyclic complexes [41]. One way is to chemically substitute the active center of the molecule with silicon [34,36,37]. In our study, five of the six PC’s, respectively 2–6 are this type of compounds. They shared a similar PS behavior with good efficacy and decreased dark toxicity. Another way to decrease Pc aggregation, while increasing lipophilicity, thus the tissue penetration, is to synthesize metal substituents coordinated to the silicon center [9], in our case Indium. As seen by the viability study, this compound showed greater photokilling properties with no toxic effects at therapeutic doses.
Conclusion
The most efficient photosensitizers for PDT in melanoma cells were the phthalocyanines, especially the Indium (III) Phthalocyanine chloride. The viability decrease induced by the PDT was accompanied in this case by low dark toxicity. This makes it suitable for further testing in order to find the molecular mechanisms that led to tumor cell photokilling.
Acknowledgments
This paper was published under the frame of European Social Found, Human Resources Development Operational Programme 2007–2013, project no. POSDRU/159/1.5/S/138776
References
- 1.NCCN Guidelines Version 4. 2014 Melanoma. 2014. Available from: http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf.
- 2.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monge-Fuentes V, Muehlmann LA, de Azevedo RB. Perspectives on the application of nanotechnology in photodynamic therapy for the treatment of melanoma. Nano Rev. 2014;5 doi: 10.3402/nano.v5.24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maduray K, Karsten A, Odhav B, Nyokong T. In vitro toxicity testing of zinc tetrasulfophthalocyanines in fibroblast and keratinocyte cells for the treatment of melanoma cancer by photodynamic therapy. J Photochem Photobiol B. 2011;103(2):98–104. doi: 10.1016/j.jphotobiol.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Robertson CA, Abrahamse H, Evans D. The in vitro PDT efficacy of a novel metallophthalocyanine (MPc) derivative and established 5-ALA photosensitizing dyes against human metastatic melanoma cells. Lasers Surg Med. 2010;42(10):766–776. doi: 10.1002/lsm.20980. [DOI] [PubMed] [Google Scholar]
- 6.Haddad R, Blumenfeld A, Siegal A, Kaplan O, Cohen M, Skornick Y, et al. In vitro and in vivo effects of photodynamic therapy on murine malignant melanoma. Ann Surg Oncol. 1998;5(3):241–247. doi: 10.1007/BF02303780. [DOI] [PubMed] [Google Scholar]
- 7.Haddad R, Kaplan O, Greenberg R, Siegal A, Skornick Y, Kashtan H. Photodynamic therapy of murine colon cancer and melanoma using systemic aminolevulinic acid as a photosensitizer. Int J Surg Investig. 2000;2(3):171–178. [PubMed] [Google Scholar]
- 8.Szurko A, Kramer-Marek G, Widel M, Ratuszna A, Habdas J, Kus P. Photodynamic effects of two water soluble porphyrins evaluated on human malignant melanoma cells in vitro. Acta Biochim Pol. 2003;50(4):1165–1174. [PubMed] [Google Scholar]
- 9.Barge J, Decreau R, Julliard M, Hubaud JC, Sabatier AS, Grob JJ, et al. Killing efficacy of a new silicon phthalocyanine in human melanoma cells treated with photodynamic therapy by early activation of mitochondrion-mediated apoptosis. Exp Dermatol. 2004;13(1):33–44. doi: 10.1111/j.0906-6705.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 10.Saczko J, Kulbacka J, Chwilkowska A, Drag-Zalesiniska M, Wysocka T, Lugowski M, et al. The influence of photodynamic therapy on apoptosis in human melanoma cell line. Folia Histochem Cytobiol. 2005;43(3):129–132. [PubMed] [Google Scholar]
- 11.Mroz P, Huang YY, Szokalska A, Zhiyentayev T, Janjua S, Nifli AP, et al. Stable synthetic bacteriochlorins overcome the resistance of melanoma to photodynamic therapy. FASEB J. 2010;24(9):3160–3170. doi: 10.1096/fj.09-152587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teng IT, Chang YJ, Wang LS, Lu HY, Wu LC, Yang CM, et al. Phospholipid-functionalized mesoporous silica nanocarriers for selective photodynamic therapy of cancer. Biomaterials. 2013;34(30):7462–7470. doi: 10.1016/j.biomaterials.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Shi J, Yu X, Wang L, Liu Y, Gao J, Zhang J, et al. PEGylated fullerene/iron oxide nanocomposites for photodynamic therapy, targeted drug delivery and MR imaging. Biomaterials. 2013;34(37):9666–9677. doi: 10.1016/j.biomaterials.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Chu M, Li H, Wu Q, Wo F, Shi D. Pluronic-encapsulated natural chlorophyll nanocomposites for in vivo cancer imaging and photothermal/photodynamic therapies. Biomaterials. 2014;35(29):8357–8373. doi: 10.1016/j.biomaterials.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 15.Camerin M, Magaraggia M, Soncin M, Jori G, Moreno M, Chambrier I, et al. The in vivo efficacy of phthalocyanine-nanoparticle conjugates for the photodynamic therapy of amelanotic melanoma. Eur J Cancer. 2010;46(10):1910–1918. doi: 10.1016/j.ejca.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Idris NM, Gnanasammandhan MK, Zhang J, Ho PC, Mahendran R, Zhang Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat Med. 2012;18(10):1580–1585. doi: 10.1038/nm.2933. [DOI] [PubMed] [Google Scholar]
- 17.Tammela T, Saaristo A, Holopainen T, Yla-Herttuala S, Andersson LC, Virolainen S, et al. Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci Transl Med. 2011;3(69):69ra11. doi: 10.1126/scitranslmed.3001699. [DOI] [PubMed] [Google Scholar]
- 18.Dabrowski JM, Urbanska K, Arnaut LG, Pereira MM, Abreu AR, Simões S, et al. Biodistribution and photodynamic efficacy of a water-soluble, stable, halogenated bacteriochlorin against melanoma. ChemMedChem. 2011;6(3):465–475. doi: 10.1002/cmdc.201000524. [DOI] [PubMed] [Google Scholar]
- 19.Arnaut LG, Pereira MM, Dabrowski JM, Silva EF, Schaberle FA, Abreu AR, et al. Photodynamic therapy efficacy enhanced by dynamics: the role of charge transfer and photostability in the selection of photosensitizers. Chemistry. 2014;20(18):5346–5357. doi: 10.1002/chem.201304202. [DOI] [PubMed] [Google Scholar]
- 20.Rapozzi V, Zorzet S, Zacchigna M, Drioli S, Xodo LE. The PDT activity of free and pegylated pheophorbide a against an amelanotic melanoma transplanted in C57/BL6 mice. Invest New Drugs. 2013;31(1):192–199. doi: 10.1007/s10637-012-9844-4. [DOI] [PubMed] [Google Scholar]
- 21.Sheleg SV, Zhavrid EA, Khodina TV, Kochubeev GA, Istomin YP, Chalov VN, et al. Photodynamic therapy with chlorin e(6) for skin metastases of melanoma. Photodermatol Photoimmunol Photomed. 2004;20(1):21–26. doi: 10.1111/j.1600-0781.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 22.Donaldson MJ, Lim L, Harper CA, Mackenzie J, Campbell GW. Primary treatment of choroidal amelanotic melanoma with photodynamic therapy. Clin Experiment Ophthalmol. 2005;33(5):548–549. doi: 10.1111/j.1442-9071.2005.01083.x. [DOI] [PubMed] [Google Scholar]
- 23.Soucek P, Cihelkova I. Photodynamic therapy with verteporfin in subfoveal amelanotic choroidal melanoma (A controlled case) Neuro Endocrinol Lett. 2006;27(1–2):145–148. [PubMed] [Google Scholar]
- 24.Barbazetto IA, Lee TC, Rollins IS, Chang S, Abramson DH. Treatment of choroidal melanoma using photodynamic therapy. Am J Ophthalmol. 2003;135(6):898–899. doi: 10.1016/s0002-9394(02)02222-5. [DOI] [PubMed] [Google Scholar]
- 25.Baldea I, Filip AG. Photodynamic therapy in melanoma--an update. J Physiol Pharmacol. 2012;63(2):109–118. [PubMed] [Google Scholar]
- 26.Garland MJ, Cassidy CM, Woolfson D, Donnelly RF. Designing photosensitizers for photodynamic therapy: strategies, challenges and promising developments. Future Med Chem. 2009;1(4):667–691. doi: 10.4155/fmc.09.55. [DOI] [PubMed] [Google Scholar]
- 27.Ion RM, Nyokong T. The use of phthalocyanines and related complexes in photodynamic therapy. In: Nyokong T, Ahsen V, editors. Photosensitizers in Medicine, Environment, and Security. 1st ed. Springer; 2012. [Google Scholar]
- 28.Ion RM. Derivative UV-Vis spectrophotometry for porphyrins interactions in photodynamic therapy. Anal Lett. 2010;43(7):1277–1286. [Google Scholar]
- 29.Nowis D, Makowski M, Stoklosa T, Legat M, Issat T, Golab J. Direct tumor damage mechanisms of photodynamic therapy. Acta Biochim Pol. 2005;52:339–352. [PubMed] [Google Scholar]
- 30.Lindsey JS, Hsu HC, Schreiman IC. Synthesis of Tetraphenylporphyrins Under Very Mild Conditions. Tetrahedron Lett. 1986;27:4969–4970. [Google Scholar]
- 31.Kashino G, Liu Y, Suzuki M, Masunaga S, Kinashi Y, Ono K, et al. An alternative mechanism for radioprotection by dimethyl sulfoxide; possible facilitation of DNA double-strand break repair. J Radiat Res. 2010;51:733–740. doi: 10.1269/jrr.09106. [DOI] [PubMed] [Google Scholar]
- 32.Baldea I, Costin GE, Shellman Y, Kechris K, Olteanu ED, Filip A, et al. Biphasic pro-melanogenic and pro-apoptotic effects of all-trans-retinoic acid (ATRA) on human melanocytes: time-course study. J Dermatol Sci. 2013;72:168–76. doi: 10.1016/j.jdermsci.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Kolarova H, Nevrelova P, Bajgar R, Jirova D, Kejlova K, Strnad M. In vitro photodynamic therapy on melanoma cell lines with phthalocyanine. Toxicol In Vitro. 2007;21:249–253. doi: 10.1016/j.tiv.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Decreau R, Richard MJ, Verrando P, Chanon M, Julliard M. Photodynamic activities of silicon phthalocyanines against achromic M6 melanoma cells and healthy human melanocytes and keratinocytes. J Photochem Photobiol B. 1999;48:48–56. doi: 10.1016/S1011-1344(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 35.Michailov N, Peeva M, Angelov I, Wohrle D, Muller S, Jori G, et al. Fluence rate effects on photodynamic therapy of B16 pigmented melanoma. J Photochem Photobiol B. 1997:37154–157. doi: 10.1016/s1011-1344(96)07401-5. [DOI] [PubMed] [Google Scholar]
- 36.Friso E, Roncucci G, Dei D, Soncin M, Fabris C, Chiti G, et al. A novel 10B-enriched carboranyl-containing phthalocyanine as a radio- and photo-sensitising agent for boron neutron capture therapy and photodynamic therapy of tumours: in vitro and in vivo studies. Photochem Photobiol Sci. 2006;5:39–50. doi: 10.1039/b506364g. [DOI] [PubMed] [Google Scholar]
- 37.Jiang XJ, Huang JD, Zhu YJ, Tang FX, Ng DK, Sun JC. Preparation and in vitro photodynamic activities of novel axially substituted silicon (IV) phthalocyanines and their bovine serum albumin conjugates. Bioorg Med Chem Lett. 2006;16:2450–2453. doi: 10.1016/j.bmcl.2006.01.075. [DOI] [PubMed] [Google Scholar]
- 38.Cicillini SA, Prazias ACL, Tedesco AC, Serra OA, da Silva RS. Nitric oxide and singlet oxygen photo-generation by light irradiation in the phototherapeutic window of a nitrosyl ruthenium conjugated with a phthalocyanine rare earth complex. Polyhedron. 2009;28:2766–2770. [Google Scholar]
- 39.Busetti A, Soncin M, Jori G, Rodgers MA. High efficiency of benzoporphyrin derivative in the photodynamic therapy of pigmented malignant melanoma. Br J Cancer. 1999;79:821–824. doi: 10.1038/sj.bjc.6690131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abels C, Fritsch C, Bolsen K, Szeimies RM, Ruzicka T, Goerz G, et al. Photodynamic therapy with 5-aminolaevulinic acid-induced porphyrins of an amelanotic melanoma in vivo. J Photochem Photobiol B. 1997;40:76–83. doi: 10.1016/s1011-1344(97)00027-4. [DOI] [PubMed] [Google Scholar]
- 41.Swavey S, Tran M. Davids Lester., editor. Porphyrin and Phthalocyanine Photosensitizers as PDT Agents: A New Modality for the Treatment of Melanoma, Recent Advances in the Biology, Therapy and Management of Melanoma. 2013. Available from: http://www.intechopen.com/books/recent-advances-in-the-biology-therapy-and-management-of-melanoma/porphyrin-and-phthalocyanine-photosensitizers-as-pdt-agents-a-new-modality-for-the-treatment-of-melanoma.