Abstract
Aim:
The aim was to evaluate the analgesic, anti-inflammatory, and anti-hyperlipidemic activities of Commiphora molmol extract (CME) and its effects on body weight and blood lipids.
Materials and Methods:
The analgesic effect was assessed using thermal (hot plate test) and chemical (writhing test) stimuli to induce central and peripheral pain in mice. The anti-inflammatory activity was determined using formalin-induced paw edema in rats. For anti-hyperlipidemic effect, 25 rats were randomly divided into five groups (n = 5). Group 1 was fed on basal diet (normal control), while the other four groups were fed on high-fat diet for 6 weeks to induce obesity and hyperlipidemia. Thereafter, Group 2 was kept obese hyperlipidemic, and Groups 3, 4 and 5 were orally given CME in doses of 125, 250, and 500 mg/kg for 6 weeks, respectively. Body weight gains of rats were calculated, and blood samples were collected for analysis of blood lipids.
Results:
CME produced a dose-dependent analgesic effect using both hot plate and writhing tests in mice. The hot plate method appeared to be more sensitive than writhing test. CME exhibited an anti-inflammatory activity as it decreased volume of paw edema induced by formalin in rats. The extract decreased body weight gain; normalized the high levels of blood lipids and decreased atherogenic index low-density lipoprotein/ high-density lipoprotein in obese hyperlipidemic rats.
Conclusion:
The results denote that C. molmol extract (myrrh) has significant analgesic, anti-inflammatory and anti-hyperlipidemic effects and reduces body weight gain and improves blood lipids profile. These results affirm the traditional use of C. molmol for the treatment of pain, inflammations, and hyperlipidemia.
KEY WORDS: Analgesic, anti-inflammatory, blood lipids, Commiphora molmol, obesity
INTRODUCTION
The use of herbal medicine represents a long history of human interactions with the environment. According to World Health Organization more than 80% of the world’s population depends upon traditional medicine for their primary healthcare needs. Medicinal plants have been used in healthcare since time immemorial, and they contain a wide range of bioactive substances that can prevent and treat many diseases. The most important of plant bioactive compounds are sterols, flavonoids, terpenes, diterpenes, sesquiterpenes, and polyphenolic compounds [1,2]. Medicinal plants represent a safer and cheaper source of drugs than chemically synthesized drugs which produce harmful or toxic side-effects [3].
Commiphora molmol, family burseraceae, is small perennial tropical trees that grown in arid and semiarid regions in East Africa, Saudi Arabia, and India [4]. Somali or Arabian myrrh is a resinous exudate (oleo-gum resin) obtained from the stem of C. molmol trees. Myrrh has been approved in USA by Food and Drug Administration as a safe natural flavoring agent in foods and beverages and as fragrance in cosmetics [5]. For many years, myrrh has been used for healing wound injuries [6]. The benefits of using myrrh in medicine have been proven in many scientific studies [7-10].
Previous studies revealed that the resinous exudates of different Commiphora tree species produced analgesic and anti-inflammatory, [11-13] antiulcer, [4,8,10] antioxidant, [14] anti-hyperlipidemic, [15,16] hypoglycemic, [17] and cardioprotective [18] effects. Extracts of Commiphora tree species were reported to possess anti-bacterial [19-21] and anti-schistosomal [22,23] activities. The resinous exudates of different Commiphora tree species have been used for arthritis, hyperlipidemia, and pain, inflammatory conditions, healing of wounds, obesity, schistosomiasis, and gastrointestinal diseases [10]. The resin of C. molmol has been used in Egypt as an effective anti-schistosomal drug under the commercial name “Mirazid” (Pharco Pharmaceuticals Company) in the form of soft gelatin capsules.
The present study was undertaken to evaluate the analgesic, anti-inflammatory, and anti-hyperlipidemic effects of C. molmol ethanol extract and its effect on body weight and blood lipids.
MATERIALS AND METHODS
C. molmol Resin
The oleo-gum resin of C. molmol (Somali myrrh, Arabian myrrh), family burseraceae, was procured from the agricultural seeds, Herbs and Medicinal Plants Company, Egypt. Myrrh resin is present in the form of brownish masses as illustrated Figure 1. It has an aromatic odor and bitter taste. Myrrh resin was grinded by a mill into a fine powder until used for alcohol extraction.
Figure 1.

Commiphora molmol resin (myrrh)
Chemicals and Drugs
The following chemicals and drugs were used: Acetic acid, formalin and Tween-80 (El Gomhoryia Company Egypt); diclofenac sodium (Voltaren, 75 mg/3 ml ampoules, Novartis Company); aspirin (Aspocid. 300 mg tablets, Cid Company, Egypt) and indomethacin (Liometacen, 50 mg/2 ml ampoules, Nile Company Egypt). The myrrh extract was dissolved by the aid of suspending agent Tween-80.
Animals
Fifty adult male Wister mice (20-25 g body weight, 5-6 weeks old) and 50 Sprague-Dawley male rats (150-155 g b.wt. and 8-10 weeks old) were used in this study. The animals were purchased from the Laboratory Animal Colony, Helwan, Egypt. The animals were kept under controlled hygienic conditions and maintained at a temperature of 25°C ± 2°C, relative humidity of 50% ± 5% and photoperiod at 12 h dark/12 h light cycles. Feed and water were provided ad-libitum. The experiments on laboratory animals were carried out according to guidelines and roles for animal experimentation approved by the Institutional Animal Care and Use Committee, National Research Center, Dokki, Egypt.
Preparation of Basal Diet
Basal diet was prepared using American Institute of Nutrition - 93 according to Reeves et al.,[24]. It consists of 20% protein (casein), 10% sucrose, 5% fat (corn oil), 3.5% salt mixture, 1% vitamin mixture, 2.5% choline chloride, and 5% fibers (cellulose). The remainder was corn starch up to 100%.
Biochemical Kits
Kits for biochemical analysis of serum total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), and very low-density lipoprotein (LDL) were purchased from Gamma Trade Co. for Pharmaceuticals and Diagnostic Chemicals, Dokki, Egypt.
Preparation of Commiphora Extract
Two hundred grams of fine powder of C. molmol resin were soaked in 1 L of 90% ethanol and kept in a refrigerator with daily shaking for 3 days. The liquid ethanol extract was filtrated using double-layer of gauze. The extract was concentrated using rotatory evaporator connected with an electric vacuum pump and metal water bath adjusted at 50°C. The method of preparation of plant extract was described by Shalaby and Hamowieh [25]. The obtained semisolid ethanol extract of C. molmol was kept in a refrigerator until further use.
Analgesic Activity
Hot plate method
This method was applied as described by Turner [26]. The test is based upon induction of thermal stimulus by putting the mouse in a glass beaker setting on the surface of the hot plate thermostatically controlled at 55°C. The time (seconds) which elapse from putting the mice on the hot plate until the mouse clicks its fore paws or jumps is the reaction time. The increase in latency (reaction) time denotes analgesic activity. For this experiment, 25 adult male Wister mice were randomly divided into five equal groups, of five mice each. Group 1 served as a normal control and orally given 1 ml distilled water (vehicle). Group 2 was orally given diclofenac sodium (standard) in a dose 20 mg/kg. Groups 3, 4 and 5 were orally given C. molmol extract in doses of 125, 250, and 500 mg/kg, respectively. One hour after administration of diclofenac or plant extract, the latency time (seconds) at time intervals of 15, 30, 60, and 120 min for each group was noted and recorded. A cut-off reaction time was set at 60 s to prevent damage to tissues of the foot.
Writhing test
This test was performed according to the method described by Gawade [27]. Twenty five adult male Wister mice were randomly divided to five equal groups, each of five mice. Group 1 was used as a normal control and given 1 ml distilled water (vehicle), and Group 2 was orally given analgesic drug aspirin (standard) in a dose 100 mg/kg. Groups 3, 4 and 5 were orally given C. molmol extract in doses 125, 250, and 500 mg/kg, respectively. One hour after administration of aspirin or extract, all mice were intraperitoneally injected with 0.1 ml of 1% acetic acid. The number of abdominal writhing for each mouse was observed and counted during a period of 30 min postinjection of acetic acid and pain inhibition percentages (PIP) were then calculated for each group.
Anti-inflammatory test
This test was carried out as described by Sugishita et al., [28]. The method depends upon induction of inflammation and edema in the hind paw of rats by subcutaneous injection of 0.1 ml of 2% formalin in the right hind paw. Twenty-five adult male rats were divided into five equal groups, of five rats each. Group 1 was orally given the vehicle (negative control), and the other four groups were injected with 0.1 ml of 2% formalin solution in the right hind paw. After induction of edema, the rats of Group 2 were intraperitoneally injected by anti-inflammatory drug indomethacin (standard) in a dose 10 mg/ kg b.wt. Groups 3, 4 and 5 were orally given C. molmol extract in doses of 125, 250 and 500 mg/kg, respectively. The volume of paw edema was measured at 1, 3, 6, and 12 h postadministration of indomethacin or extract.
Induction of Obesity and Hyperlipidemia
Experimental obesity and hyperlipidemia were induced by feeding the rats for 6 weeks on high-fat diet (HFD) which supplies 59% calories from fat; 21% calories from carbohydrate and 20% calories from protein. A 4-6 week, HFD is sufficient to induce obesity and hyperlipidemia and this obese model in rats closely resembles the reality of obesity in humans according to Bhatt et al., [29].
Body Weight and Blood Lipids
Twenty-five adult male rats were randomly divided into five equal groups, each of five animals. Group 1 was fed on basal diet and kept as a negative control. The other four groups were fed on HFD for 6 weeks for induction of obesity and hyperlipidemia. Thereafter, Group 2 was kept obese hyperlipidemic (positive control) and the other three groups were orally given C. molmol extract in daily doses of 125, 250, and 500 mg/kg for 6 weeks. During feeding period (6 weeks), the rats were weighed at weeks 0, 3, and 6 and changes in body weight gains were calculated as percentages. At end of the experiment, blood samples were collected for estimation of serum TC [30], TG [31], and HDL cholesterol [30]. Estimations of blood lipids were carried out chemically using specific diagnostic kits and measurements were performed using ultraviolet-visible spectrophotometer. LDL
cholesterol was calculated according to formula of Friedewald et al. [32] and the atherogenic index (LDL/HDL) was recorded.
Blood Sampling
Blood samples were withdrawn by puncture of retro-orbital plexus of veins in the inner canthus of eye using microcapillary tubes and collected into dry plastic centrifuge tubes. The samples were kept standing for 15 min to clot then centrifuged at 5000 rpm for 10 min for separation of the serum which kept frozen at − 18°C until used for biochemical analysis.
Statistical Analysis
Data were expressed as means ± standard error. Comparisons between the control and experimental groups were carried out using Student’s t-test according to Snedecor and Cochran [33]. The difference between the experimental groups was considered significant at P < 0.05.
RESULTS
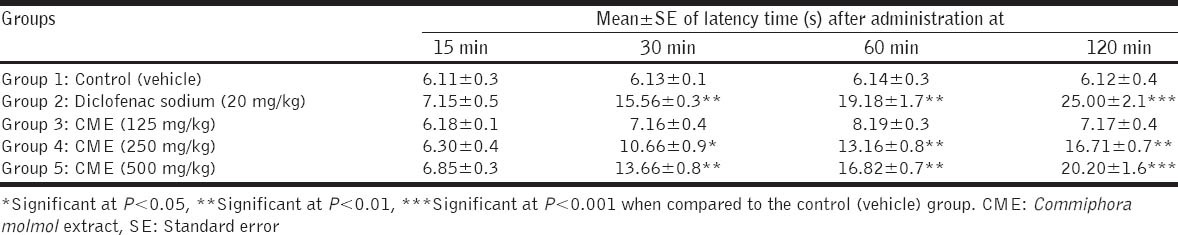
The results showed that oral administration of diclofenac sodium (20 mg/kg) to mice increased the latency time to thermal stimuli at 30, 60, and 120 min post-administration when compared with the control (vehicle) group. Commiphora molmol extract (CME) in doses of 250 and 500 mg/kg increased the latency time to thermal stimuli at 30, 60, and 90 min post-oral dosage, in a dose-dependent manner, when compared with the control group. The small dose (125 mg/kg) of CME did not show significant changes in latency time as depicted in Table 1.
Table 1.
Effect of CME on latency time using hot plate test in mice (n=5 mice)
The analgesic drug aspirin (100 mg/kg) when given to mice, 1 h prior to intraperitoneal injection of acetic acid, significantly (P < 0.001) decreased the number of abdominal writhings when compared with the control (vehicle) group. CME in doses of 250 and 500 mg/kg significantly (P < 0. 001) decreased the number of abdominal writhings. PIP were 37.33 and 49.33% for the dose 250 and 500 mg/kg of the extract, respectively, versus to 66.66% in mice given aspirin. Mice given the small dose of CME showed no significant changes in number of abdominal writhings and PIP was 5.33% versus to 66.66% in mice given aspirin as illustrated in Figure 2.
Figure 2.
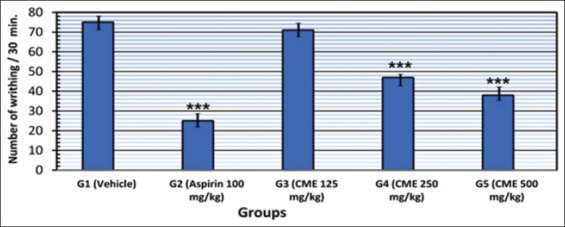
Graphical presentation of analgesic effect of Commiphora molmol extract using writhing test in mice
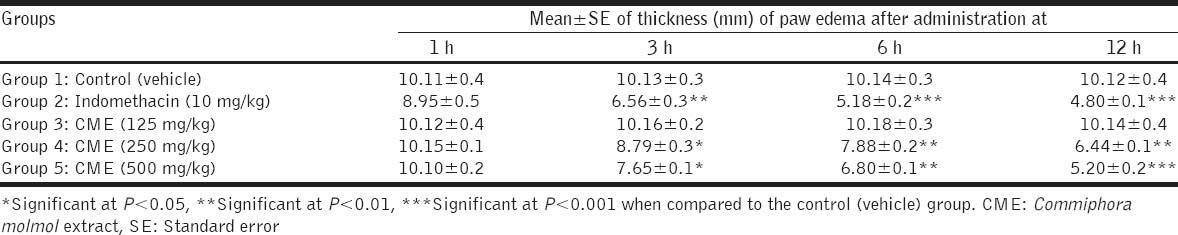
In rats with hind paw edema induced by formalin, the intraperitoneal injection of indomethacin (10 mg/kg) caused significant decreases in volume (thickness) of paw edema at 3, 6, and 12 h postinjection when compared with the control (vehicle) group. CME in doses of 250 and 500 mg/kg significantly decreased the volume of paw edema in rats at 3, 6, and 12 h postoral administration as compared with the control group. The small dose of showed no significant changes in volume of paw edema until 12 h postadministration as recorded in Table 2.
Table 2.
Effect of CME on volume (mm) of edema induced by formalin solution in hind paw of rats (n=5 rats) Groups Mean±SE of thickness (mm) of paw edema after administration at
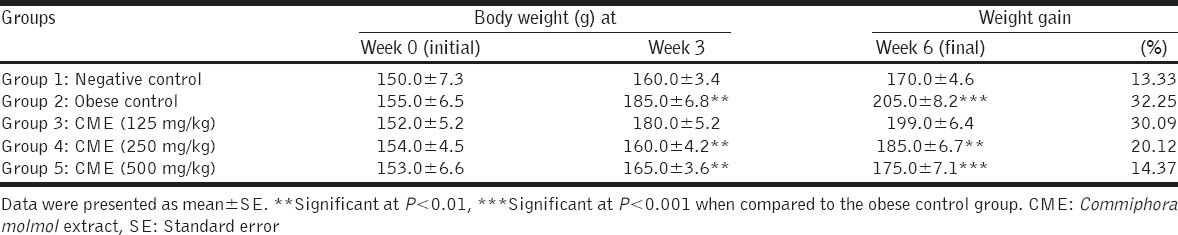
The results showed that feeding rats on HFD significantly (P < 0.001) increased the body weight at the end of 6 weeks (final weight). The body weight gain of rats fed on HFD was 32.25% versus to 13.33% in control rats fed on basal diet. Oral administrations of CME in doses of 250 and 500 mg/kg to obese rats for 6 weeks decreased the body weight gain to 20.12 and 14.37%, respectively, versus to 32.25% of control obese rats. Rats given the small dose of CME showed no significant changes in body weight as recorded in Table 3.
Table 3.
Effect of CME on body weight of rats fed on high-fat diet (n=5 rats)
Feeding of rats on HFD for 6 weeks significantly increased serum levels of TC and TG as compared to control rats fed on basal diet (negative control). Oral administration of CME in doses of 125, 250 and 500 mg/kg for 6 weeks significantly decreased the elevated serum levels of TC and TG when compared to obese control rats as shown in Figure 3. The lowering effect of CME on TC and TG appeared to be dose-dependent and more pronounced on TG than on TC.
Figure 3.
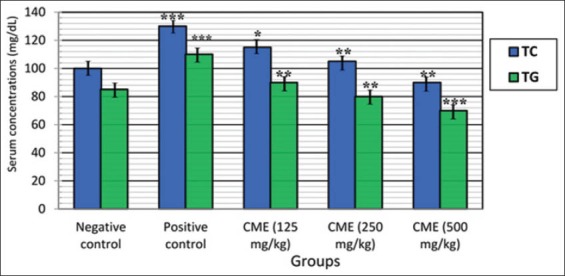
Effect of Commiphora molmol on serum levels of total cholesterol and triglycerides in obese hyperlipidemic rats
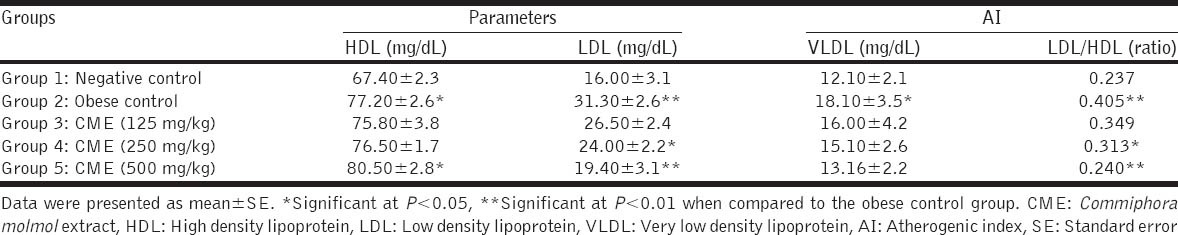
Oral administration of CME in doses of 250 and 500 mg/kg to obese hyperlipidemic rats significantly decreased serum levels of LDL and reduced atherogenic index (LDL/HDL) when compared with the obese control group. The large dose (500 mg/ kg) of CME significantly (P < 0.05) increased serum level of HDL as depicted in Table 4.
Table 4.
Effect of CME on serum HDL, LDL, VLDL and AI in rats (n=5 rats)
DISCUSSION
The main goal of the present study is to evaluate the analgesic, anti-inflammatory and anti-hyperlipidemic activities of CME as well as its effect on body weight and blood lipids in rats fed on HFD.
In the present study, two tests for studying analgesia were used. The hot plate thermal stimulation and chemical irritation by acetic acid-induced writhing in mice were used to examine both central and peripheral mechanisms of analgesic effect.
Pains and inflammations are frequently accompanying the degenerative diseases including cancers, rheumatoid arthritis and peripheral vascular disease, which cause disaster to the patient. Nonsteroidal anti-inflammatory drugs (NSAIDs) and immunosuppressant drugs have been used for a long time all over the world to relief inflammatory conditions. These drugs are often associated with severe harmful and adverse side-effects. Many natural bioactive substances derived from medicinal plants are more effective and safer than chemically synthesized drugs for the treatment of various diseases including pain and inflammations [34,35].
The results of the present study denoted that CME produced significant analgesic activity as measured by both hot plate and writhing tests in mice. The analgesic effect of CME was found to be a dose dependent. This effect was similar to that previously demonstrated [11,12,35]. Moreover, the present study showed that thermal hot plate test was more sensitive than chemical writhing test. This finding agreed with that previously reported by Shanmugasundaram and Venkataraman [36] who compared between the two tests for studying analgesic activity of Hygrophila auriculata extract. The previous authors have reported that thermal hot plate method was more sensitive than the chemical writhing test by acetic acid.
The mechanism of analgesic activity of C. molmol extract could be probably due to its bioactive substances that raised pain threshold by depressing pain receptors centrally in the brain [27]. A second possible mechanism of analgesic effect of C. molmol might be due to an inhibition of release of prostaglandins (PGs), which are mediators that produce a wide variety of effects including pain and peripheral inflammation [35]. C. molmol extract appeared to produce analgesic effect through both central and peripheral mechanisms.
Inflammation is a local response of living mammalian tissues due to an injury or any irritant chemical substance. There are various components to the inflammatory reaction that can contribute to the associated symptoms and tissue injury. Edema formation, leukocyte infiltration and granuloma formation represent the main components of inflammation [35].
CME induced an anti-inflammatory effect as evident by the decrease in thickness (volume) of paw edema induced by formalin in rats. This effect of CME was in accord with that the previously reported [7,12,35]. The mechanism of anti-inflammatory activity of CME could be probably due to an inhibition of release of inflammatory mediator PGs. This explanation was confirmed by the finding of Su et al., [35] who reported that C. molmol significantly decreased levels of inflammatory factor PGE2 in the edema of paw tissue at the 4th h postformalin injection. However, NSAIDs act by reducing the formation of PGs [37-39].
Results of the current study revealed that C. myrrha significantly decreased the body weight gain in obese hyperlipidemic rats. The decrease in weight gain of rats was inversely proportional to the administered dose. This finding agreed with that mentioned by Lv et al. [40]. who mentioned that guggulsterones, plant sterol, from C. molmol, has been used to treat hyperlipidemia and obesity and its anti-inflammatory and anti-hyperlipidemic effects have been well-documented.
In the present study, CME caused anti-hyperlipidemic effect as it significantly lowered the elevated serum levels of TC, TG, and LDL and reduced atherogenic index in obese rats. Moreover, the effect of CME appeared to be more effective on TG than on TC in this study.
The bioactive steroids guggulsterones have been attracted attention for the potent hypolipidemic effect of C. molmol resin [10]. Previous studies have been reported that guggulsterone, the active substance of C. mukul, is highly hypolipidemic agent [41,42].
CONCLUSION
The results denote that C. molmol extract (myrrh) has a dose-dependent analgesic effect and effective anti-inflammatory and anti-hyperlipidemic activities. Myrrh reduces body weight gain and improves lipids profile in obese hyperlipidemic rats. These data affirm its traditional use for the treatment of painful and inflammatory conditions, obesity and hyperlipidemia. Therefore, C. molmol extract (myrrh) may be beneficial for obese hyperlipidemic patients who suffer from pain and inflammatory conditions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4:685–8. [Google Scholar]
- 2.Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med. 2013;10:210–29. doi: 10.4314/ajtcam.v10i5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur A, Nain P, Nain J. Herbal plants used in treatment of rheumatoid arthritis: A Review. Int J Pharm Pharm Sci. 2012;4:44–57. [Google Scholar]
- 4.Haffor AS. Effect of myrrh (Commiphora molmol) on leukocyte levels before and during healing from gastric ulcer or skin injury. J Immunotoxicol. 2010;7:68–75. doi: 10.3109/15476910903409835. [DOI] [PubMed] [Google Scholar]
- 5.Hall BL, Oser BL. Recent progress in the consideration of flavoring ingredients under the food additive amendment. 3. GRAF substances. Food Technol. 1965;19:151–97. [Google Scholar]
- 6.Duwiejua M, Zeitlin IJ, Waterman PG, Chapman J, Mhango GJ, Provan GJ. Anti-inflammatory activity of resins from some species of the plant family burseraceae. Planta Med. 1993;59:12–6. doi: 10.1055/s-2006-959594. [DOI] [PubMed] [Google Scholar]
- 7.Tariq M, Ageel AM, Al-Yahya MA, Mossa JS, Al-Said MS, Parmar NS. Anti-inflammatory activity of Commiphora molmol. Agents Actions. 1986;17:381–2. doi: 10.1007/BF01982655. [DOI] [PubMed] [Google Scholar]
- 8.al-Harbi MM, Qureshi S, Raza M, Ahmed MM, Afzal M, Shah AH. Gastric antiulcer and cytoprotective effect of Commiphora molmol in rats. J Ethnopharmacol. 1997;55:141–50. doi: 10.1016/s0378-8741(96)01488-2. [DOI] [PubMed] [Google Scholar]
- 9.El-Ashmawy IM, Ashry KM, El-Nahas AF, Salama OM. Protection by turmeric and myrrh against liver oxidative damage and genotoxicity induced by lead acetate in mice. Basic Clin Pharmacol Toxicol. 2006;98:32–7. doi: 10.1111/j.1742-7843.2006.pto_228.x. [DOI] [PubMed] [Google Scholar]
- 10.Shen T, Li GH, Wang XN, Lou HX. The genus Commiphora: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2012;142:319–30. doi: 10.1016/j.jep.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117–24. doi: 10.1016/s0378-8741(97)00137-2. [DOI] [PubMed] [Google Scholar]
- 12.Tipton DA, Lyle B, Babich H, Dabbous MKh. In vitro cytotoxic and anti-inflammatory effects of myrrh oil on human gingival fibroblasts and epithelial cells. Toxicol In Vitro. 2003;17:301–10. doi: 10.1016/s0887-2333(03)00018-3. [DOI] [PubMed] [Google Scholar]
- 13.Al-Howiriny T, Al-Sohaibani M, Al-Said M, Al-Yahya M, El-Tahir K, Rafatullah S. Effect of Commiphora opobalsamum (L.) Engl. (Balessan) on experimental gastric ulcers and secretion in rats. J Ethnopharmacol. 2005;98:287–94. doi: 10.1016/j.jep.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 14.Ashry KM, El-Sayed YS, Khamiss RM, El-Ashmawy IM. Oxidative stress and immunotoxic effects of lead and their amelioration with myrrh (Commiphora molmol) emulsion. Food Chem Toxicol. 2010;48:236–41. doi: 10.1016/j.fct.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Verma SK, Bordia A. Effect of Commiphora mukul (gum guggulu) in patients of hyperlipidemia with special reference to HDL-cholesterol. Indian J Med Res. 1988;87:356–60. [PubMed] [Google Scholar]
- 16.Lata S, Saxena KK, Bhasin V, Saxena RS, Kumar A, Srivastava VK. Beneficial effects of Allium sativum, Allium cepa and Commiphora mukul on experimental hyperlipidemia and atherosclerosis - A comparative evaluation. J Postgrad Med. 1991;37:132–5. [PubMed] [Google Scholar]
- 17.Al-Harbi NO, Al-Ashban RM, Shah AH. Saudi traditional medicine: Studies on herbal drugs with anti-diabetic potential. J Pharmacogn Phytother. 2011;3:1–7. [Google Scholar]
- 18.Ojha S, Bhatia J, Arora S, Golechha M, Kumari S, Arya DS. Cardioprotective effects of Commiphora mukul against isoprenalineinduced cardiotoxicity: A biochemical and histopathological evaluation. J Environ Biol. 2011;32:731–8. [PubMed] [Google Scholar]
- 19.Saeed MA, Sabir AW. Antibacterial activities of some constituents from oleo-gum-resin of Commiphora mukul. Fitoterapia. 2004;75:204–8. doi: 10.1016/j.fitote.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Rahman MM, Garvey M, Piddock LJ, Gibbons S. Antibacterial terpenes from the oleo-resin of Commiphora molmol (Engl.) Phytother Res. 2008;22:1356–60. doi: 10.1002/ptr.2501. [DOI] [PubMed] [Google Scholar]
- 21.Kuete V, Wiench B, Hegazy ME, Mohamed TA, Fankam AG, Shahat AA, et al. Antibacterial activity and cytotoxicity of selected Egyptian medicinal plants. Planta Med. 2012;78:193–9. doi: 10.1055/s-0031-1280319. [DOI] [PubMed] [Google Scholar]
- 22.Ramzy F, Mahmoud S, William S. Further assessment of Mirazid as antischistosomal drug in experimental schistosomiasis hematobium. Pharm Biol. 2010;48:775–9. doi: 10.3109/13880200903074635. [DOI] [PubMed] [Google Scholar]
- 23.El-Malky MA, Lu SH, El-Beshbishi SN, Saudy NS, Ohta N. Effect of Mirazid in Schistosoma japonicum-infected mice: Parasitological an pathological assessment. Parasitol Res. 2013;112:373–7. doi: 10.1007/s00436-012-3145-x. [DOI] [PubMed] [Google Scholar]
- 24.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 25.Shalaby MA, Hamowieh AR. Safety and efficacy of Zingiber officinale roots on fertility of male diabetic rats. Food Chem Toxicol. 2010;48:2920–4. doi: 10.1016/j.fct.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Turner RA. Analgesics: Screening Methods in Pharmacology. In: Turner RA, Hebban P, editors. New York: Academic Press; 1965. p. 100. [Google Scholar]
- 27.Gawade SP. Acetic acid induced painful endogenous infliction in writhing test on mice. J Pharmacol Pharmacother. 2012;3:348. doi: 10.4103/0976-500X.103699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugishita E, Amagaya S, Ogihara Y. Anti-inflammatory testing methods:Comparative evaluation of mice and rats. J Pharmacobiodyn. 1981;4:565–75. doi: 10.1248/bpb1978.4.565. [DOI] [PubMed] [Google Scholar]
- 29.Bhatt BA, Dube JJ, Dedousis N, Reider JA, O’Doherty RM. Dietinduced obesity and acute hyperlipidemia reduce IkappaBalpha levels in rat skeletal muscle in a fiber-type dependent manner. Am J Physiol Regul Integr Comp Physiol. 2006;290:R233–40. doi: 10.1152/ajpregu.00097.2005. [DOI] [PubMed] [Google Scholar]
- 30.Richmond N. Colorimetric determination of total cholesterol and high density lipoprotein cholesterol (HDL-c) Clin Chem. 1973;19:1350–6. [Google Scholar]
- 31.Jacob NJ, Van-Denmark PJ. A chemical method for the determination of triglycerides. Arch Biochem Biophys. 1963;88:250–5. [Google Scholar]
- 32.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 33.Snedecor GW, Cochran WG. 7th ed. Ames, USA: Lowa State University Press; 1986. Statistical Methods; p. 90. [Google Scholar]
- 34.Sheir Z, Nasr AA, Massoud A, Salama O, Badra GA, El-Shennawy H, et al. A safe, effective, herbal antischistosomal therapy derived from myrrh. Am J Trop Med Hyg. 2001;65:700–4. doi: 10.4269/ajtmh.2001.65.700. [DOI] [PubMed] [Google Scholar]
- 35.Su S, Hua Y, Wang Y, Gu W, Zhou W, Duan JA, et al. Evaluation of the anti-inflammatory and analgesic properties of individual and combined extracts from Commiphora myrrha and Boswellia carterii. J Ethnopharmacol. 2012;139:649–56. doi: 10.1016/j.jep.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Shanmugasundaram P, Venkataraman S. Anti-nociceptive activity of Hygrophila Auriculata. Tradit Complement Altern Med. 2005;2:62–9. [Google Scholar]
- 37.Goodwin JS. Are prostaglandins proinflammatory, antiinflammatory, both or neither? J Rheumatol Suppl. 1991;28:26–9. [PubMed] [Google Scholar]
- 38.Purushoth PT, Panneerselvam P, Vijaykumar R, Clement AW, Balasubramanian S. Anti-inflammatory, anti-arthritis and analgesic effect of ethanol extract of whole plant of Merremia Emarginata Brum F. Cent Euro J Exper Biol. 2012;1:94–9. [Google Scholar]
- 39.Bacchi S, Palumbo P, Sponta A, Coppolino MF. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Antiinflamm Antiallergy Agents Med Chem. 2012;11:52–64. doi: 10.2174/187152312803476255. [DOI] [PubMed] [Google Scholar]
- 40.Lv N, Song MY, Kim EK, Park JW, Kwon KB, Park BH. Guggulsterone, a plant sterol, inhibits NF-kappaB activation and protects pancreatic beta cells from cytokine toxicity. Mol Cell Endocrinol. 2008;289:49–5. doi: 10.1016/j.mce.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Wu J, Xia C, Meier J, Li S, Hu X, Lala DS. The hypolipidemic natural product guggulsterone acts as an antagonist of the bile acid receptor. Mol Endocrinol. 2002;16:1590–7. doi: 10.1210/mend.16.7.0894. [DOI] [PubMed] [Google Scholar]
- 42.Urizar NL, Moore DD. Gugulipid: A natural cholesterol-lowering agent. Annu Rev Nutr. 2003;23:303–13. doi: 10.1146/annurev.nutr.23.011702.073102. [DOI] [PubMed] [Google Scholar]


