Abstract
Aim:
Our previous works have demonstrated that Chinese herb medicine yanhusuo (Corydalis yanhusuo W. T. Wang) has strong anti-cancer proliferation effect in MDA-MB-231 cells. The goal of this study was to find out the synergic cytotoxicity effect of three natural compounds, tetrahydropalmatine (THP), berberine (Ber), and dehydrocorydaline (DHC), isolated from C. yanhusuo W. T. Wang.
Materials and Methods:
The IC50 of THP Ber and DHC in single use, as well as in combination use at fixed ratios and doses was measured by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay. Isobologram, combination index and modified coefficient of drug interaction (CDI) methods were used for evaluation the combination effects of THF! Ber, and DHC in different ratio and concentration.
Results:
The results indicated that the combination of THP and Ber shown the strongest anti-cancer cell proliferation effect at the ratio of 2:3 (Ber: THF the average CDI value was 0.5795). DHC and THP have additive cytotoxicity in MDA-MB-231 cells. However, there wasn’t any synergistic effect between Ber and DHC, and it even exhibited antagonistic effect when the percentage of DHC was >50%.
Conclusion:
Our findings suggested that the combination of THP and Ber might be beneficial for anti-proliferation of MDA-MB-231 breast cancer cells through a significant synergy effect.
KEY WORDS: Coefficient of drug interaction, combination effect, Corydalis Yanhusuo W. T. Wang, natural products
INTRODUCTION
Yanhusuo (Corydalis yanhusuo W. T. Wang.) is a well-known plant of corydalis, which is a group of herbs used in different parts of the world to relieve pain. As an important Chinese remedy, yanhusuo has been used for hundreds of years to help “invigorate the blood” and relieve almost any painful condition. In China, people thought yanhusuo could promote circulation of blood and qi, and relieve pain, such as chest pain, epigastric pain, amenorrhea, dysmenorrheal, blood stasis after childbirth, and traumatic swelling pain [1]. Nowadays, yanhusuo is widely used to relieve menstrual cramps, chest and abdominal pains in clinical, not only in analgesic, antiseptic, and antispasmodic and antitussive, but also in combination with other herbs in formulae to treat pains in the traditional system of Chinese medicine.
Alkaloids contained in yanhusuo may the responsible for its activities. Published researches indicted that there are many active alkaloids in yanhusuo. For example, dl-tetrahydropalmatine (dl-THP) has neuroprotective effect, and it also has anti-multi-drug resistance (MDR) effect to the MCF-7 cell lines [2]. It could interact with P-gp and alters its ATPase activity to reverse MDR and enhances vincristine’s ability to inhibit the proliferation of human leukemia cell lines [3]. dl-THP also depresses lipopolysaccharide (LPS)-induced overexpression of intercellular adhesion molecule-1 and E-selectin in human umbilical vein endothelium cells (HUVEC)[4].
Berberine (Ber), another alkaloid in yanhusuo, not only induces the apoptosis of human cancer cells, such as HONE1 cells, HepG2, HCT116 and SW480 cells [5-9], but also induces the apoptosis of HUVEC cell [10]. Ber also inhibits cell invasion in non-small lung cancer [11]. Previous reported also indicated that Ber was effective MDR and/or P-gp modulator. Ber modulated the expression and function of pgp-170 that leads to reduce the response to Paclitaxel in the digestive track cancer cells [12].
Dehydrocorydaline (DHC) could inhibit breast cancer cells proliferation by inducing apoptosis in MCF-7 cells [13], and DHC also inhibited the elevation of mitochondrial membrane potential and induced ATP depletion in LPS-stimulated macrophages, but neither affected basal mitochondrial membrane potential nor ATP content in non-stimulated macrophages [14].
Nevertheless, the studies on the combination effect of the components in Chinese herbs were limited, in this study, the synergy of THP, Ber and DHC was evaluated by isobologram, combination index (CI) and modified coefficient of drug interaction (CDI) methods in a fixed ratio and different concentrations. As a result, THP and Ber produced the strongest synergy effect on anti-cancer cell proliferation activity at the ratio of 2:3 (Ber:THP, the average CDI value is 0.5795), and there were no significant synergistic effect between THP and DHC, and DHC and Ber.
MATERIALS AND METHODS
Materials
Roswell Park Memorial Institute (RPMI) 1640, fetal bovine serum (FBS), phosphate-buffered saline, penicillin-streptomycin and 0.25% (w/v) trypsin/1 mM ethylenediaminetetraacetic acid were purchased from Invitrogen (Carlsbad, CA, USA). Dimethyl sulfoxide (DMSO) was supplied by Sigma (St. Louis, MO). Ber and dl-THP were purchased from International Laboratory (San Bruno, CA, USA) or ChromaDex (Irvine, CA, USA). DHC was isolated from crude plant of C. yanhusuo, and identified by high performance liquid chromatograph, infrared, nuclear magnetic resonance and mass spectrometry. The C. yanhusuo was purchased from the Huadong Medicine Group Co., Ltd., (Hangzhou, Zhejiang, P. R. China).
Cell Lines
MDA-MB-231 cells (human breast cancer cell line) were purchased from ATCC (Manassas, VA, USA) and cultured in a monolayer at 37°C and 5% CO2 in RPMI 1640 medium supplemented with 10% FBS, 100 mg/mL streptomycin, and 100 U/mL penicillin. MDA-MB-231 cells in exponential growth phase were seeded to the plates or dishes. After 24 h, the cells were attached to the bottom of the plate, and different concentration of drug-containing medium was added.
Evaluation of Cytotoxicity
Cell viability was estimated with MTT assay. The method was described in our previous paper [15]. Briefly, MDA-MB-231 cells were seeded at 2 × 104 cells/well density in 96 well plates. 100 mL of drug-containing medium were added to treat for 48 h. Cell inhibition was monitored by the classical MTT assay at 570 nm using a Multilabel counter (Perkin Elmer, 1420 Multilabel Counter VICTOR3, Wellesley, USA). The relative growth rate was defined as the percentage of the absorbance of the treated cells compared to that of the untreated cells. Dose-response curves were generated. The cytotoxicity of the designed mixtures was detected. Subsequently, refer the result from the first screening, the cell viability in different ratios were also detected.
Evaluation of Combination Effect by CI Method
Drug combination effect was analyzed by the method of Chou and Talalay [16,17], which was the most popular method to evaluate the combination effect by median effect analysis. In brief, two drugs were administered at a fixed ratio, the dose of the combination required to produce fractional survival f could be divided into the component doses (D) and (D)2 of drug 1 and drug 2, respectively. For each level of cytotoxicity, the CI was then calculated according to the following equation:
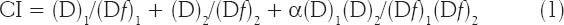
Where (D)1 and (D)2 are the concentrations of the combination required to produce survival f, (Df)1 and (Df)2 are the concentrations of the individual drugs required to produce f. The CIs were calculated based on the most conservative assumption of drug interactions as followed: if the effect of two agents is ’mutually exclusive’ (similar mode of action), then α = 0, otherwise, α = 1 (nonexclusive, differ in their action). In this method, the CI indicates antagonism (CI > 1), additivity (CI = 1), or synergism (CI < 1). The linear correlation coefficient r was generated for each curve to determine the applicability of the data to this method of analysis. In all experiments, R2 was > 0.9.
Evaluation of Combination Effect by Isobologram Methodology
Isobologram is another mathematical approach, which has been described in order to determine the level of drug interaction [18-20]. Cell viability results were analyzed by plotting an “equivalent line” on the isobologram. If data points for combinations fall to the left of the line, synergy is indicated, if the data fall on the line, drug interaction is said to be additive (summation of effects). If the data points fall to the right of the line then the combination is considered subadditive (antagonistic).
Evaluation of Combination Effect by Modified CDI
The CDI was used to analyze effects of drug combinations. The foundation of CDI is (E)1,2 = E1 × E2, where (E)1,2 is the measured effect of combination effect; E1 and E2 are the drug effects of each agent when separate application. CDI is calculated as follows: CDI = AB/(A × B). According to the absorbance of each group, AB is the ratio of the combination groups to control group; A or B is the ratio of the single agent group to control group. Thus, CDI <1, = 1 or >1 indicates that the drugs are synergistic, additive or antagonistic, respectively. CDI <0.7 indicates that the drug is significantly synergistic [21].
However, it is un-comprehensive to evaluate the drug interaction by the CDI only in one concentration. We modified the classical CDI method, namely calculate the average CDI value of several drug concentrations, to evaluate the total drug interaction of the agents. Briefly, a dosage range (from Cmix to Cmax) is designated according as the actual drugs effect. Subsequently, we selected a series of dosages (6 points, n = 6) in the above range, to calculate the CDI by 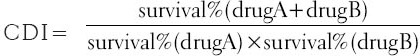 , K is defined as the interval between two consecutive dosage points,
, K is defined as the interval between two consecutive dosage points, 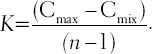 . Finally, aver-CDI, defined as
. Finally, aver-CDI, defined as 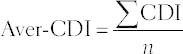 , was used to evaluate the total drug combination effect.
, was used to evaluate the total drug combination effect.
Statistical Analysis
Unless otherwise indicated, experiments were repeated until three replicates yielded coefficients R > 0.9 for all three median effect lines. Results of multiple experiments were summarized by indicating the means ± standard deviation of the indicated level of growth inhibition. Significances were determined using Student’s t-test and were accepted when P < 0.05.
RESULTS
DHC and THP
As shown in Table 1, modified CDI method was used for evaluation the combined effect between DHC and THP. DHC (40 μm in DMSO) and THP (20 μm in DMSO) were mixed in 24:1, 12:1, 4:1, 2:1, 1:1, and 1:3 (DHC:THP), then diluted to 100, 150, 200, 300, 400, 600, 800, 1200, 1600, and 2400 folds for cell culture.
Table 1.
The CDI values in different ratios of DHC and THP mixture on their cytotoxicity effect in MDA-MB-231 cells
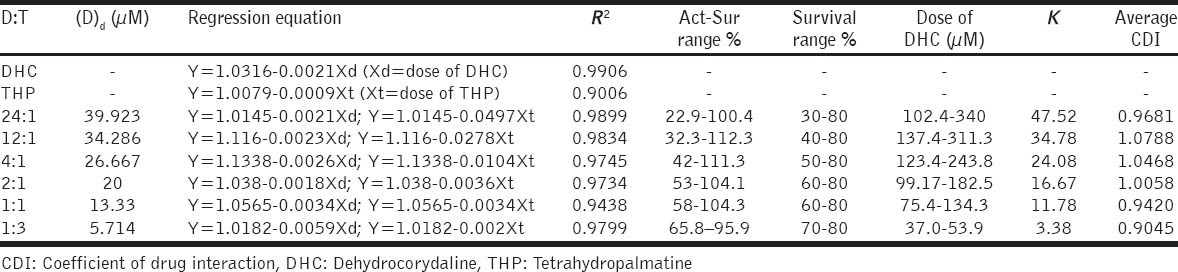
As a result, under the experimental conditions, DHC and THP hardly exhibited combined growth inhibitory effect in MDA-MB-231 cells [Table 1], the average CDI values were from 0.90 to 1.08, indicated an additivity effect.
Ber and THP
To investigate the synergistic inhibitory effects of Ber and THP on the proliferation of MDA-MB-231 cell lines, six different ratios, namely 12:1, 4:1, 3:2, 1:1, 1:3, and 1:9 (Ber:THP), were used to analyze the synergistic inhibitory effect of drug combination. Ber (30 μm in DMSO) and THP (20 μm in DMSO) were mixed and diluted to 100, 150, 200, 300, 400, 600, 800, 1200, 1600, and 2400 folds for treatment.
As shown in Table 2, the combination effects of Ber and THP in the 3:2 and 1:1 have strong synergistic effect. Therefore, we further studied the synergistic interactions in several specifically ratio between Ber and THP, from 2:3 to 2:1 [Figure 1].
Table 2.
CDI values of Ber and THP mixture in different ratios on their cytotoxicity effect in MDA-MB-231 cells
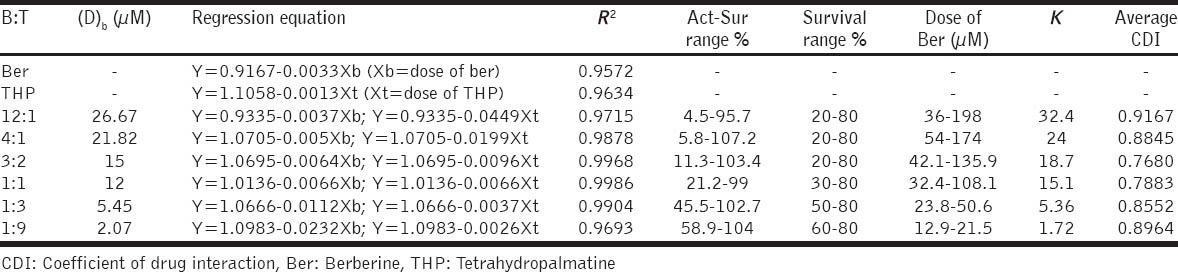
Figure 1.
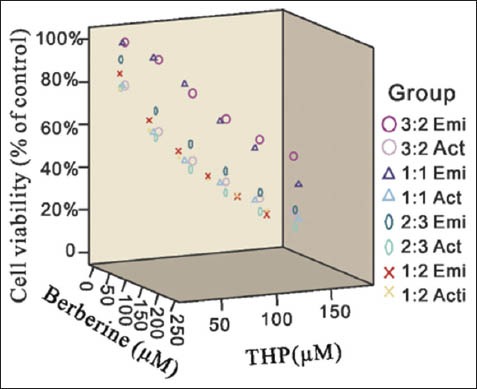
The combination effect of Berberine and Tetrahydropalmatine (THP). Y bar shown as the cell viability (% of control) of different groups. × and Z bars shown the concentrations of Berberine and THP μm). Emi= the estimated cell viability of the groups, Act= the active cell viability of the groups calculated from MTT assay results
As shown in Figure 1, Ber and THP yielded synergistic interactions across a wide concentration range (CDI <0.7), especially between the B: T = 2:3 and 1:1.
DHC and Ber
To investigate the synergistic inhibitory effects of Ber and DHC on the proliferation of MDA-MB-231 cell lines, five different ratios, namely 9:1, 3:1, 1:1, 1:3, and 1:9 (Ber:DHC), were used to analyze the synergy of Ber and DHC combination. Ber and DHC (40 μm in DMSO) were mixed and diluted to 100, 150, 200, 300, 400, 600, 800, 1200, 1600 and 2400 folds for cell culture.
Combination of Ber and DHC was synergistic when the ratio of B:D was low than 3:1 in MDA-MB-231 cells, and it even exhibited antagonistic effect when the percentage of DHC was >50%.
Furthermore, we compared the three methods, namely CI [Table 3], isobolograms [Figure 2] and CDI [Table 3], by evaluating the combination effect between Ber and DHC. Taken together, our results indicate that the values calculated with three different methods were similar, and pointed to the same type of combination effect.
Table 3.
Aver-CDI values and CI values of different Ber and DHC mixtures on their cytotoxicity effect in MDA-MB-231 cells
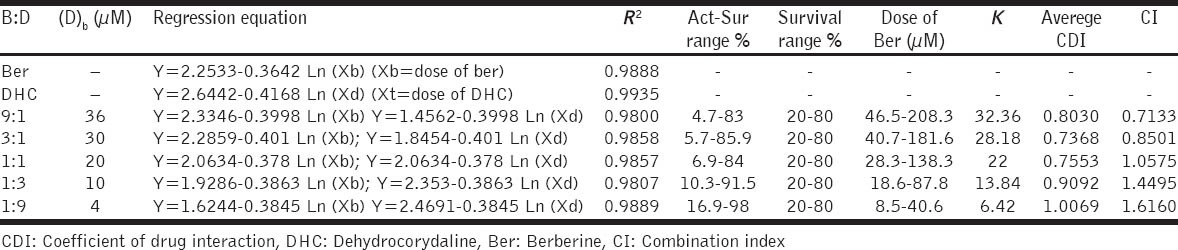
Figure 2.
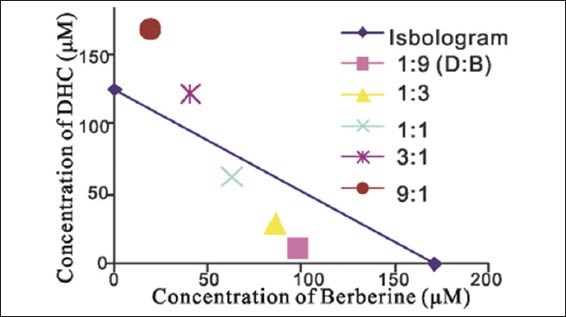
The synergic anti-proliferation effect of berberine and dehydrocorydaline in MDA-MB-231 cells by classical isobolograms method. Data points fall to the left of the line indicate synergy
DISCUSSION
Combination therapy with multiple drugs is a common practice in cancer treatment. It is the best strategy to reduce cancer in clinical chemotherapy. In fact, the possible favorable outcomes for synergism include: (1) Increasing the efficacy of the therapeutic effect, (2) decreasing the dosage but increasing or maintaining the same efficacy to avoid toxicity, (3) minimizing or slowing down the development of drug resistance, and (4) providing selective synergism against target (or efficacy synergism) versus host (or toxicity antagonism)[22].
Therefore, evaluation of drug-drug interaction is important in all areas of medicine, especially in cancer chemotherapy. More than eight methods were developed to quantitatively and qualitatively evaluate the drug interaction, including loewe additivity model, fractional analysis, isobologram methodology, medium effect polt (also known as CI method), reflection method, parameter method, response surface method, weighted modification method, and so on [23,24]. Isobologram and CI methods were the most popular methods for evaluating drug interactions in combination cancer chemotherapy [25,26]. However, these methods were less used in the quantity evaluating of combination effect in other areas, such as ethnological medicine [27].
In this research, we evaluated the drug-drug interactions between THP, Ber or DHC using CI method, modified CDI and isobologram methodology. Because of the anti-MDR effect of THP and the cytotoxicy effect of Ber in cancer cells, the combination of THP and Ber shown the strongest anti-cancer cell proliferation effect at the ratio of 2:3 (Ber:THP, the average CDI value is 0.5795). DHC and THP showed additive effect after combination. Nevertheless, DHC and Ber even exhibited antagonistic effect when the percentage of DHC was >50%.
Presently, although the combination of three or more agents was a common method in many clinical settings, the mathematical method is less for quantitative evaluation their synergy effect. Evaluating the combination effect among three drugs, the quantitative research for drug interaction and the integrative estimate in multi-dosages and multi-levels are the future direction in the area. We described our success in generating a systemic evaluation method, modified CDI method, the modified CDI method is based on the assumption that a drug cannot interact with itself and the max survival of cells was
100% even in low dosage. It is easy for studying the combination effects among three agents. The foundation of CDI method is (E)1,2,3 = E1 × E2 × E3, where (E)1,2,3 is the measured effect of combination effect, E1, E2 and E3 are the drug effect of each agents when separate application. We subsequently compared the modified CDI method with other two methods, and listed the characteristic of modified CDI method: (1) Based on the drug efficiency, (2) multi-dosages and multi-ratios, (3) quantitative analysis method, (4) easy for application in three drugs interaction but unsuitable for antagonistic agents, (5) ignored sigmoidal shape of the concentration-effect relationship, (6) the result is inaccurate when out of the treatment doses.
ACKNOWLEDGMENTS
The reported work was supported by China National Natural Science Foundation (no. 81102852 to JL Gao), young academic leaders project of high school in Zhejiang Province (pd2013208 to JL Gao), the Zhejiang Provincial Key Laboratory Project (no. 2012E10002), the Macao Science and Technology Development Fund (029/2007/A2) and the Innovation Group Project of Zhejiang Chinese Medical University.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.2005 ed. I. Beijing: Chemical Industry Press; 2005. Pharmacopoeia Commission of PRC, editors. Pharmacopoeia of the People's Republic of China. [Google Scholar]
- 2.Zhang XL, Cao GX, Yu HX, Jin J. Reversal effect of tetrahydropalmatine on multidrug resistance of human breast cancer cells MCF-7. Pharmacol Clin Chin Mater Med. 2005;21:19–21. [Google Scholar]
- 3.He L, Liu GQ. Effects of various principles from Chinese herbal medicine on rhodamine 123 accumulation in brain capillary endothelial cells. Acta Pharmacol Sin. 2002;23:591–6. [PubMed] [Google Scholar]
- 4.Zhang ZM, Jiang B, Zheng XX. Effect of l-tetrahydropalmatine on expression of adhesion molecules induced by lipopolysaccharides in human umbilical vein endothelium cell. Zhongguo Zhong Yao Za Zhi. 2005;30:861–4. [PubMed] [Google Scholar]
- 5.Tsang CM, Lau EP, Di K, Cheung PY, Hau PM, Ching YP, et al. Berberine inhibits Rho GTPases and cell migration at low doses but induces G2 arrest and apoptosis at high doses in human cancer cells. Int J Mol Med. 2009;24:131–8. doi: 10.3892/ijmm_00000216. [DOI] [PubMed] [Google Scholar]
- 6.Hwang JM, Kuo HC, Tseng TH, Liu JY, Chu CY. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells. Arch Toxicol. 2006;80:62–73. doi: 10.1007/s00204-005-0014-8. [DOI] [PubMed] [Google Scholar]
- 7.Lin CC, Ng LT, Hsu FF, Shieh DE, Chiang LC. Cytotoxic effects of Coptis chinensis and Epimedium sagittatum extracts and their major constituents (berberine, coptisine and icariin) on hepatoma and leukaemia cell growth. Clin Exp Pharmacol Physiol. 2004;31:65–9. doi: 10.1111/j.1440-1681.2004.03951.x. [DOI] [PubMed] [Google Scholar]
- 8.He BC, Kang Q, Yang JQ, Shang JC, He TC, Zhou QX. Correlation between antitumor activity of berberine and the inhibition of Wnt/β-catenin signal pathway. Chin Pharmacol Bull. 2005;21:1108–11. [Google Scholar]
- 9.Letasiová S, Jantová S, CipáK L, Múcková M. Berberine-antiproliferative activity in vitro and induction of apoptosis/necrosis of the U937 and B16 cells. Cancer Lett. 2006;239:254–62. doi: 10.1016/j.canlet.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Gao JL, Shi JM, Lee SM, Zhang QW, Wang YT. Angiogenic pathway inhibition of Corydalis yanhusuo and berberine in human umbilical vein endothelial cells. Oncol Res. 2009;17:519–26. doi: 10.3727/096504009789745575. [DOI] [PubMed] [Google Scholar]
- 11.Peng PL, Hsieh YS, Wang CJ, Hsu JL, Chou FP. Inhibitory effect of berberine on the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Toxicol Appl Pharmacol. 2006;214:8–15. doi: 10.1016/j.taap.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Lin HL, Liu TY, Wu CW, Chi CW. Berberine modulates expression of mdr1 gene product and the responses of digestive track cancer cells to Paclitaxel. Br J Cancer. 1999;81:416–22. doi: 10.1038/sj.bjc.6690710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Chen X, Fu S, Bao J, Dang Y, Huang M, et al. Dehydrocorydaline inhibits breast cancer cells proliferation by inducing apoptosis in MCF-7 cells. Am J Chin Med. 2012;40:177–85. doi: 10.1142/S0192415X12500140. [DOI] [PubMed] [Google Scholar]
- 14.Ishiguro K, Ando T, Maeda O, Watanabe O, Goto H. Dehydrocorydaline inhibits elevated mitochondrial membrane potential in lipopolysaccharide-stimulated macrophages. Int Immunopharmacol. 2011;11:1362–7. doi: 10.1016/j.intimp.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Gao JL, Ji X, He TC, Zhang Q, He K, Zhao Y, et al. Tetrandrine suppresses cancer angiogenesis and metastasis in 4T1 tumor bearing mice. Evid Based Complement Alternat Med. 2013;2013:265061. doi: 10.1155/2013/265061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships:The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 17.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 18.Wagenpfeil S, Treiber U, Lehmer A. Statistical analysis of combined dose effects for experiments with two agents. Artif Intell Med. 2006;37:65–71. doi: 10.1016/j.artmed.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Naruse T, Nishida Y, Ishiguro N. Synergistic effects of meloxicam and conventional cytotoxic drugs in human MG-63 osteosarcoma cells. Biomed Pharmacother. 2007;61:338–46. doi: 10.1016/j.biopha.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Gao JL, He TC, Li YB, Wang YT. A traditional Chinese medicine formulation consisting of rhizoma Corydalis and rhizoma curcumae exerts synergistic anti-tumor activity. Oncol Rep. 2009;22:1077–83. doi: 10.3892/or_00000539. [DOI] [PubMed] [Google Scholar]
- 21.Hao JQ, Li Q, Xu SP, Shen YX, Sun GY. Effect of lumiracoxib on proliferation and apoptosis of human nonsmall cell lung cancer cells in vitro. Chin Med J (Engl) 2008;121:602–7. [PubMed] [Google Scholar]
- 22.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 23.Tallarida RJ. Drug synergism:its detection and applications. J Pharmacol Exp Ther. 2001;298:865–72. [PubMed] [Google Scholar]
- 24.Tallarida RJ. Revisiting the isobole and related quantitative methods for assessing drug synergism. J Pharmacol Exp Ther. 2012;342:2–8. doi: 10.1124/jpet.112.193474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L, Wientjes MG, Au JL. Evaluation of combination chemotherapy:integration of nonlinear regression, curve shift, isobologram, and combination index analyses. Clin Cancer Res. 2004;10:7994–800. doi: 10.1158/1078-0432.CCR-04-1087. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L, Au JL, Wientjes MG. Comparison of methods for evaluating drug-drug interaction. Front Biosci (Elite Ed) 2010;2:241–9. doi: 10.2741/e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan WJ, Lai MD, Zhou JG. Anticancer effects of Chinese herbal medicine, science or myth? J Zhejiang Univ Sci B. 2006;7:1006–14. doi: 10.1631/jzus.2006.B1006. [DOI] [PMC free article] [PubMed] [Google Scholar]


