Abstract
Aim:
The antioxidant and pharmacological effects of hawthorn have mainly been attributed to the polyphenolic contents. The aim of this research is to determine some bioactive compounds and antioxidant properties of hawthorn aqueous and ethanol extracts of leaves, flowers, and ripened fruits.
Materials and Methods:
For this purpose, antioxidant activities of extracts were assessed on DPPH•, ABTS•+, superoxide scavenging, reducing power and ferrous metal chelating activity assays and phenolic content of extracts was determined by Folin—Cioacalteu’s reagent.
Results:
The flavonoids including rutin, apigenin, myricetin, quercetin, naringenin and kaempferol, were identified by high-performance liquid chromatography in the hawthorn extract.
Conclusion:
It was observed the aqueous and ethanol extracts of Crataegus monogyna subsp. monogyna fruits showed the highest activity in reducing power and metal chelating activity assays. In addition, it was determined that the aqueous flower extract showed higher flavonoid content than aqueous leaves extract. The antioxidant and pharmacological effects of hawthorn have mainly been attributed to the polyphenolic contents.
KEY WORDS: Antioxidant activity, Crataegus monogyna subsp. monogyna, flavonoid, polyphenolic content
INTRODUCTION
Crataegus monogyna subsp. monogyna Jacq. (Hawthorn) is very important for folk medicine, and some parts (such as flowers, flower buds, and leaf) are being been used to the treatment of some diseases including irritability, insomnia, migraines, confusion, and memory loss [1-5]. The unripe and ripe fruit juices of hawthorn were prepared in the traditional medicine and cosmetic applications. These juices are used to some skin applications, arthritis and muscle pains. Dried fruits also have diuretic properties [3,5]. Hawthorn leaves, flowers and fruits are used for coronary vasodilatoric, cardiotonic, and hypotensive drug. The antioxidant property of medicinal plants can be attributed to the polyphenolic contents. Flavonoids are natural benzo-y-pyran derivatives [6]. The hawthorn contains the aromatic amines, essential oils, phenolic acids, flavonoids (hyperin, quercetin, spirein, rutin, and apigenin), proanthocyanidins as bioactive compounds [7]. It also has anti-carcinogen properties. In recent years, hawthorn fruit concentrates are prepared and used as a food supplement in developed countries. Despite hawthorn has a large amount in nature in itself, its’ values as fruit is not understood in Turkey [8].
The aim of this study was to determine polyphenolic and flavonoid contents and to investigate in vitro antioxidant properties of aqueous and ethanol extracts of leaves, flowers, and ripened fruits of Crataegus monogyna subsp. monogyna.
MATERIALS AND METHODS
Chemicals and Standards
All chemicals were used analytical reagent and these chemicals were obtained from Sigma-Aldrich.
Plant Materials and Extraction Procedures
Crataegus monogyna subsp. monogyna Jacq. (Hawthorn) leaves, flowers and ripened fruits were collected from Gaziantep in Turkey. Region characteristics are N 37° 09.415’ and E0 37° 12.864’; altitude: 1090 m. All samples were dried in the dark at room temperature. For extraction (ethanol or water), 25 g powder of samples (leaves, flowers or fruits) were mixed with 100 mL solvent (water or ethanol). Extraction was continued until the extraction solvents became colorless. The obtained extracts were filtered, and the filtrate was collected, and then solvent was removed [9]. The dried extracts and standard antioxidants were dissolved in extraction solvents at μg/mL concentration.
Determination of Antioxidant Properties of Hawthorn Extracts
ABTS•+ radical scavenging capacity
The spectrophotometric analysis of ABTS•+ radical scavenging capacity was determined according to the method of Re et al. [10]. ABTS•+ was produced by reacting 2 mM ABTS in H2O with 2.45 mM K2S2O8, and it was stored for 12 h at room temperature in the dark. The ABTS•+ solution was diluted to give an absorbance of 0.750 ± 0.025 at 734 nm in 0.1 M sodium phosphate buffer (pH 7.4). Then, 1 mL of ABTS•+ solution was added to 3 mL of hawthorn extracts at 100 μg/mL concentrations. The absorbance was recorded for 0.5 h at 734 nm. The extent of decolorization was calculated as a percentage reduction of absorbance.
DPPH• radical scavenging capacity
The DPPH radical scavenging capacity was measured by using the method of Shimada et al. [11]. Briefly, 0.1 mM solution of DPPH• in ethanol was prepared, and 1 mL of this solution was added to 3 mL of hawthorn extracts solution at 100 μg/mL concentration. Absorbance at 517 nm was determined after 0.5 h against a blank solution containing the ethanol. Lower absorbance of the reaction mixture indicates the higher DPPH radical scavenging activity.
Superoxide Anion Scavenging Capacity
The measurements of superoxide anion scavenging capacity were based on the method described by Liu et al. [12] with slight modification. 1 mL of nitroblue tetrazolium (NBT) solution (156 mmol/L NBT in 100 mmol/L phosphate buffer, pH = 7.4), 1 mL NADH solution (468 mmol/L in 100 mmol/L phosphate buffer, pH = 7.4) and 100 μL of sample solution of hawthorn extracts were mixed. The reaction was started by adding 100 μL of phenazine methosulfate (PMS) solution (60 mmol/L PMS in 100 mmol/L phosphate buffer, pH = 7.4) to the mixture. The reaction mixture was incubated at 25°C for 5 min and the absorbance at 560 nm was measured. Decreased absorbance of the reaction mixture shows an increase in the superoxide anion scavenging capacity.
Measurement of Chelating Activity on Metal Ions
The chelating of ferrous was estimated by the method of Dinis et al. [13]. Briefly, extracts 100 μg/mL concentration was added to a solution of 2 mM FeCl2 (0.05 mL). The reaction was started by the addition of 5 mM ferrozine (0.2 mL) and the mixture was shaken vigorously and then it was kept at room temperature for 10 min. Absorbance of the solution was measured at 562 nm.
Reducing Power Assay
The reducing power activities were determined by the method of Oyaizu [14]. Briefly, 100 μg/mL of hawthorn extract in 1 mL of distilled water were mixed with phosphate buffer (2.5 mL, 0.2 M, pH = 6.6) and potassium ferricyanide (2.5 mL, 1%). The mixture was incubated at 50°C for 20 min. Trichloroacetic acid (2.5 mL, 10%) were added to the mixture, and then centrifuged for 10 min at 1000 ×g. The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%), and the absorbance was measured at 700 nm.
All tests and analyses were repeated 3 times and average values were calculated. The antioxidant activities of samples were estimated by the following equation: Percentage of scavenging activity = ([A0–A1)/A0]× 100.
Where A0 is the absorbance of control, and is the absorbance of the sample in the presence of extracts or standards.
Determination of Polyphenolic Contents
Total polyphenolic contents of the extracts were determined with Folin–Ciocalteu reagent according to the method of Slinkard and Singleton [15] using pyrocatechol and quercetin as standard phenolic compounds. Briefly, 1 mL of the hawthorn extracts solution in a volumetric flask diluted with distilled water (46 mL). 1 mL of Folin–Ciocalteu reagent was added, and the content of the flask was mixed thoroughly. After 3 min, 3 mL of Na2CO3 (2%) was added and then it was intermittent shaken for 2 h. The absorbance was measured at 760 nm. The total concentration of phenolic contents of the hawthorn extracts determined as milligram of pyrocatechol and quercetin equivalent by using an equation that was obtained from standard pyrocatechol and quercetin graph:
Absorbance = 0.00053 × total phenols (quercetin equivalent [mg])+ 0.00019.
Absorbance = 0.00198 × total phenols (pyrocatechol equivalent [mg])+ 0.00158.
Chromatographic Conditions for Flavonoid Analysis
Chromatographic analysis was carried out using PREVAIL C 18 reversed-phase (RP) column (150 × 4.6 mm × 5 μm) diameter particles. The mobile phase was methanol/water/acetonitrile (46/46/8, v/v/v) containing 1.0% acetic acid [16]. This phase was filtered through a 0.45 μm membrane filter (Millipore), then deaerated ultrasonically prior to use. Quercetin, rutin, apigenin, myricetin, naringenin and kaempferol were quantified by diode array detector following RP-high-performance liquid chromatography separation at 280 nm for quercetin and naringenin, 254 nm for rutin and myricetin, 306 nm for apigenin and 265 nm for kaempferol. Flow rate and injection volume were 1.05 mL/min and 10 μL, respectively. The chromatographic peaks of the analyses were confirmed by comparing their retention time and ultraviolet spectra with those of the reference standards. Quantification was carried out by the integration of the peak using the external standard method. All chromatographic operations were carried out at 25°C.
RESULTS AND DISCUSSION
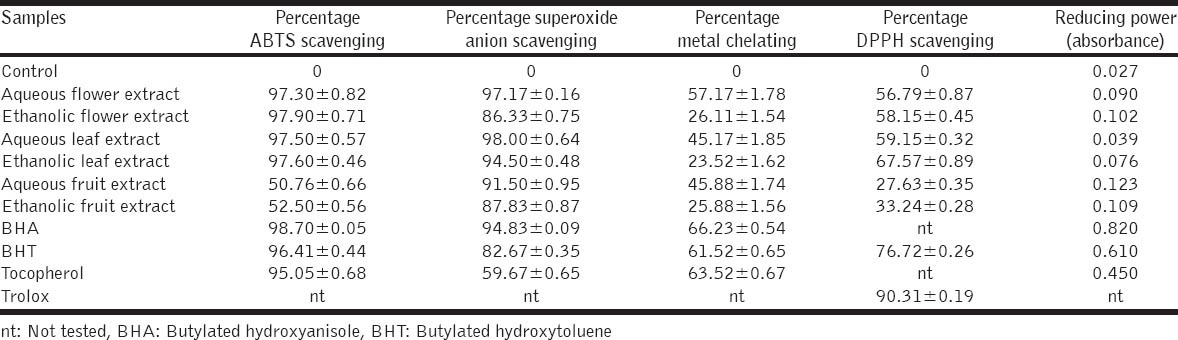
In this study, the antioxidant activity of hawthorn extracts was compared to the standard antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), tocopherol and trolox. The antioxidant activity of the extracts and standards were evaluated by a series of the following in vitro tests: ABTS•+ radical, DPPH free radical and superoxide anion radical scavenging, total reducing activity and metal chelating activity [Table 1]. Furthermore, the flavonoid contents and phenolic compounds (as pyrocatechol and quercetin) of hawthorn extracts were determined and calculated.
Table 1.
Results of ABTS, superoxide, DPPH radical scavenging, metal chelating and reducing power activities of hawthorn extracts (100 μg/mL) and standard antioxidants
ABTS•+ Radical Scavenging Capacity
In this study, hawthorn extracts showed in the range of 50.76-97.90% scavenging activity on ABTS radical. ABTS scavenging activities of hawthorn ethanolic flower, ethanolic leaf, aqueous leaf and aqueous flower extracts higher than standard antioxidants BHT and tocopherol. The scavenging effect of hawthorn extracts at the 100 μg/mL concentration on the ABTS•+ is sorted as follows: BHA > ethanolic flower extract > ethanolic leaf extract > aqueous leaf extract > aqueous flower extract > BHT > tocopherol > ethanolic fruit extract > aqueous fruit extract.
DPPH• Radical Scavenging Capacity
The scavenging activity of hawthorn extracts in the range of 23.63-67.57%. DPPH radical scavenging activities of all extracts lower than trolox and BHT: Trolox > BHT > ethanolic leaf extract > aqueous leaf extract > ethanolic flower extract > aqueous flower extract > ethanolic fruit extract > aqueous fruit extract at the 100 μg/mL concentration.
Superoxide Anion Scavenging Capacity
The scavenging activity of hawthorn extracts in the range of 86.33-98.00%. In comparison to BHA, the aqueous leaf and flower extracts have high superoxide radical scavenging activity. Inhibition of superoxide radical of all samples on the percentage by 100 μg/mL concentration is found to order as follows: Aqueous leaf extract > aqueous flower extract > BHA > ethanolic leaf extract > aqueous fruit extract > ethanolic fruit extract > ethanolic flower extract > BHT > tocopherol.
Metal Chelating Activity
The ferrous ion chelating of hawthorn extracts showed in the range of 23.52-57.17%. All extracts showed a low metal chelating activity in comparison to BHA, BHT and tocopherol. Effect of samples on the percentage chelating of ferrous by 100 μg/mL concentration in the decreased order: BHA > tocopherol > BHT > aqueous flower extract > aqueous fruit extract > aqueous leaf extract > ethanolic flower extract > ethanolic fruit extract > ethanolic leaf extract.
Reducing Power Assay
The reducing activity of the hawthorn extracts and standards was detected using the potassium ferricyanide reduction method. The total reducing capacities of all extracts lower than BHA, BHT and tocopherol as follows: BHA > BHT > tocopherol > aqueous fruit extract > ethanolic fruit extract > ethanolic flower extract > aqueous flower extract > ethanolic leaf extract > aqueous leaf extract.
Total Phenolic Contents
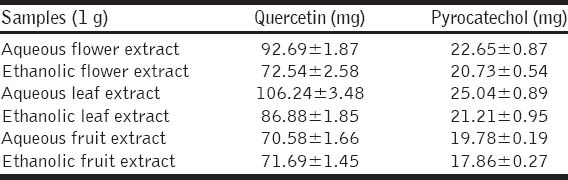
The equivalent of phenolics was determined, which belong to 70.58-106.24 mg quercetin/1 g of dried weight of extract and 17.86-25.04 mg pyrocatechol/1 g of dried weight of extract [Table 2].
Table 2.
Polyphenolic contents of hawthorn extracts
Flavonoid Contents
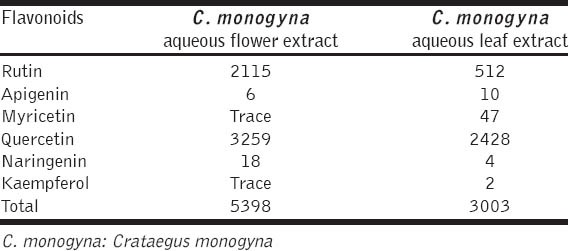
Flavonoid contents of hawthorn flower and leaf extracts were shown in Table 3. Rutin, apigenin, quercetin, naringenin, myricetin, and kaempferol were determined in the extracts.
Table 3.
Flavonoids contents of hawthorn aqueous extracts (μg/g)
The antioxidant activities of hawthorn aqueous and ethanol extracts were evaluated by five in vitro chemical assays including, DPPH, ABTS and superoxide anion radical scavenging effects, reducing power, and metal chelating activity.
Froehlicher et al. [17] have studied that the antioxidant activities of fresh fruit, dried fruit, flowering tops and flowers of C. monogyna from France, and they determined that total phenol, proanthocyanidin, and flavonoid contents of these extracts. According to their results, hawthorn flower buds and flowering tops are the highest bioactive compounds, and fresh and dried fruit extracts showed lower antioxidant effect compared to other extracts. In our study, we determined that fruit extracts had lower antioxidant activity than flower and leaf extracts.
In the present study, it was observed that there is a correlation between antiradical activity and phenolic contents. The hawthorn aqueous leaves extract revealed the highest content in phenolics (106.24 mg quercetin/1 g, 25.04 mg pyrocatechol/1 g), and this extract showed the highest DPPH and ABTS radical scavenging activity compared to other hawthorn extracts.
Tadic et al. [18] have reported that flower buds, flowers and unripe fruits revealed a higher DPPH scavenging activity than the hawthorn berries from Serbia. In fact, flower buds, flowers and, mostly unripe fruits gave better results for DPPH radical scavenging effects and reducing power than the trolox. They investigated the amount of total phenolic compound, and reported it as 35.4 mg gallic acid/g. In our study, while total polyphenolic contents are higher than their results, DPPH radical scavenging and reducing power are lower than standard antioxidants.
Bernatoniene et al. [19] studied that DPPH radical scavenging activity of ethanolic and aqueous extracts of hawthorn fruits and individual substances, such as chlorogenic acid, hyperoside, rutin, quercetin, vitexin-O-rhamnoside, epicatechin, catechin, and procyanidins and they reported that fruit extracts lower free radical scavenging properties than a combination of these substances. Our results are in agreement with their study. Hawthorn aqueous and ethanolic fruit extracts showed lower free radical scavenging activity than other hawthorn extracts in the DPPH, ABTS and superoxide radical scavenging activity.
Barros et al. [5] investigated that the DPPH radical scavenging activity and reducing power of petroleum ether extracts of hawthorn flower buds, flowers, unripe fruits, ripened fruits and over ripened fruits, and they reported flower buds, flowers and unripe fruits were more effective than a standard antioxidant trolox. The present study was showed that flower aqueous and ethanol extracts are high antioxidant property than fruit extracts for DPPH and ABTS radical scavenging activities. The differences between our and their results may be originated from the solvent used for the preparing of extracts.
Egea et al. [20] determined that ABTS, OH radical and H2O2 scavenging activity of hawthorn fruit extract. They reported that the extracts were showed 81.04% OH radical inhibition and 86.39% H2O2 inhibition. In addition, these researchers showed that ABTS radical scavenging activity of hawthorn fruit extract was significantly lower than BHA. In their studies results are similar to our results. We determined that aqueous and ethanolic fruit extracts of hawthorn have lower activity than BHA and BHT for the ABTS radical scavenging test.
Keser et al. [21] studied that H2O2 scavenging activity and inhibition of lipid peroxidation of hawthorn aqueous and ethanol extracts of leaf, flowers and ripened fruits. They showed that hawthorn extracts are high H2O2 scavenging and inhibition of lipid peroxidation when compared to BHA and tocopherol.
CONCLUSION
In the present study, it was investigated that in vitro antioxidant properties of hawthorn aqueous and ethanol extracts based on DPPH, ABTS, superoxide radical scavenging, reducing power and metal chelating activity. In conclusion, the leaves water extract which had the highest total phenolic content as pyrocatcehol and quercetin equivalent, and leaves water and ethanol extracts showed high antiradical activity than aqueous and ethanolic fruit extracts in ABTS, superoxide and DPPH scavenging assays. It was observed that hawthorn fruit water and ethanol extracts showed the highest activity in reducing power and metal chelating activity assays. Additionally, aqueous flower extract showed higher total flavonoid content than leaves water extract.
ACKNOWLEDGMENTS
This work was supported by TUBITAK, under grand number 107T898, and it was supported by Firat University, under grand number FÜBAP 1936.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Camejo-Rodrigues J, Ascensão L, Bonet MA, Vallès J. An ethnobotanical study of medicinal and aromatic plants in the Natural Park of “Serra de São Mamede” (Portugal) J Ethnopharmacol. 2003;89:199–209. doi: 10.1016/s0378-8741(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 2.Novais MH, Santos I, Mendes S, Pinto-Gomes C. Studies on pharmaceutical ethnobotany in Arrabida Natural Park (Portugal) J Ethnopharmacol. 2004;93:183–95. doi: 10.1016/j.jep.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho AM. Madrid: Universidad Autonoma; 2005. Etnobotánica del Parque Natural de Montesinho. Plantas, Tradición y saber Popular en un Territorio del Nordeste de Portugal. [Google Scholar]
- 4.Neves JM, Matos C, Moutinho C, Queiroz G, Gomes LR. Ethnopharmacological notes about ancient uses of medicinal plants in Trás-os-Montes (Northern of Portugal) J Ethnopharmacol. 2009;124:270–83. doi: 10.1016/j.jep.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 5.Barros L, Carvalho AM, Ferreira IC. Comparing the composition and bioactivity of Crataegus monogyna flowers and fruits used in folk medicine. Phytochem Anal. 2011;22:181–8. doi: 10.1002/pca.1267. [DOI] [PubMed] [Google Scholar]
- 6.Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983;32:1141–8. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 7.Bruneton J. 2nd ed. Paris, France: Lavoisier Publishing; 1999. Pharmacognosy, Phytochemistry, Medicinal Plants. [Google Scholar]
- 8.Batu A. Evaluation of hawthorn fruits in production of functional foods and importance for human health. Türk Bilimsel Derlemeler Derg. 2012;5:1–5. [Google Scholar]
- 9.Keser S, Celik S, Turkoglu S, Yilmaz O, Turkoglu I. Determination of antioxidant capacities of ethanol and water extracts of Achillea millefolium L.(Yarrow) Asian J Chem. 2011;23:3172–6. [Google Scholar]
- 10.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 11.Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–8. [Google Scholar]
- 12.Liu F, Ooi VE, Chang ST. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997;60:763–71. doi: 10.1016/s0024-3205(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 13.Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–9. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 14.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 15.Slinkard K, Singleton VL. Total phenol analysis-automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- 16.Zu Y, Li C, Fu Y, Zhao C. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J Pharm Biomed Anal. 2006;41:714–9. doi: 10.1016/j.jpba.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 17.Froehlicher T, Hennebelle T, Martin-Nizard F, Cleenewerck P, Hilbert JL, Trotin F, et al. Phenolic profiles and antioxidative effects of hawthorn cell suspensions, fresh fruits, and medicinal dried parts. Food Chem. 2009;115:897–903. [Google Scholar]
- 18.Tadić VM, Dobrić S, Marković GM, Dordević SM, Arsić IA, Menković NR, et al. Anti-inflammatory, gastroprotective, free-radicalscavenging, and antimicrobial activities of hawthorn berries ethanol extract. J Agric Food Chem. 2008;56:7700–9. doi: 10.1021/jf801668c. [DOI] [PubMed] [Google Scholar]
- 19.Bernatoniene J, Masteikova R, Majiene D, Savickas A, Kevelaitis E, Bernatoniene R, et al. Free radical-scavenging activities of Crataegus monogyna extracts. Medicina (Kaunas) 2008;44:706–12. [PubMed] [Google Scholar]
- 20.Egea I, Sánchez-Bel P, Romojaro F, Pretel MT. Six edible wild fruits as potential antioxidant additives or nutritional supplements. Plant Foods Hum Nutr. 2010;65:121–9. doi: 10.1007/s11130-010-0159-3. [DOI] [PubMed] [Google Scholar]
- 21.Keser S, Celik S, Turkoglu S, Yilmaz Ö, Turkoglu I. Hydrogen peroxide radical scavenging and total antioxidant activity of hawthorn. Chem J. 2012;2:9–12. [Google Scholar]


