Abstract
Objective:
The objective of the present study was to evaluate the in vivo wound healing effect of water extract of Tetrapleura tetraptera in stem-bark.
Materials and Methods:
The healing activity was studied in 40 male rats using excision and incision wounds on normal and dexamethasone-suppressed wound healing. For each model, rats were divided in four groups as follows: control, dexamethasone, T. tetraptera and dexamethasone combined with T. tetraptera. Results: Data recorded exhibited a significant effect by the extract in the epithelialization time within 14 and 18 days of the normal and dexamethasone-induced healing delay rats, respectively (P < 0.05). The extract also significantly increased the wound tensile strength in dexamethasone treated rats. Histological examination of incision wounds of the extract-treated group showed many fibroblasts and the same rats presented significant cutaneous tensile strength, suggesting important collagen crosslinkage.
Conclusion:
This study illustrated an excellent potential of the bark of T. tetraptera therapy on dermal wound healing with a possible mechanism of action related to epithelialization, contraction, and tensile strength improvement.
KEY WORDS: Epithelialization, excision wound, incision wound, skin, tensile strength, Tetrapleura tetraptera
INTRODUCTION
The outermost (epidermis) and inner deeper layer (dermis) of the skin always exists in steady-state equilibrium to form a protective barrier of internal organs against entry of infectious and other noxious agents from the environment. Any damage to the skin layers initiates complex biochemical response that leads to tissue repair or wound healing, which is characterized by dynamic, interactive events described in three phases: inflammation, proliferation, and remodeling [1]. As a natural response, wound healing occurs whenever there is a loss of continuity in the skin or any body tissue, as a result of trauma, infection, or pathological process [2]. Due to simplicity in the measurement of wound healing responses, the excision and incision skin-wound healing models in animals are by far the most convenient and reliable methods of study for potential therapeutic agents. Hence, a number plant extracts from herbal medicine have been recently shown to be beneficial for treatment of wounds using these experimental model [3]. Medicinal plants have also generated much interest in recent years for treating skin ailments as they are affordable and purportedly safe due to less hypersensitivity reactions [4].
Tetrapleura tetraptera (family, mimosaceae) is a single-stemmed, robust, perennial tree with dark green leaves and thick, woody base and spreading branches. Various preparations of the plant are known to be used in folk medicine for treating human ailments, including cardiovascular disorders such as hypertension, asthma, diabetes mellitus, epilepsy, and schistosomiasis [5]. The plant is also frequently used in Tropical African traditional medicine for the management and/or control of several women’s diseases such as breast and uterine cancers, as well as inflammatory conditions [6]. The fruit extracts of the plant have been shown to possess hypocholesterolemic effect in rats [7] as well as alteration of various metabolic parameters in rabbits [8]. Cardiovascular and neuromuscular actions of scopeletin isolated from the fruits of T. tetraptera were also described [6]. The pods and/or fruits have been shown to have an antibacterial effect against Bacillus sp., Enterococcus faecalis, Escherichia coli, Klebsiella pnemonium, Staphylococcus aureus, Salmonella typhi, Pseudomonas aeruginosa and Shigella [9-11]. On the other hand, the bark extracts have been shown to possess antiplasmodial activity in vitro [12]. The preventive as well as curative effects of T. tetraptera bark extracts on hypertension, dyslipidemia, and oxidative stress have also been published recently [13,14]. The present study was designed to evaluate for the first time the wound healing effect of the water extract of T. tetraptera stem-bark.
MATERIALS AND METHODS
Plant Material and Extraction
The stem-bark of T. tetraptera was collected in Lobo in the ’Center Region, 32 km from Yaoundé city, altitude 690 m, latitude 3°45’, longitude 11°13’ of Cameroon, and authenticated at the national herbarium of Cameroon where a voucher specimen (No 31310/HNC) was deposited. The dried powder of the plant materials (200 g) were soaked in 3 L distilled water for 24-h. The aqueous extract was the filtered and concentrated at 70°C to give 12 g of a dark brown powder (yield 6%).
Animal Husbandry and Ethical Considerations
All animal procedures were conducted with strict adherence to the NIH Guide for the care and use of Laboratory Animals (NIH Publication #85-23 Rev. 1985). Male albino-Wistar rats weighing 150-180 g, fed on standard chow pellet diet and allowed water ad libitum were used. Animals were caged under laboratory environment with 12-h dark and light cycles.
Drugs
Ketamine (Rotexmedica-Tritau-Germany), diazepam (Renaudin-France), dexamethasome (Guangdong Medicine and Health Products I/E Corp.) and nylon surgical treat size 1 (Agary Pharmaceutical Ltd.) were purchased from a local pharmacy store). All other chemicals were of laboratory grade and freshly prepared.
Determination of In Vivo Cicatrizant Activity
Grouping of animals
Animals were divided into four groups consisting five rats each as follows: group 1: Water (2 mL); Group 2: Dexamethasone (0.34 mg/kg i.m. on 1st day, thereafter 0.17 mg/kg on alternate days); Group 3: T. tetraptera (50 mg topically); and Group 4: Dexamethasone (0.34 mg/kg i.m. on 1st day, thereafter 0.17 mg/kg on alternate days)+ T. tetraptera (50 mg topically).
Excisional wound model
Animals were anesthetized by intramuscular injection of ketamine/diazepam (ketamine 25 mg/kg and diazepam 10 mg/kg). An area (4 cm2) was marked using a frame and marker pen. The required area of the dorsal fur of the animals was shaved with an electric clipper and area sterilization achieved by spraying with 70% alcohol in water. A full-thickness skin (4 cm2) was excised from the predetermined area by removing the epidermis and dermis layer until the subcutaneous fat (avoiding panniculus carnosus and the muscle layer)(punch biopsy) as described by Frank and Kamfer [15]. All treatments in the four groups were given every 2 days until the wound was completely healed. Special care was taken to avoid variation in the dose given.
Animals were monitored on a daily basis. Wound diameter was recorded in vertical and horizontal planes as well as epithelialization time that indicate the formation of new epithelial tissue to cover the wound. The lesions on each rat were also rated using the following parameters, (1) the presence and type of exudates, (2) erythema, (3) swelling, (4) ulceration, and (5) crust formation [16]. The degree of wound healing was calculated using the formula:
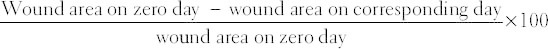
The number of days for complete epithelialization was noted. Wounds were considered closed (completely healed) if moist granulation tissue was no longer apparent and the wound was covered with new epithelium.
Incisional wound model
A 5-cm incision was made perpendicular to the axis of symmetry of the animal and the two borders of the wound were stitched together at its center with interrupted sutures at a distance of 1-cm [17]. Treatment started immediately and the experimental agent being tested was applied to the wound every 48-h. On the 10th day of post wounding measurements, animals were sacrificed by chloroform overdose and wound areas from each animal were dissected carefully. Stripes of equal size (width) from one side were cut, and a line was drawn on either side, 3 mm away from the wound, for breaking strength determination. One piece of tissue was fixed in 10% formalin for histopathological examination, and the other was used to quantify the wound breaking strength (WBS).
Determination of wound tensile strength
Both ends of each skin stripe were fixed with a pair of steel clip, one clip was allowed hanging on a stand and other clip with a freely suspended polyethylene bag through a string run over the pulley. It was then gradually filled with water from a polyethylene reservoir until the wound stripe was broken at the site of the wound. The amount of water required to break the wound was noted and expressed as tensile strength of wound in grams [18]. The tensile strength was calculated according to the following equation:

For preliminary screening, an activity >25% was considered a positive wound healing activity. The percentage of activity was calculated according to the following formula:
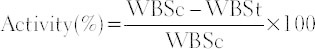
WBSt = Average of force necessary to open the wound of a treated rats
WBSc = Average of force necessary to open the wound of untreated rats
Histopathological Studies
Skin specimens were immediately fixed in 10% (v/v) neutral formalin until the tissues hardened. Each specimen was embedded in a paraffin block, and thin sections (5 μm) were prepared and stained with hematoxylin and eosin (for general morphological observations). Slides were examined qualitatively under a light microscope, for collagen formation, fibroblast (Fb) proliferation, angiogenesis, and epithelialization.
Statistical Analysis
All data were expressed as mean ± standard deviation statistical analyses were evaluated by one-way ANOVA followed by Dunnett test using SPSS 16.0 software (SPSS Inc., Chicago, USA). P < 0.05 were considered to be significant.
RESULTS
Wound Contraction and Epithelialization Time
As shown in Figure 1, the extract-treated group demonstrated significantly higher wound contracting ability than untreated and dexamethasone-treated groups from day 6 to 14. As expected, dexamethasone-induced an important delay in wound healing that lasted more than a month. When compared to the extract treated group, one more week was necessary for the untreated animals (control) to completely recover from the injury [Table 1].
Figure 1.

Effect of the water extract of Tetrapleura tetraptera stem-bark on wound contraction in rats. Data represents mean ± standard error of mean, n = 5. ***P < 0001: Significantly different when compared with the control group
Table 1.
Effects of the water extract of T. tetraptera on epithelialization time in rats
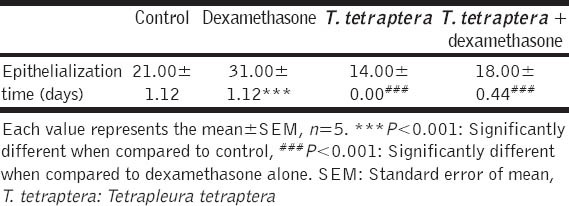
Wound Tensile Strength
As shown in Figure 2, tensile strength of the scared tissue was 672.90 g/cm2 in the control group. T. tetraptera applied alone slightly enhanced the tensile strength of 10 days old wounds as compared with the control group, while dexamethasone decreased this force by 44.44%. It was also evident from Figure 2 that the plant extract did not prevent dexamethasone-induced inhibition on the tensile strength.
Figure 2.

Effects of the water extract of Tetrapleura tetraptera stem-bark on the tensile strength of rats. Data represents the mean ± standard error of mean, n = 5. *P < 0.05: Significantly different when compared with the control group. #P < 0.05: Significantly different when compared to the dexamethasone group
Histological Study
Histological observations of scared tissue from the various groups of experimental animals of the study are illustrated below. The negative control rats showed a thick granular cell layer [Figure 3a]. Granulation tissue of the dexamethasone-treated group contains less collagen, Fb, and dilated blood capillary, when compared with control group [Figure 3b]. The plant extract treated group exhibited improvement in the skin structure with a thin well-formed epidermis and a well-organized dermis, with more collagen and Fb, no inflammatory cells and numerous blood vessels (Bvs) [Figure 3c]. In dexamethasone and T. tetraptera treated group, these positive effects were less pronounced when compared to extract treated group alone [Figure 3d].
Figure 3.

Microscopic view of the section of skin tissue after 10 days of treatment. (a) Negative control group, (b) dexamethasone treated group, (c) Tetrapleura tetraptera extract treated group and (d) T. tetraptera extract + dexamethasone treated group (H and E stain, ×400). Control animals show thick granular cell layer. Granulation tissue of dexamethasone treated rats contains less collagen, less fibroblasts (Fb), and dilated blood capillary. Arrow indicates inflammatory cells. A thin well-formed epidermis is displayed in plant extract treated skin. The dermis is well-organized, Fb longitudinally to the incision, and no inflammatory pattern. Numerous blood vessels also arise from this specimen. In the extract + dexamethasone treated group, granulation tissue contains more Fb collagen than dexamethasone-treated wounds alone, and capillary buds. Ep: Epithelial layer
DISCUSSION
The stem-bark of T. tetraptera has been shown to possess antiulcerative, antibacterial and antioxidant potential that could be attributed to a wound healing potential [13,19,20]. On this basis, the present study was designed to evaluate the wound healing effect of the water extract of the bark on excisional and incisional wound models. Wound healing process constitutes three interactive phases of inflammation, proliferation, and remodeling [3]. Re-epithelialization is generally achieved by migration, proliferation, and differentiation of epidermal keratinocytes. Simultaneous to the tissue remodeling phase, contraction begins a few days after injury primarily through myofibroblasts [21]. Since the water extract of the T. tetraptera stem-bark significantly improved the epithelialization time and the rate of contraction, it is likely to act on the three phases of the wound healing process.
Therapeutic agents that modulate wound repair can be evaluated based on their influence on the development of wound strength [17]. The increasing amount of stable collagen and the alignment of its fibers gradually increase the strength of the healing wound [21]. Rats treated with the water extract of T. tetraptera developed tensile strength that suggested good amount of mature collagen deposition. Histopathological examination further provided additional evidence on the wound healing potential of T. tetraptera stem-bark. Fixation of tissues with formalin followed by hematoxylin eosin staining is a common method, which displays a broad range of cytoplasmic, nuclear, and extracellular matrix features [17]. Such data provide additional insights into the status of the healing process, particularly in studies of impaired wound healing [17]. In agreement with previous studies on dexamethasone at a higher dose [22], poor wound healing associated with less epithelialization, and Fb density were observed in the present study. As with previous studies [23], the vascularity and the inflammatory pattern recorded for the dexamethasone group were higher than the control group. Our data unequivocally showed that the water extract of T. tetraptera stem-bark inhibited vascularity as observed in the dexamethasone-treated group. The granulation tissue of animal skins treated with the extract also exhibited numerous Bvs. Many Fb arranged longitudinally to the incision and significant cutaneous tensile strength were also observed when compared with the dexamethasone treated rats, suggesting an important collagen crosslinkage [17,24].
Even though the extract was effective on the cutaneous wound healing action of normal and dexamethasone treated rats, the epithelialization time was slightly different in rats receiving both dexamethasone and the plant extract as compared to those treated the plant extract alone. These observations were confirmed by the tensile strength measurement and the presence of capillary buds in the scared tissue. Although the possible mechanism remains to be elucidated, similar wound healing effect were reported on many other medicinal plants [25]. Recent phytochemical studies also revealed the existence of carotenoids-like substances in fruits and the seeds of T. tetraptera [26,27] but whether these compounds account for wound healing effect of the stem-bark remains to be proved. Furthermore, the activity reported in this study probably comes from the synergistic effect of compounds present in the extract and their antioxidant potential as demonstrated elsewhere [13].
CONCLUSION
Our study shows that an aqueous extract of T. tetraptera stem-bark accelerates cutaneous wound healing in normal and dexamethasone-treated rats. Our findings also indicate that the extract’s effect is based on the development of new capillaries and on collagen crosslinkage. Nevertheless, further researches needed to address the precise underlying molecular mechanisms of T. tetraptera stem-bark wound healing effects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Martin M. Physiology of wound healing. In: Flanagan M, editor. Wound Healing and Skin Integrity: Principles and Practice. Chicester: Wiley-Blackwell; 2013. p. 312. [Google Scholar]
- 3.Tsala DE, Dawe A, Habtemariam S. Natural wound healing and bioactivenatural products. Phytopharmacology. 2013;4:532–60. [Google Scholar]
- 4.Raina R, Parwez S, Verma PK, Pankaj NK. Medicinal plants and their role in wound healing. Online Vet J. 2008;3:1–7. [Google Scholar]
- 5.Aladesanmi AJ. Tetrapleura tetraptera: Molluscicidal activity and chemical constituents. Afr J Tradit Complement Altern Med. 2006;4:23–36. [PMC free article] [PubMed] [Google Scholar]
- 6.Ojewole JA, Adesina SK. Cardiovascular and neuromuscular actions of scopoletin from fruit of Tetrapleura tetraptera. Planta Med. 1983;49:99–102. doi: 10.1055/s-2007-969824. [DOI] [PubMed] [Google Scholar]
- 7.Ajayi OB, Fajemilehin AS, Dada CA, Awolusi OM. Effect of Tetrapleura tetraptera fruit on plasma lipid profile and enzyme activities in some tissues of hypercholesterolemic rats. J Nat Prod Plant Resour. 2011;1:47–55. [Google Scholar]
- 8.Odesanmi OS, Lawal RA, Ojokuku SA. Effects of ethanolic extract of Tetrapleura tetraptera fruit on serum lipid profile and kidney function in male Dutch-white rabbits. Nig Q J Hosp Med. 2011;21:299–302. [PubMed] [Google Scholar]
- 9.Aboaba OO, Ezeh AR, Anabuike CL. Antimicrobial activities of some Nigerian spices on some pathogens. Agric Biol J North Am. 2011;2:1187–93. [Google Scholar]
- 10.Ekwenye UN, Okorie CF. Antibacterial activity of Tetrapleura tetraptera TAUB. pod extracts. Int J Pharma Bio Sci. 2010;1:734–41. [Google Scholar]
- 11.Awofisayo OS, Udoh IE, Herbert O, Mbagwu C. Antibacterial effects of the aqueous and ethanolic extracts of Tetrapleura tetraptera pods on the pathogens in nosocomial wound infections. IJPI's J Biotechnol Biotherapeutic. 2010;1:18–23. [Google Scholar]
- 12.Lekana-Douki JB, Oyegue Liabagui SL, Bongui JB, Zatra R, Lebibi J, Toure-Ndouo FS. In vitro antiplasmodial activity of crude extracts of Tetrapleura tetraptera and Copaifera religiosa. BMC Res Notes. 2011;4:506. doi: 10.1186/1756-0500-4-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bella NM, Ngo LT, Ngo LT, Aboubakar OB, Tsala DE, Dimo T. Aqueous extract of Tetrapleura tetraptera (Mimosaceae) prevents hypertension, dyslipidemia and oxidative stress in high salt-sucrose induced hypertensive rats. Pharmacologia. 2012;3:397–405. [Google Scholar]
- 14.Bella NM, Tsala DE, Ngo LT, Aboubakar OB, Bilanda DC, Dimo T. Protective effects of Tetrapleura tetraptera extract on high salt-induced hypertension in male rats. Int J Trop Med. 2013;8:54–61. [Google Scholar]
- 15.Frank S, Kamfer H. Excisional wound healing: An experimental approach. In: Dipietro LA, Burns AL, editors. Wound Healing/Method and Protocols. Totowa, New Jersey: Humana Press; 2003. pp. 3–16. [Google Scholar]
- 16.Masoko P, Picard J, Eloff JN. The use of a rat model to evaluate the in vivo toxicity and wound healing activity of selected Combretum and Terminalia (Combretaceae) species extracts. Onderstepoort J Vet Res. 2010;77:E1–7. doi: 10.4102/ojvr.v77i1.2. [DOI] [PubMed] [Google Scholar]
- 17.Garmelli RL, He LK. Incisional wound healing: Model and analysis of wound breaking strength. In: Dipietro LA, Burns AL, editors. Wound Healing/Method and Protocols. Totowa, New Jersey: Humana Press; 2003. pp. 37–54. [DOI] [PubMed] [Google Scholar]
- 18.Kumar N, Prakash D, Kumar P. Wound healing activity of Solanum xanthocarpum Schrad. and Wendl. fruit. Indian J Nat Prod Resour. 2010;1:470–5. [Google Scholar]
- 19.Noamesi BK, Mensah JF, Bogale M, Dagne E, Adotey J. Antiulcerative properties and acute toxicity profile of some African medicinal plant extracts. J Ethnopharmacol. 1994;42:13–8. doi: 10.1016/0378-8741(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 20.Okoronkwo NE, Echeme JO. Cholinesterase and microbial inhibitory activities of Tetrapleura tetraptera. J Appl Nat Sci. 2012;4:156–63. [Google Scholar]
- 21.Shai A, Maibach HI. Berlin, Heidelberg: Springer-Verlag; 2005. Wound Healing and Ulcers of the Skin: Diagnosis and Therapy - The Practical Approach; p. 270. [Google Scholar]
- 22.Al-Refu K. Khopkar U, editor. General methods in preparation of skin biopsies for haematoxylin and eosin stain and immunohistochemistry. Skin Biopsy –Perspectives. InTech. 2011. pp. 19–30. Available from: http://www.intechopen.com/books/skin-biopsy-perspectives/general-methods-in-preparation-of-skin-biopsies-for-haematoxylin-eosin-stain-and-immunohistochemistry .
- 23.Murti K, Kumar U. Reversal of dexamethasone depressant action in wound healing by Ficus benghalensis L. roots. Am J Pharmacol Toxicol. 2011;6:68–75. [Google Scholar]
- 24.Vidinsk B, Gàl P, Toporcer T, Longauer F, Lenhardt L, Bobrov N, et al. Histological study of the first seven days of skin wound healing in rats. Acta Vet Bras. 2006;75:197–202. [Google Scholar]
- 25.Durmus M, Karaaslan E, Ozturk E, Gulec M, Iraz M, Edali N, et al. The effects of single-dose dexamethasone on wound healing in rats. Anesthsx Analg. 2003;97:1377–80. doi: 10.1213/01.ANE.0000080611.29106.9E. [DOI] [PubMed] [Google Scholar]
- 26.Tsala DE, Nnanga N, Mendimi NJ, Godwe EK, Edmond J, Ze Ze PV, et al. Adermal wound healing effect of water extract of the stem bark of Alafia multiflora Stapf. Phytopharmacology. 2013;4:114–22. [Google Scholar]
- 27.Abugri DA, Pritchett G. Determination of chlorophylls, carotenoids, and fatty acid profiles of Tetrapleura tetraptera seeds and their health implication. J Herbs Spices Med Plants. 2013;19:391–400. [Google Scholar]


