Abstract
Aim:
Aristolochia longa (from the family Aristolochiaceae) is widely used in Algerian traditional medicine. Here, we document ethnomedicinal uses by local population of Mascara province (West Algeria) and we evaluate the antifungal activity, the phytochemical composition and total phenolic content of aqueous extract (decoction) of the roots of A. longa from Algeria.
Materials and Methods:
The ethnobotanical investigation was carried out in Mascara Province (West Algeria). Antifungal activity was assessed against Saccharomyces cerevisiae. Total phenolic content was measured using the Folin–Ciocalteu’s reagent.
Results:
Our results showed that A. longa is widely used to treat several ailments such as cancer (38%), skin infections (14%), and diabetes (11%). Crushed roots are commonly used (89%) mixed with honey, milk, water or other medicinal plants. A. longa aqueous extract induced growth inhibition of S. cerevisiae cells in a dose - and time - dependent manner. An effective suppression of S. cerevisiae (97.06% inhibition of proliferation) was obtained at the 500 µg/mL after 72 h. Results of the phytochemical screening revealed that A. longa aqueous extract contained various bioactive compounds, including polyphenols and flavonoids. Total phenolic content in A. longa aqueous extract was found to be 6.07 ± 0.12 mg (gallic acid equivalents)/g.
Conclusion:
A. longa may be considered as a promising source of new drugs for treating cancer and as a good antifungal agent.
KEY WORDS: Algeria, antifungal, Aristolochia longa, ethnobotany, Mascara, phenolic, phytochemistry
INTRODUCTION
Medicinal plants are one of major resources of therapeutic agents. They are used by 80% of the world population in health care [1]. Medicinal plant use knowledge developed by local populations in Africa is now disappearing. There is, therefore, an urgent need to document this traditional knowledge [2].
Aristolochia, Family Aristolochiaceae, is a large genus of herbs and twining plants, found in the tropical and temperate regions of the world [3]. Aristolochia species have well known beneficial effects. Some of them were found to possess anticancer activity in several bioscreening studies [4]. The Aristolochia species are cultivated as ornamentals and popularly used as sources of abortifacient, emmenagogue, sedative, analgesic, anti-inflammatory, anti-feedant, muscle relaxant, antihistaminic, and anti-allergic drugs [5]. We have recently published a review on biological activities of Aristolochia plants [6]. Phytochemical studies on plants of the Aristolochia genus have yielded various types of compounds with antitumor, antiplatelet aggregation, immunomodulating and antifertility activities [7]. Aristolochia longa, commonly known as “Berrostom” to the local population in Algeria, is widely used in traditional medicine. In our previous study, we demonstrated that the aqueous extract of A. longa induced cell growth inhibition of Burkitt’s lymphoma (BL41) cells in a dose-dependent manner. The extract induced apoptosis, loss of mitochondrial membrane potential and the activation of caspases-9 and -3 followed by poly(adenosine diphosphate-ribose) polymerase cleavage [8].
As part of our continuing work to investigate and biologically evaluate Algerian medicinal plants used in cancer treatment, the present study aimed to document ethnomedicinal uses, and to evaluate phytochemical composition and antifungal activity of A. longa.
MATERIALS AND METHODS
Ethnobotanical Study
The ethnobotanical investigation was carried out in Mascara Province. Mascara (5941 km²) is located in the north-west of Algeria, (at 360 km of Algiers) with Mediterranean climate and mean annual precipitations of about 450 mm. In 2010, the total population was 826,334, with male/female ratio of about 1.04. Ethnomedicinal uses of A. longa information were collected and documented through casual conversations and semi-structured interview technique [9] with local herbal practitioners and knowledgeable residents of the study area. The main focus was to collect the oral information about the ethnomedicinal uses of A. longa by local population.
Preparation of A. longa Aqueous Extract
Roots of A. longa were collected in March 2009; in “Tissemssilet,” an administrative region located in western Algeria. Botanic identification and authentication were made by Dr. Kada Righi (Department of Agriculture, Faculty of Nature and Life Sciences, Mascara University, Algeria). The roots were dried, pulverized and finely sieved. The aqueous extract of A. longa was prepared as follows: The dried roots were boiled for 20 min at 100°C, cooled to room temperature, and then filtered. The solution passing through the filter was collected, concentrated, lyophilized and stored in a desiccator at +4°C until use.
Antifungal Activity Evaluation
Antifungal activity was evaluated using Saccharomyces cerevisiae from our laboratory collection (Département de Biologie, Faculté SNV, Université de Mascara). Sabouraud’s dextrose broth was used for the preparation of fungal cultures and for the antifungal activity evaluation. A volume of 50 µl of overnight liquid cultures of yeast (density of 2 × 108 cells/mL) was added to each well of 96-well plates containing 50 µl media and various concentrations (0-500 µg/mL) of the extract in triplicate. The plates were incubated at 37°C. Absorbance was measured at 620 nm using a spectrophotometer (Shimadzu, Japan).
Phytochemical Screening
A. longa roots were screened for the presence of phytochemical constituents, such as alkaloids, terpenoids, anthraquinones, flavonoids, tannins, saponins, steroids and glycosides, with the standard qualitative phytochemical procedures described [10].
Determination of Total Phenolic Content
Total phenolic content was measured using the Folin–Ciocalteu’s reagent as described [11]. Absorbance was measured at 760 nm using an ultraviolet spectrophotometer (Shimadzu, Japan). Gallic acid was used as a standard. A dose response linear regression was generated using the gallic acid standard absorbance, and the total phenolic content was expressed as mg gallic acid equivalents (GAE)/g dry weight of extract. Values were determined in triplicate.
Statistics Analysis
Mean data values are presented, with their standard deviations (mean ± SD). All statistical comparisons were made by Student’s t-test, and statistical significance was defined as P < 0.05.
RESULTS
Ethnobotanical Study
In the present study, we interviewed 100 informants (female: 92; male: 8) in different locations of Mascara province (West Algeria). Almost all of the respondents knew and/or used A. longa for medicinal purposes. According to our results [Figure 1], A. longa is recognized to treat several ailments and health disorders. Cancer is the first ailment treated with the plant (39%), followed by skin infections (14%), diabetes (11%) and gastro-intestinal ailments (9%). Moreover, infertility and gynecological troubles are also treated with A. longa.
Figure 1.
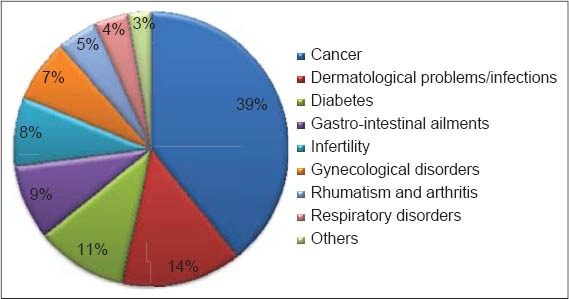
Ailments treatd with Aristolochia longa
As shown in Figure 2, roots are the most frequently used part by local populations of Mascara (89%). Leaves (9%) and the entire plant (2%) are also used. The most usually method of preparation used is the mixture crushed roots - honey (63%), taken orally. Mixture with milk (13%) or other medicinal plants (12%) and decoction in water (11%) are also used to treat internal ailments. The paste (plant grinded with olive oil or water) is applied externally for skin infections and rheumatism [Figure 3].
Figure 2.
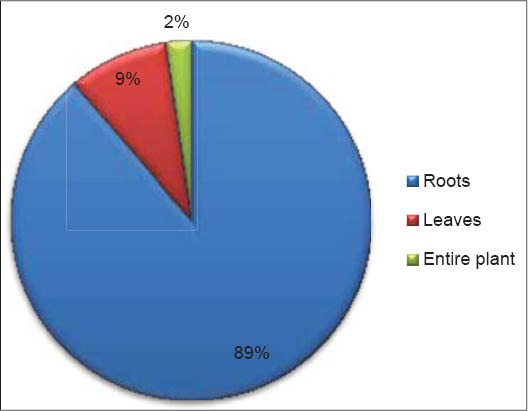
Plant parts used
Figure 3.
Preparation methods
Antifungal Activity of A. longa Aqueous Extract
To investigate the antifungal activity of the A. longa aqueous extract, S. cerevisiae cells were incubated with increasing concentrations (0-500 µg/mL). After 2, 48 and 72 h, cell viability was determined. Percent survival was determined when compared to untreated cells.
Cells were treated with increasing concentrations (from 0.00 to 500 µg/mL) of A. longa aqueous extract for 72 h and cell growth was measured as described in M and M. Percent survival was determined when compared to untreated cells. The difference in cell growth between untreated and A. longa aqueous extract-treated cells was found to be significant (P < 0.001). Data are represented as the mean ± standard deviation of three experiments per treatment group.
According to our results [Figure 4], A. longa aqueous extract induced growth inhibition of S. cerevisiae cells in a dose- and time- dependent manner. At 250 µg/mL, A. longa aqueous extract resulted in 30% and 67.65% inhibition of cell viability of S. cerevisiae cells after 48 and 72 h, respectively. At 500 µg/mL, the maximal inhibitory dose on the cell growth, the extract suppressed effectively the growth of S. cerevisiae cells after 72 h of treatment (97.06% inhibition of proliferation). We demonstrate a strong antifungal effect of A. longa aqueous extract as assessed against S. cerevisiae cells.
Figure 4.
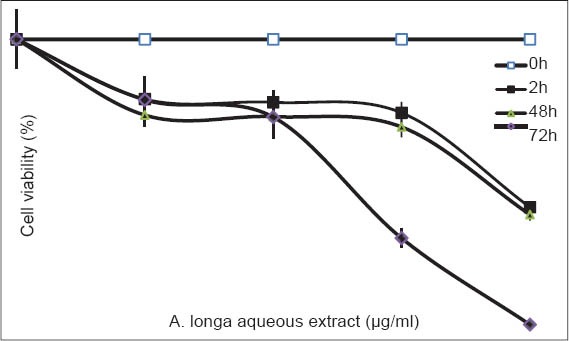
Effect of Aristolochia longa aqueous extract on growth Saccharomyces cerevisiae cells
Phytochemical Screening of A. longa
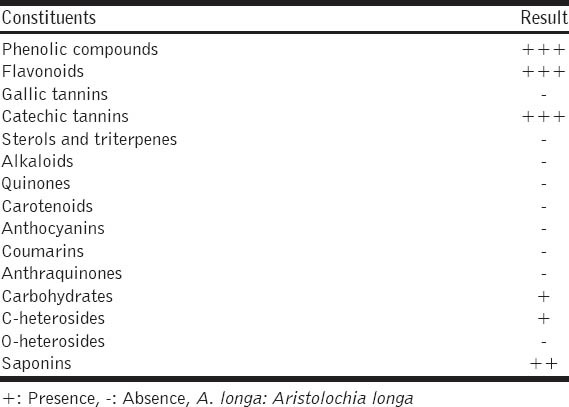
Phytochemical screening of A. longa aqueous extract showed the presence polyphenols, flavonoids, tannins, C-heterosides, carbohydrates, and saponins. However, alkaloids, coumarins and O-heterosides were not detected [Table 1].
Table 1.
Preliminary phytochemical screening of A. longa roots
Total Phenolic Content
Total phenolic content was estimated by using Folin–Ciocalteu reagent. Folin–Ciocalteu is a widely used and reported method for a simple and rapid estimation of total phenolics [12]. A standard curve [Figure 5] was obtained using gallic acid as a standard.
Figure 5.
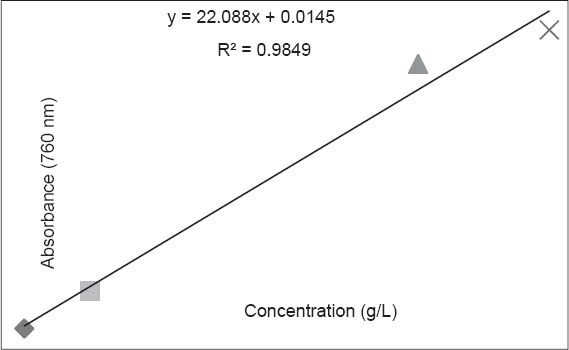
Calibration curve of gallic acid
Total phenolic content in A. longa roots was found to be 6.07 ± 0.12 mg (GAE)/g.
DISCUSSION
Plants have played a vital role in the prevention and treatment of disease since prehistoric times [13]. Although local populations of Mascara have a long history of traditional use of medicinal plants, no studies have documented the traditional knowledge, which it is disappearing. The present study aimed to record the ethnomedicinal uses and to investigate the antifungal and the phytochemical profile of A. longa. The latter is widely used in Algeria.
Our results showed that the first ailment treated by local population using A. longa is cancer. Our findings are in consistence with previous studies. It has been reported that the most widely uses of A. longa in Algeria are in cancer treatment [14-16]. The use of this plant as an anti-cancer has been also reported in Morocco [17]. Arthroleptis brevipes, Arachis monticola and Agrimonia pilosa are used as anticancer plants in Mexico [18]. We have recently demonstrated that an aqueous extract of A. longa induced apoptosis of BL41 cell line in a dose-dependent manner, by triggering the mitochondrial pathway [8]. Furthermore, our results revealed that skin infections are the second ailment group treated with A. longa in Mascara. Similar findings have been reported for Aristolochia bracteolate and Acacia albida used in Nigeria [19]. Current antifungal drugs are insufficient due to the limited selection of medications, their adverse side-effects and the emergence of resistance to them by fungal pathogens [20]. According to our results, A. longa aqueous extract induced growth inhibition of S. cerevisiae cells in a dose- and time- dependent manner. At 500 µg/mL, the maximal inhibitory dose on the cell growth, the extract almost abolished the proliferation of S. cerevisiae cells after 72 h of treatment (97.06% inhibition of proliferation). A. longa is considered as good antimicrobial drug [21]. Aristolochic acids, isolated from this plant inhibited Escherichia coli, Pseudomonas aeruginosa, Streptococcus faecalis, Staphylococcus aureus and Staphylococcus epidermidis [22]. Several plant extracts from the genus Aristolochia have been shown to possess strong in vitro antimicrobial activity [23-25].
Secondary metabolites produced by plants possess several interesting biological activities, and are a source of pharmacologically active principles against pathogenic microorganisms [26]. Since biological activities of medicinal plants are closely related to their chemical compounds [27], thus strong antifungal activity of the A. longa aqueous extract may be due to the detected compounds. Phytochemical screening of A. longa aqueous extract revealed the presence of polyphenols, flavonoids, tannins, c-heterosides, carbohydrates, and saponins. Phenolic compounds, flavonoids and saponins may be responsible for the antifungal activity. The mechanisms thought to be responsible for phenolic toxicity to microorganisms include enzyme inhibition by the oxidized compounds, possibly through reaction with sulfhydryl groups or through more nonspecific interactions with the proteins. On the other hand, saponins appear to act by disrupting the membrane integrity of fungal cells [28]. In the current study, total phenolic content of the A. longa aqueous extract was measured and found to be 6.07 ± 0.12 mg (GAE)/g. In A. longa total phenolics and flavonoids were estimated as 1.47 mg/g and 0.8 mg/g, respectively [29].
CONCLUSION
We present evidence that A. longa is widely used by local populations of Mascara province (West Algeria) to treat several ailments such as cancer. Crushed roots are commonly used mixed with honey, milk, water or other medicinal plants. Moreover, a strong antifungal effect (dose- and time- dependent manner) of A. longa aqueous extract as assessed against S. cerevisiae cells was demonstrated. Phytochemical screening revealed the presence of bioactive compounds that may contribute to the antifungal activity of the A. longa aqueous extract, such as flavonoids or saponins. Thus, A. longa may be considered as a promising source of new drugs for treating cancer and as a good antifungal agent.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
REFERENCES
- 1.Oliveira SG, de Moura FR, Demarco FF, Nascente Pda S, Pino FA, Lund RG. An ethnomedicinal survey on phytotherapy with professionals and patients from basic care units in the Brazilian Unified Health System. J Ethnopharmacol. 2012;140:428–37. doi: 10.1016/j.jep.2012.01.054. [DOI] [PubMed] [Google Scholar]
- 2.Asase A, Akwetey GA, Achel DG. Ethnopharmacological use of herbal remedies for the treatment of malaria in the Dangme West District of Ghana. J Ethnopharmacol. 2010;129:367–76. doi: 10.1016/j.jep.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Shirwaikar A, Somashekar AP, Udupa AL, Udupa SL, Somashekar S. Wound healing studies of Aristolochia bracteolata Lam. with supportive action of antioxidant enzymes. Phytomedicine. 2003;10:558–62. doi: 10.1078/094471103322331548. [DOI] [PubMed] [Google Scholar]
- 4.Alali FQ, Tawaha K, Shehadeh MB, Telfah S. Phytochemical and biological investigation of Aristolochia maurorum L. Z Naturforsch C. 2006;61:685–91. doi: 10.1515/znc-2006-9-1013. [DOI] [PubMed] [Google Scholar]
- 5.Pacheco AG, Machado de Oliveira P, Piló-Veloso D, Flávio de Carvalho Alcântara A. 13C-NMR data of diterpenes isolated from Aristolochia species. Molecules. 2009;14:1245–62. doi: 10.3390/molecules14031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benarba B, Meddah B. Biological activities of Aristolochia plants: A mini review. J Physiobiochem Metab. 2013;2:1–4. [Google Scholar]
- 7.Chen YG, Yu LL, Huang R, Liu JC, Lv YP, Zhao Y. 3“-hydroxyamentoflavone and its 7-O-methyl ether, two new biflavonoids from Aristolochia contorta. Arch Pharm Res. 2005;28:1233–5. doi: 10.1007/BF02978204. [DOI] [PubMed] [Google Scholar]
- 8.Benarba B, Ambroise G, Aoues A, Meddah B, Vazquez A. Aristolochia longa aqueous extract triggers the mitochondrial pathway of apoptosis in BL41 Burkitt's lymphoma cells. Int J Green Pharm. 2012;6:45–9. [Google Scholar]
- 9.Rajakumar N, Shivanna MB. Ethno-medicinal application of plants in the eastern region of Shimoga District, Karnataka, India. J Ethnopharmacol. 2009;126:64–73. doi: 10.1016/j.jep.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Trease GE, Evans I. 12th ed. London: Bailliere Tindall; 1983. Pharmacognosy. [Google Scholar]
- 11.Kumbhare MR, Guleha V, Sivakumar T. Estimation of total phenolic content, cytotoxicity and in-vitro antioxidant activity of stem bark of Moringa oleifera. Asian Pac J Trop Dis 2012. 2012:144–50. [Google Scholar]
- 12.Dhaouadi K, Raboudi F, Estevan C, Barrajón E, Vilanova E, Hamdaoui M, et al. Cell viability effects and antioxidant and antimicrobial activities of Tunisian date syrup (Rub El Tamer) polyphenolic extracts. J Agric Food Chem. 2011;12(59):402–6. doi: 10.1021/jf103388m. [DOI] [PubMed] [Google Scholar]
- 13.Assefa B, Glatzel G, Buchmann C. Ethnomedicinal uses of Hagenia abyssinica (Bruce) J.F. Gmel. among rural communities of Ethiopia. J Ethnobiol Ethnomed. 2010;6:20. doi: 10.1186/1746-4269-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherif HS, Saidi F, Boutoumi H, Rouibi A, Chaouia C. Identification and characterization of some chemicals from Aristolochia longa L. Agricultura. 2009;3:76–82. [Google Scholar]
- 15.Saidi F, Cherif HS, Lazouri H, Aid K, Rouibi A, Bele C, Matea C. Determination of the lipid compounds of Aristolochia Longa L. from Algeria. Bull Univ Agric. 2009;66:17–23. [Google Scholar]
- 16.Saidi F, Cherif HS, Metidji H, Rouibia A, Chaouia C, Abdulhussain MS. In vitro multiplication by direct organogenesis of a medicinal plant Aristolochia longa L. Agricultura. 2009;3:53–66. [Google Scholar]
- 17.Benzakour G, Benkirane N, Amrani M, Oudghiri M. Immunostimulatory potential of Aristolochia longa L. induced toxicity on liver, intestine and kidney in mice. J Toxicol Environ Health Sci. 2011;3:214–22. [Google Scholar]
- 18.Alonso-Castro AJ, Villarreal ML, Salazar-Olivo LA, Gomez-Sanchez M, Dominguez F, Garcia-Carranca A. Mexican medicinal plants used for cancer treatment:Pharmacological, phytochemical and ethnobotanical studies. J Ethnopharmacol. 2011;133:945–72. doi: 10.1016/j.jep.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 19.Ezuruike UF, Prieto JM. The use of plants in the traditional management of diabetes in Nigeria:Pharmacological and toxicological considerations. J Ethnopharmacol. 2014;155:857–924. doi: 10.1016/j.jep.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 20.Tebbets B, Yu Z, Stewart D, Zhao LX, Jiang Y, Xu LH, et al. Identification of antifungal natural products via Saccharomyces cerevisiae bioassay:Insights into macrotetrolide drug spectrum, potency and mode of action. Med Mycol. 2013;51:280–9. doi: 10.3109/13693786.2012.710917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinou J, Demetzos C, Harvala C, Roussakis C. Cytotoxic and antimicrobial principles from the roots of Aristolochia longa. J Ethnopharmacol. 1990;28:149–51. [Google Scholar]
- 22.Lans C. Comparison of plants used for skin and stomach problems in Trinidad and Tobago with Asian ethnomedicine. J Ethnobiol Ethnomed. 2007;3:3. doi: 10.1186/1746-4269-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarro-García VM, Luna-Herrera J, Rojas-Bribiesca MG, Álvarez-Fitz P, Ríos MY. Antibacterial activity of Aristolochia brevipes against multidrug-resistant Mycobacterium tuberculosis. Molecules. 2011;16:7357–64. doi: 10.3390/molecules16097357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alviano WS, Alviano DS, Diniz CG, Antoniolli AR, Alviano CS, Farias LM, et al. In vitro antioxidant potential of medicinal plant extracts and their activities against oral bacteria based on Brazilian folk medicine. Arch Oral Biol. 2008;53:545–52. doi: 10.1016/j.archoralbio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Camporese A, Balick MJ, Arvigo R, Esposito RG, Morsellino N, De Simone F, et al. Screening of anti-bacterial activity of medicinal plants from Belize (Central America) J Ethnopharmacol. 2003;87:103–7. doi: 10.1016/s0378-8741(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 26.Damián-Badillo LM, Salgado-Garciglia R, Martínez-Muñoz RE, Martínez-Pacheco MM. Antifungal properties of some Mexican medicinal plants. Open Nat Prod J. 2008;1:27–33. [Google Scholar]
- 27.Hashemi SR, Zulkifli I, Hair Bejo M, Farida A, Somchit MN. Acute toxicity study and phytochemical screening of selected herbal aqueous extract in broiler chickens. Int J Pharmacol. 2008;4:352–60. [Google Scholar]
- 28.Arif T, Bhosale JD, Kumar N, Mandal TK, Bendre RS, Lavekar GS, et al. Natural products –Antifungal agents derived from plants. J Asian Nat Prod Res. 2009;11:621–38. doi: 10.1080/10286020902942350. [DOI] [PubMed] [Google Scholar]
- 29.Djeridane A, Yousfi M, Nadjemi B, Maamri S, Djireb F, Stocker P. Phenolic extracts from various Algerian plants as strong inhibitors of porcine liver carboxylesterase. J Enzyme Inhib Med Chem. 2006;21:719–26. doi: 10.1080/14756360600810399. [DOI] [PubMed] [Google Scholar]


