Abstract
Aims:
The present study was designed to assess some pharmacological effects of cinnamon (CAE) and ginger (GAE) aqueous extracts in obese diabetic rats, and to elucidate the potential mechanisms.
Materials and Methods:
Forty-two Sprague-Dawley rats were randomized into 6 equal groups. Group 1 was a negative control and the other groups were rendered obese by feeding rats on high-fat diet for 4 weeks. The obese rats were subcutaneously injected with alloxan for 5*days to induce diabetes. Group 2 was a positive control, and Groups 3, 4, 5 and 6 were orally given CAE in doses 200 and 400 mg/kg and GAE in the same doses, respectively for 6 weeks. Blood samples were collected for serum biochemical analyses. Kidneys were dissected out to assay activity of tissue antioxidant enzymes: Superoxide dismutase, glutathione peroxidase and catalase.
Results:
CAE and GAE significantly reduced body weight and body fat mass; normalized serum levels of liver enzymes; improved lipid profile; decreased blood glucose and leptin and increased insulin serum levels in obese diabetic rats. Both extracts also increased activity of kidney antioxidant enzymes.
Conclusion:
CAE and GAE exhibit anti-obesity, hepatoprotective, hypolipidemic, antidiabetic and anti-oxidant effects in obese diabetic rats. These results confirm the previous reports on both extracts. The potential mechanisms underlying these effects are fully discussed and clarified. Our results affirm the traditional use of cinnamon and ginger for treating patients suffering from obesity and diabetes. The obese diabetic rat model used in this study is a novel animal model used in pharmacology researches.
KEY WORDS: Anti-obesity, anti-diabetic, anti-oxidant, biochemical analyses, cinnamon, ginger, hepatoprotective, hypolipidemic
INTRODUCTION
Obesity and diabetes are among the most challenging global health problems. Obesity is an excessive fat accumulation in the body that results from an imbalance between energy intake and energy expenditure. It is associated with genetic, metabolic, and behavioral components, and the rapid development of obesity might reflect other risk factors such as dietary fat intake, fat storage and metabolism, and lifestyle [1]. Obesity increases the risk for many diseases including diabetes mellitus, and there is a positive association between obesity and insulin resistance and infiltration of adipose tissues by inflammatory cells [2,3]. Insulin resistance that commonly accompanies obesity is a major risk factor for the incidence of diabetes mellitus [4].
Diabetes mellitus is a metabolic disease characterized by hyperglycemia due to insulin deficiency, insulin resistance, or both. The increased extracellular and intracellular glucose concentration results in oxidative stress that plays a key role in the onset and development of serious diabetes complications, notably diabetic nephropathy [4,5].
Cinnamon (Cinnamomum zeylanicum L.) is one of the most important spices used to flavor most foods in both Arabian and European countries. In animal studies, the aqueous extract of cinnamon potentiated insulin-regulated glucose utilization via enhancing insulin signaling pathway [6,7] and prevented insulin resistance induced by a high-fructose diet [8]. Cinnamon is used in folk medicine for its hepatoprotective [9], anti-oxidant [10], anti-obesity [11], antihyperlipidemic [12] and antidiabetic [8,13] activities.
Ginger (Zingiber officinale) is widely used as a culinary spice and has a long history for its health benefits. Ginger is used medicinally for its hepatoprotective and anti-oxidant [14], antidiabetic and antihyperlipidemic [15,16] and anti-obesity [17] effects.
The present study aimed to assess some pharmacological effects of cinnamon (CAE) and ginger aqueous extracts (GAE) in obese diabetic rat model and to elucidate the potential mechanisms.
MATERIALS AND METHODS
Plant Materials
Dried cinnamon (C. zeylanicum L., Family Lauraceae) barks and ginger rhizomes (Z. officinale, Family Zingiberaceae) were purchased from the local market of Agricultural Herbs, Spices and Medicinal plants, Cairo, Egypt. The dried plant materials were grinded into a fine powder and kept till the preparation of aqueous extracts.
Alloxan and Biochemical Kits
Alloxan was purchased from El-Gomhoryia Company for Chemicals; Cairo, Egypt as a white powder packed in brown bottles each containing 25 g alloxan monohydrate. Glucose enzymatic kits for estimating blood glucose (BG) and radioimmunoassay kits for leptin and insulin hormones were procured from Gamma Trade Company, Egypt. The other biochemical kits were obtained from Biodiagnostics Company, Dokki, Egypt.
Rats
Forty-two adult male Sprague-Dawley rats weighing 200-210 g body weight (B.wt) and 10-12 weeks old were used in this study. Animals were obtained from Laboratory Animal Colony, Agricultural Research Center, Giza, Egypt. Rats were housed in a well-ventilated animal room under controlled hygienic conditions of 24°C temperature, 50% relative humidity and 12 h light/12 h dark cycles. Basal diet and water were provided ad libitum. The experiment on rats was carried out in accordance with the recommendations of the National regulations on animal welfare and Institutional Animal Ethical Committee (IAEC).
Preparation of Basal Diet
The dietary supply of protein, fat, carbohydrates, vitamins and minerals were in accordance to the recommended dietary allowances for rats [18]. Basal diet was consisted of 20% protein, 10% sucrose, 5% corn oil, 2% choline chloride, 1% vitamin mixture, 3.5% salt mixture and 5% fibers. The remainder was corn starch up to 100%.
Preparation of Aqueous Extracts
A total of 200 g of each powder of cinnamon barks and ginger rhizomes were dissolved in 1 L of distilled water and boiled for 10 min, cooled and filtered using double layers of gauze to obtain 20% aqueous extracts [19].
Induction of Obesity and Diabetes
Obesity and acute hyperlipidemia were induced by feeding rats on high-fat diet (HFD) which supplies 45% calories from hog fat (lard) for 4 weeks. A 3-4 weeks HFD feeding is sufficient to induce obesity, and this model of obese rats is closely resembled the reality of obesity in humans [20]. The obese rats were then rendered diabetic by subcutaneous injection of alloxan (120 mg/kg) for 5 days to induce acute diabetes [21].
Design of Experiment
Forty-two mature male Sprague-Dawley rats were randomized into 6 groups of 7 rats each. Group 1 was negative (normal) control, and the other 5 groups were fed on HFD for 4 weeks to induce obesity. The obese rats were then rendered diabetic by subcutaneous injection of alloxan (120 mg/kg) for 5 days. Group 2 was kept obese diabetic (positive control) and groups 3,4,5 and 6 were orally given CAE in doses 200 and 400 mg/kg and GAE in the same doses respectively for 6 weeks. At the end of the experiment, B.wts of rats were recorded, and rats were then euthanized by prolonged exposure to ether anesthetic. The abdomen was opened, and body fats, including mesenteric, visceral, epididymal and retroperitoneal fats were carefully dissected out and total fat mass was weighed. The adiposity index (Ad. I) was calculated by dividing total body fat mass by B.wt and multiplied by 100 (Ad. I = fat weight [F.wt]/B.wt × 100) as described by Pichon et al. [22]. Blood samples were collected for serum biochemical analyses and kidneys were dissected out to assay the activity of tissue antioxidant enzymes.
Biochemical Analyses
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) [23], alkaline phosphatase (ALP) [24], total cholesterol (TC) [25], triglycerides (TG) [26] and high-density lipoprotein cholesterol (HDL) [25] were chemically determined using specific diagnostic kits and measured using a spectrophotometer. Low-density lipoprotein (LDL) cholesterol was calculated according to Friedewald [26]. BG was determined using glucose enzymatic kits according to Siest et al., [27]. Serum insulin was estimated using specific antibody radioimmunoassay kit [28] and leptin hormone was determined using enzyme-linked immunosorbent assay [29].
Preparation of Kidney Homogenate and Enzymes Assay
One gram of kidney tissue was washed with ice-cooled 0.9% NaCl solution and homogenized in 100 ml of ice-cooled 1.5% solution of potassium chloride and 50 mM potassium phosphate buffer solutions (pH 7.4) to yield 10% homogenate (w/v). Kidney homogenates were centrifuged at 4000 rpm for 10 min. at 4°C and the supernatants were used to assay the activity of antioxidant enzymes GPX, SOD and catalase (CAT) according to Paglia and Valentine [30], Spitz and Oberley [31] and Sinha [32], respectively.
Statistical Analysis
Data were presented as means ± standard error. Differences between control and treated groups were tested for significance using one-way analysis of variance followed by Duncan’s multiple range test [33]. Statistical analysis was performed using computerized software program Statistical Package for Social Sciences version 15 (Chicago, IL 60606-6412,USA).
RESULTS
Feeding of rats on HFD for 4 weeks significantly (P < 0.05) increased B.wt, body fat mass weight (F.wt) and Ad. I when compared to negative control rats fed on the basal diet. Oral administration CAE and GAE in doses 200 and 400 mg/kg given to obese diabetic rats for 6 weeks induced significant decreases in B.wt, F.wt and Ad. I when compared to the positive control group, in a dose-dependent manner as shown in Table 1.
Table 1.
Effect of CAE and GAE on B.wt, F.wt and Ad. I in obese diabetic rats (n=7)

Rats fed on HFD for 4 weeks had significant (P < 0.05) increases in serum levels of liver enzymes AST, ALT and ALP when compared with negative control rats. CAE and GAE in doses 200 and 400 mg/kg when orally given to obese diabetic rats significantly (P < 0.05) lowered the high serum levels of AST, ALT and ALP enzymes when compared to the positive control group, in a dose-dependent fashion, as illustrated in Figure 1.
Figure 1.

Effect of cinnamon aqueous extract and ginger aqueous extract on serum levels of aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase liver enzymes in obse diabetic rats
As demonstrated in Figure 2, feeding of rats on HFD for 4 weeks produced significant (P < 0.05) increases in serum levels of TC and TG when compared to rats fed on the basal diet. CAE and GAE in doses 200 and 400 mg kg when given orally to obese diabetic rats significantly lowered the high levels of serum TC and TG in a dose-dependent manner, when compared with the positive control group.
Figure 2.

Effect of cinnamon aqueous extract and ginger aqueous extract on serum total cholesterol and triglycerides on obese diabetic rats
Feeding of rats on HFD for 4 weeks significantly (P <0.05) decreased serum HDL and increased both LDL and At. I when compared to negative control rats. Oral administration of CAE and GAE in doses of 200 and 400 mg/kg to obese diabetic rats induced a significant (P <0.05) increase in serum HDL and decreased in both LDL and At. I when compared with the positive control groups as depicted in Table 2.
Table 2.
Effect of CAE and GAE on HDL, LDL and At. I in obese diabetic rats (n=7 rats)

Data in Table 3 showed that rats fed on HFD had significant (P < 0.05) increases in BG and leptin hormone and a decrease in insulin serum levels when compared to the negative control group. CAE and GAE when given orally in doses 200 and 400 mg/kg to obese diabetic rats for 6 weeks significantly (P < 0.05) decreased BG and leptin hormone and increased insulin serum levels when compared with the positive control group, in a dose-dependent manner.
Table 3.
Effect of CAE and GAE on BG and leptin and insulin hormones levels in obese diabetic rats (n=7 rats)

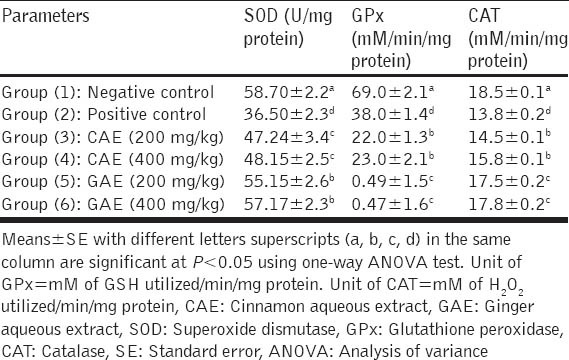
Feeding of HFD to rats for 4 weeks significantly (P < 0.05) decreased the activity of renal tissue levels of SOD, GPX and CAT antioxidant enzymes as compared to negative control rats. CAE and GAE when given in doses of 200 and 400 mg/kg to obese diabetic rats normalized the elevated renal tissue levels of SOD, GPX and CAT enzymes when compared with the positive control group, in a dose-dependent manner as recorded in Table 4.
Table 4.
Effect of CAE and GAE on activities of kidney SOD, GPx and CAT anti-oxidant enzymes in obese diabetic rats (n=7 rats)
DISCUSSION
The present study aimed to assess some pharmacological effects of CAE and GAE herbs in obese diabetic rats, and to elucidate the potential mechanisms.
Obesity is a major health problem that enhances the incidence of some diseases such as diabetes [2]. The oxidative stress plays an important role in the development of diabetes in obese individuals [3]. Both oxidative stress and the decreased antioxidant body defense mechanisms contribute to decreased insulin sensitivity and impaired insulin secretory response in obese diabetic rats [34,35]. In this study, obesity was induced by feeding rats on HFD for 4 weeks. This obese rat model closely resembles the reality of obesity in humans [20]. However, the experimental obesity could be also induced in rats and mice by other methods such as feeding on HFD [8], damage in the anterior hypothalamus and genetically induced-obesity. The obese diabetic rat model used in this study a novel animal model in pharmacology researches.
Results of the present study showed that CAE when given orally in 200 and 400 mg/kg to obese diabetic rats for 6 weeks produced a dose-dependent antiobesity effect. This effect was closely similar to the previous reports [11,12]. Similarly, GAE induced an antiobesity activity in obese diabetic rats and this effect agreed with the previous reports [17,36].
The potential mechanism underlying the antiobesity effect of CAE and GAE could be possibly attributed to their hyperinsulinimic effect that evident in the present study. Previous studies showed that hyperinsulinemia and insulin resistance are common features of obesity in rats [37] and in humans [38]. A second possible mechanism of the antiobesity activity of CAE and GAE could be due to the low serum level of leptin hormone reported in the current study. In this concern, Friedman [39] mentioned that leptin is a peptide hormone secreted by adipose tissues in proportion to body fat mass. When leptin circulates in the blood it acts on the brain to regulate food intake (appetite) and energy expenditure. When body fat mass decreases the serum leptin level decreases so stimulating appetite and suppressing energy expenditure till fat mass is restored. On this basis, the antiobesity activity and decreased Ad. I of CAE and GAE when given obese diabetic rats could be possibly attributed to the low serum leptin level that reported in the study. Conclusively, the antiobesity effect of CAE and GAE could be explained by their hyperinsulinimic activity and decreased serum leptin levels.
The hepatoprotective effect of CAE and GAE reported in this study was evident from the significant decrease in serum levels of liver enzymes (AST, ALT and ALP) in obese diabetic rats. This effect was in accordance with the previous reports for cinnamon extract and its polyphenols [11,12] and for ginger [14] extract. The potential mechanism underlying the hepatoprotective effect of CAE and GAE could be attributed to the antioxidant activity of cinnamon [40] and of ginger [14]. The oxidative stress was reported to increase serum levels of liver enzyme (AST, ALT and ALP) in diabetic rats because hyperglycemia increases the production of ROS, which induces oxidative stress [4,5].
The hypolipidemic effects of CAE and GAE reported in this study were similar to the previous reports for cinnamon [11,12] and for ginger [16] extracts. The previous authors concluded that both extracts can lower the elevated levels of TC, TG and LDL in man and rats. The possible potential mechanism underlying the hypolipidemic effect of CAE and GAE could be due to their high contents of polyphenols (cinnamon) and of gingerols and shogaols (ginger) which inhibit the intestinal absorption of cholesterol with subsequent hypocholesterolemic activity.
Oral administration of CAE and GAE to obese diabetic rats caused hyperinsulinemia. This effect was also reported in previous studies [13,16] in rats. Some previous studies revealed that obesity is commonly associated with hyperinsulinemia and insulin resistance in rats [37] and in humans [38]. The hyperinsulinimic effect of CAE and GAE could be possibly explains their antiobesity effect, which was evident in this study, as obesity is commonly linked with hyperinsulinemia.
Leptin hormone plays a key role in regulating energy intake and energy expenditure. The level of circulating leptin is proportional to the total body fat mass. Oral administration of CAE and GAE to obese diabetic rats significantly decreased serum leptin levels in obese diabetic rats. This result agreed with the results of previous studies that revealed CAE and GAE decreased serum leptin level and depressed appetite in obese rats fed HFD [12] and human volunteers [41].
In obese diabetic rats, alloxan injection to rats induced hyperglycemia that caused renal oxidative stress which plays a key role in the onset and development of diabetes [4,5]. Oral administration of CAE and GAE produced a dose-dependent antioxidant effect. The mechanism underlying the antioxidant effect of CAE and GAE could be attributed to their antidiabetic activity, which reported in this study and previous studies on cinnamon [8,13] and on ginger [15,16].
CONCLUSION
CAE and GAE herbs exhibit significant anti-obesity, hepatoprotective, hypolipidemic, antidiabetic and anti-oxidant effects in obese diabetic rats. These effects confirm the previous reports on cinnamon and ginger extracts. The mechanisms underlying these effects are fully discussed and clarified. This study provides a scientific evidence to substantiate the traditional use of cinnamon and ginger herbs in folk medicine for treating obesity and diabetes. The obese diabetic rat model used in this study is a novel animal model in pharmacology researches and it closely resembles the reality of obesity in humans.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
REFERENCES
- 1.Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity:Possible evolutionary origins. Br J Nutr. 2008;99:931–40. doi: 10.1017/S0007114507853347. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–34. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raza H, John A, Howarth FC. Increased metabolic stress in Zucker diabetic fatty rat kidney and pancreas. Cell Physiol Biochem. 2013;32:1610–20. doi: 10.1159/000356597. [DOI] [PubMed] [Google Scholar]
- 4.Luis-Rodríguez D, Martínez-Castelao A, Górriz JL, De-Álvaro F, Navarro-González JF. Pathophysiological role and therapeutic implications of inflammation in diabetic nephropathy. World J Diabetes. 2012;3:7–18. doi: 10.4239/wjd.v3.i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang GG, Lu XH, Li W, Zhao X, Zhang C. Protective Effects of Luteolin on Diabetic Nephropathy in STZ-Induced Diabetic Rats. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1155/2011/323171. 323171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract. 2003;62:139–48. doi: 10.1016/s0168-8227(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 7.Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, et al. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36:340–4. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 8.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract prevents the insulin resistance induced by a high-fructose diet. Horm Metab Res. 2004;36:119–25. doi: 10.1055/s-2004-814223. [DOI] [PubMed] [Google Scholar]
- 9.Moselhy SS, Ali HK. Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol Res. 2009;42:93–8. [PubMed] [Google Scholar]
- 10.Azab KSh, Mostafa AH, Ali EM, Abdel-Aziz MA. Cinnamon extract ameliorates ionizing radiation-induced cellular injury in rats. Ecotoxicol Environ Saf. 2011;74:2324–9. doi: 10.1016/j.ecoenv.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Vafa M, Mohammadi F, Shidfar F, Sormaghi MS, Heidari I, Golestan B, et al. Effects of cinnamon consumption on glycemic status, lipid profile and body composition in type 2 diabetic patients. Int J Prev Med. 2012;3:531–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Shatwan IA, Ahmed LA, Badkook MM. Effect of barley flour, crude cinnamon, and their combination on glycemia, dyslipidemia, and adipose tissue hormones in type 2 diabetic rats. J Med Food. 2013;16:656–62. doi: 10.1089/jmf.2012.0083. [DOI] [PubMed] [Google Scholar]
- 13.Lee SC, Xu WX, Lin LY, Yang JJ, Liu CT. Chemical composition and hypoglycemic and pancreas-protective effect of leaf essential oil from indigenous cinnamon (Cinnamomum osmophloeum Kanehira) J Agric Food Chem. 2013;61:4905–13. doi: 10.1021/jf401039z. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Azeem AS, Hegazy AM, Ibrahim KS, Farrag AR, El-Sayed EM. Hepatoprotective, antioxidant, and ameliorative effects of ginger (Zingiber officinale Roscoe) and vitamin E in acetaminophen treated rats. J Diet Suppl. 2013;10:195–209. doi: 10.3109/19390211.2013.822450. [DOI] [PubMed] [Google Scholar]
- 15.Akhani SP, Vishwakarma SL, Goyal RK. Anti-diabetic activity of Zingiber officinale in streptozotocin-induced type I diabetic rats. J Pharm Pharmacol. 2004;56:101–5. doi: 10.1211/0022357022403. [DOI] [PubMed] [Google Scholar]
- 16.ElRokh el-SM, Yassin NA, El-Shenawy SM, Ibrahim BM. Antihypercholesterolaemic effect of ginger rhizome (Zingiber officinale) in rats. Inflammopharmacology. 2010;18:309–15. doi: 10.1007/s10787-010-0053-5. [DOI] [PubMed] [Google Scholar]
- 17.Mahmoud RH, Elnour WA. Comparative evaluation of the efficacy of ginger and orlistat on obesity management, pancreatic lipase and liver peroxisomal catalase enzyme in male albino rats. Eur Rev Med Pharmacol Sci. 2013;17:75–83. [PubMed] [Google Scholar]
- 18.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents:Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 19.Shalaby MA, Hamowieh AR. Safety and efficacy of Zingiber officinale roots on fertility of male diabetic rats. Food Chem Toxicol. 2010;48:2920–4. doi: 10.1016/j.fct.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt BA, Dube JJ, Dedousis N, Reider JA, O’Doherty RM. Diet-induced obesity and acute hyperlipidemia reduce IkappaBalpha levels in rat skeletal muscle in a fiber-type dependent manner. Am J Physiol Regul Integr Comp Physiol. 2006;290:R233–40. doi: 10.1152/ajpregu.00097.2005. [DOI] [PubMed] [Google Scholar]
- 21.Ashok DC, Shrimant NP, Panadeep MG, Akalpita UA. Optimization of alloxan dose is essential to induce stable diabetes mellitus for long period. Asian J Biochem. 2007;2:402–8. [Google Scholar]
- 22.Pichon L, Huneau JF, Fromentin G, Tomé D. A high-protein, high-fat, carbohydrate-free diet reduces energy intake, hepatic lipogenesis, and adiposity in rats. J Nutr. 2006;136:1256–60. doi: 10.1093/jn/136.5.1256. [DOI] [PubMed] [Google Scholar]
- 23.Bergmeyer HU, Scheibe P, Wahlefeld AW. Optimization of methods for aspartate aminotransferase and alanine aminotransferase. Clin Chem. 1978;24:58–73. [PubMed] [Google Scholar]
- 24.Roy SE. Colorimetric determination of serum alkaline phosphatase. Clin Chem. 1970;16:431–2. [Google Scholar]
- 25.Richmond N. Colorimetric determination of total cholesterol and high density lipoprotein cholesterol (HDL) Clin Chem. 1973;19:1350–6. [Google Scholar]
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 27.Siest G, Henny F, Schiele F. Enzymatic determination of glucose. Interpret Exam Lab. 1981;2:206–13. [Google Scholar]
- 28.Yallow R, Bauman WA. Plasma insulin in health and disease. In: Ellenberg M, Rifkin H, editors. Diabetes Mellitus:Theory and Practice. Vol. 15. New York: Excerpta Medica; 1983. pp. 119–20. [Google Scholar]
- 29.Xiong Y, Shen L, Liu KJ, Tso P, Xiong Y, Wang G, et al. Antiobesity and antihyperglycemic effects of ginsenoside Rb1 in rats. Diabetes. 2010;59:2505–12. doi: 10.2337/db10-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 31.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 32.Sinha KA. Colorimetric assay of catalase enzyme. Anal Biochem. 1972;47:328–30. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 33.Snedecor GW, Cochran WG. 7th ed. Ames, USA: Iowa State University Press; 1986. Statistical Methods; pp. 90–9. [Google Scholar]
- 34.Kocic R, Pavlovic D, Kocic G, Pesic M. Susceptibility to oxidative stress, insulin resistance, and insulin secretory response in the development of diabetes from obesity. Vojnosanit Pregl. 2007;64:391–7. doi: 10.2298/vsp0706391k. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda M, Shimomura I. Increased oxidative stress in obesity:Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7:e330–41. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Nammi S, Sreemantula S, Roufogalis BD. Protective effects of ethanolic extract of Zingiber officinale rhizome on the development of metabolic syndrome in high-fat diet-fed rats. Basic Clin Pharmacol Toxicol. 2009;104:366–73. doi: 10.1111/j.1742-7843.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- 37.Amin KA, Nagy MA. Effect of Carnitine and herbal mixture extract on obesity induced by high fat diet in rats. Diabetol Metab Syndr. 2009;1:17. doi: 10.1186/1758-5996-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kay JP, Alemzadeh R, Langley G, D’Angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism. 2001;50:1457–61. doi: 10.1053/meta.2001.28078. [DOI] [PubMed] [Google Scholar]
- 39.Friedman JM. Leptin and the regulation of body weight. Keio J Med. 2011;60:1–9. doi: 10.2302/kjm.60.1. [DOI] [PubMed] [Google Scholar]
- 40.Roussel AM, Hininger I, Benaraba R, Ziegenfuss TN, Anderson RA. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. J Am Coll Nutr. 2009;28:16–21. doi: 10.1080/07315724.2009.10719756. [DOI] [PubMed] [Google Scholar]
- 41.Wadikar DD, Premavalli KS. Effect of appetizer administration on plasma leptin level in human volunteers. Int J Food Sci Nutr. 2011;62:148–51. doi: 10.3109/09637486.2010.511606. [DOI] [PubMed] [Google Scholar]


