Abstract
Aim:
The fight against infectious diseases and antimicrobial resistances needs the exploration of new active compounds with new proprieties like disrupting quorum sensing (QS) mechanisms, which is a cell-to-cell communication that regulates bacterial virulence factors. In this work, leaves and root barks extracts of a Congolese medicinal plant, Cordia gilletii, were investigated for their effect on the production of Pseudomonas aeruginosa major virulence factors regulated by QS.
Materials and Methods:
The effect of C. gilletii extracts on virulence factors of P. aeruginosa PAO1 was studied by the evaluation of the production of pyocyanine, elastase and biofilm; and by the measurement of the expression of QS-related genes.
Results:
The dichloromethane extract from root barks was found to quench the production of pyocyanin, a QS-dependent virulence factor in P. aeruginosa PAO1. Moreover, this extract specifically inhibits the expression of several QS-regulated genes (i.e. lasB, rhlA, lasI, lasR, rhlI, and rhlR) and reduces biofilm formation by PAO1.
Conclusion:
This study contributes to explain the efficacy of C. gilletii in the traditional treatment of infectious diseases caused by P. aeruginosa.
KEY WORDS: Antimicrobial, Cordia gilletii, quorum-sensing
INTRODUCTION
Pseudomonas aeruginosa is one of the major causes of nosocomial diseases; it can secrete a diversity of virulence factors and in parallel forms biofilms to ensure the infection success. The production of key virulence factors in P. aeruginosa and other important pathogenic bacteria is regulated by a cell-to-cell communication mechanism known as quorum sensing (QS). This mechanism enables bacteria to detect their population density through the production, release, and perception of small diffusible molecules called autoinducers and to coordinate gene expression accordingly [1]. In P. aeruginosa, two QS systems (las and rhl) drive the production (by the synthetases LasI and RhlI) and the perception (by the transcription factors LasR and RhlR) of the acyl-homoserine lactones (AHLs) N-(3-oxododecanoyl)-L-homoserinelactone (3-oxo-C12-HSL) and N-butanoyl-L-homoserine lactone (C4-HSL), respectively [2]. Once LasR interacts with 3-oxo-C12-HSL, it induces the las system (by increasing lasI expression) and triggers the production of LasB elastase, LasA protease, Apr alkaline protease, and exotoxin A [3]. RhlR interacts with C4-HSL, resulting in an enhancement of the production of rhamnolipids, pyocyanin, LasB elastase, hydrogen cyanide, and cytotoxic lectins [3-5]. In addition, biofilm formation and maturation is also regulated by las system [6,7] and indirectly by rhl system under nutritional condition [8,9]. Indeed, some studies demonstrated the role of rhamnolipids in biofilm architecture and maintenance [10-12]. The las and the rhl systems are organized in a hierarchical manner where the las system regulates the rhl system at the transcriptional and posttranscriptional levels [2,13,14]. In addition, P. aeruginosa releases a third intercellular signal, 2-heptyl-hydroxy-4-quinolone (designated the Pseudomonas quinolone signal), which interacts with the AHL systems in an intricate way [15] and acts as a link between the las and rhl quorum-sensing systems [16].
Since fundamental virulence processes in many pathogenic bacteria are regulated by QS systems, an interesting strategy to overcome the emergence of antibiotic-resistant microorganisms is to interfere with this cell-to-cell communication mechanism in order to attenuate their virulence [17]. Thus, medicinal plants traditionally used to treat infectious diseases should be screened, not only for their antimicrobial properties, but also for their capacity to inhibit QS mechanisms in bacteria.
In this study, we investigated the QS inhibitory (QSI) effects of extracts from a Congolese medicinal plant, Cordia gilletii De Wild. The root barks extracts from this plant species are used for the treatment of malaria and diarrhea (decoction), for wounds and skin diseases (topical application), whereas leaves decoction is used against malaria [18].
MATERIALS AND METHODS
Plant Material and Extracts Preparation
Root barks and leaves of C. gilletii were collected in Kisantu area the (Democratic Republic of Congo) in January 2005, and voucher specimen has been deposited under the number BR-SP.627986 at the National Botanical Garden of Meise, Belgium. Powders of the two-plant parts were exhaustively and successively extracted with solvents of increasing polarity (n-hexane, dicholoromethane, ethyl acetate and methanol). The evaporation of solvents in Buchi® rotavapor yielded crude extracts, which were dissolved in dimethyl sulfoxide (DMSO) at a final concentration of 10 mg/ml.
Bacterial Strains and Oulture Conditions
P. aeruginosa PAO1 wild-type and reporter strains were grown in liquid LB cultures (5 ml) supplemented with 50 mM 3-(N-morpholino) propanesulfonic acid (MOPS; pH 7.0) at 37°C supplemented with carbenicillin (300 µg/ml) when appropriate as described previously [19,20]. For the detection of anti-QS activity, we used reporter strains including six PAO1-derived strains harboring QS-related promoter-lacZ fusions (lasB-lacZ; rhlA-lacZ; rhlI-lacZ, rhlR-lacZ; lasI-lacZ and lasR-lacZ), and PAO1-derived strains harboring QS-independent aceA gene (aceA-lacZ), described previously [19,20].
Quantitative Analysis of Pyocyanin and Elastase Production in P. aeruginosa PAO1
Inhibition of pyocyanin and elastase production in P. aeruginosa PAO1 wild type was assessed according to previously described procedures [21,22]. Briefly, P. aeruginosa PAO1 were grown overnight polystyrene tube containing 5 ml of LB-MOPS medium (37°C and agitation at 175 r.p.m). The cells were washed twice in fresh LB-MOPS medium, and the pellets were suspended in LB-MOPS medium. Then, 50 µl portions of the cell suspension were added to 940 ml of LB-MOPS, spectrometrically evaluated at 600 nm (in order to obtain a A600 ranging between 0.020 and 0.025, corresponding to ~107 CFU/ml) using a SpectraMax M2 device (Molecular Devices, California, USA) and supplemented with 10 µl of DMSO (1% [vol/vol], final concentration) or 10 µl of plant extract dissolved in DMSO (100 µg/ml, final concentration). After 18 h of growth, samples were taken to assess the growth (A600). After centrifugation (16,000 ×g, 5 min), 900 µl of supernatant were mixed with 500 µl of chloroform in eppendorf tube. The organic phase was transferred in a new eppendorf tube and pyocyanin was extracted with 300 µl of HCl 0.2 N and quantified spectrometrically at 380 nm [19]. LasB elastase production was assessed through the measurement of elastase activity using elastin-Congo Red (A495 nm). The statistical significance of each test (n = 6) was evaluated by conducting Student’s t-tests using the GraphPad Prism software (GraphPad software Inc., CA, USA), and a P ≤ 0.01 was considered significant.
Gene Expression and Beta-galactosidase Measurements
PAO1 reporter strains were prepared as described for pyocyanin quantification (see previous section). PAO1 strains (50 µl) were grown in 940 µl of LB medium at 37°C under agitation (175 r.p.m), supplemented with 10 µl of plant extract or naringenin (4 mM, final concentration) or DMSO (1% [vol/vol], final concentration) and incubated for 18 h. After incubation, the cell growth was assessed as previously and the absorbance of the medium after centrifugation of the bacteria (16,000 ×g, 5 min) was used as a blank. The sample used for cell growth assessment was used to perform the b-galactosidase assay with o-nitrophenyl-b-D-galactopyranoside as previously described [11]. Promoterless lacZ fusion strains were used as controls. The A600 values were measured to account for the differences in cell density [19]. All experiments were performed in six replicates.
Biofilm Quantification
Quantification of biofilm formation by P. aeruginosa PAO1 was assessed according to previously described procedures [23]. PAO1 cells were incubated statically for 24 h at 37°C in 24-well polystyrene plates containing biofilm broth medium (Na2HPO4 1.25 g/L, FeSO4.7H2O 0.0005 g/L, glucose 0.05 g/L, (NH4)2SO4 0.1 g/L, MgSO4.2H2O 0.2 g/L, KH2PO4 0.5 g/L) supplemented with plant extract (100 µg/ml) or DMSO 1% or naringenin (4 mM). After 24 h of incubation, biofilm biomass was quantified via crystal violet staining. All experiments were performed in six replicates.
RESULTS
C. gillettii Root Barks and Leaves Extracts Reduce Pyocyanin and Elastase Production in P. aeruginosa PAO1
C. gilletii root barks and leaves extracts were investigated for their effect on pyocyanin production. As shown in Figure 1a, all tested extracts decreased drastically pyocyanin production with no significant effect on P. aeruginosa PAO1 growth when compared to the DMSO control. Besides, all extracts (except root barks methanol and leaves dichloromethane extracts) decrease significantly elastase production in PAO1 although less spectacular compared to pyocyanin reduction [Figure 1b]. In addition, no elastase-like activities (which could interfere with the tests) were observed when the extracts were used in bacteria-free control tests (data not shown).
Figure 1.
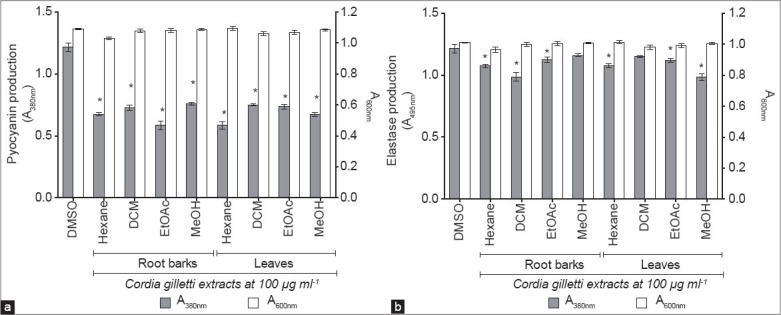
Effect of Cordia gilletii root barks and leaves extracts on pyocyanin and elastase production in Pseudomonas aeruginosa PAO1. (a) Effect of C. gilletii root barks and leaves extracts on pyocyanin production in P. aeruginosa PAO1. (b) Effect of C. gilletii root barks and leaves extracts on elastase production in P. aeruginosa PAO1 (Hexane: n-hexane, DCM: Dichloromethane, EtOAc: Ethyl acetate, MeOH: Methanol). Dimethyl sulfoxide (DMSO): control. *Significance at P < 0.001. All experiments were performed in six replicates
Root Barks Dichloromethane (RBDCM) Extract and Leaves Methanol Extract Reduce lasB and rhlA Gene Expression in P. aeruginosa PAO1
In the case of the decrease of pyocyanin and elastase production was due to the interference of C. gilletii extracts with QS mechanisms, we assessed the impact of C. gilletii extracts on QS-regulated genes lasB and rhlA genes (coding for lasB elastase and rhamnolipid, respectively) expression. Therefore, the effect of C. gilletii extracts on lasB and rhlA genes expression was monitored by using two PAO1 reporter strains harboring QS-related (lasB and rhlA) promoter-lacZ fusions. PAO1 reporter strain harboring QS-independent aceA gene (coding for isocitrate lyase) was used to verify that the drop in b-galactosidase activity was indeed associated with a reduction in QS-related gene expression rather to a general effect on transcription/translation mechanisms. Naringenin, a flavanone, which is known to affect QS signaling in P. aeruginosa PAO1 without affecting bacterial growth [20], was used as a positive control. As shown in Figure 2, the results highlight that RBDCM extract and leaves methanol extract, at final concentration of 100 µg/ml reduce QS-regulated lasB and rhlA genes expression without affecting PAO1 cells growth. Indeed, colony-forming unit of P. aeruginosa PAO1 wild-type and reporter strains grown in the presence of extracts for 18 h were similar to those of DMSO-treated cells (data not shown). More interesting, RBDCM extract does not affect the expression of the control gene aceA [Figure 2c], contrarily to the leaves methanol extract. However, effects of leaves methanol extract on QS-independent aceA gene and QS-regulated (lasB and rhlA) genes may be the results of two or more different active compounds. Indeed, some compounds could do affect specifically the expression of QS-related genes and others the expression of QS-independent aceA gene and/or the transcription machinery without affecting PAO1 cells growth.
Figure 2.
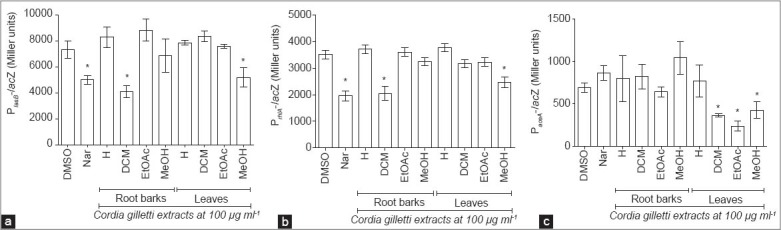
Effect of Cordia gilletii extracts on quorum sensing (QS)-regulated genes (a: lasB; b: rhlA) expressions, and QS-independent aceA gene (c) in Pseudomonas aeruginosa PAO1. Gene expression was measured as the b-galactosidase activity of the lacZ gene fusions expressed in Miller units. Root barks and leaves extracts were tested at 100 µg/ml (Hexane: n-hexane, DCM: Dichloromethane, EtOAc: Ethyl acetate, MeOH: Methanol). Dimethyl sulfoxide (DMSO) (1% [vol/vol], final concentration) was used as solvent control and naringenin (Nar: 4 mM, final concentration) as QS inhibitory control. *Significance at P < 0.05. All experiments were performed in six replicates
RBDCM Extract Affects the Expression of QS Regulator Genes in P. aeruginosa
Since QS-regulated (lasB and rhlA) genes expression is impaired by RBDCM extract of C. gillettii, we were interested in its effect on QS systems (lasRI and rhlRI) in P. aeruginosa PAO1. Therefore, the effect of root barks extract was further characterized by evaluating the expression of the AHL synthetase genes lasI and rhlI and the QS regulator genes lasR and rhlR. The results highlight that the RBDCM affects both QS systems (lasRI and rhlRI) [Figure 3]. Indeed, RBDCM inhibits significantly the expression of AHL synthetase genes lasI (24% ± 5% of inhibition) and rhlI (52% ± 5% of inhibition), and of the QS regulator genes lasR (25% ± 3% of inhibition) and rhlR (23% ± 4% of inhibition).
Figure 3.
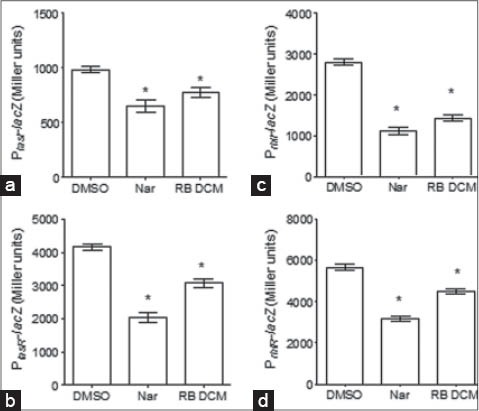
Effect of root barks dichloromethane (RBDCM) extract in Pseudomonas aeruginosa quorum sensing (QS) regulator genes (a: lasI; b: lasR; c: rhlI; d: rhlR). RBDCM extract was tested at 100 µg/ml. Dimethyl sulfoxide (1% [vol/vol], final concentration) was used as solvent control and naringenin (Nar: 4 mM, final concentration) as QS inhibitory control. *Significance at P < 0.05. All experiments were performed in six replicates
RBDCM Extract Inhibit in Biofilm Formation by P. aeruginosa PAO1
Since biofilm formation is partially controlled by QS mechanisms [6,7], the effect of RBDCM extract on P. aeruginosa PAO1 biofilm formation was assessed after 24 h. Noticeably, there were a significant decrease (21% ± 5% of inhibition) in biofilm formation when strain PAO1 was grown in the presence of RBDCM extract (100 µg/ml) compared with that of the negative control (DMSO) [Figure 4].
Figure 4.
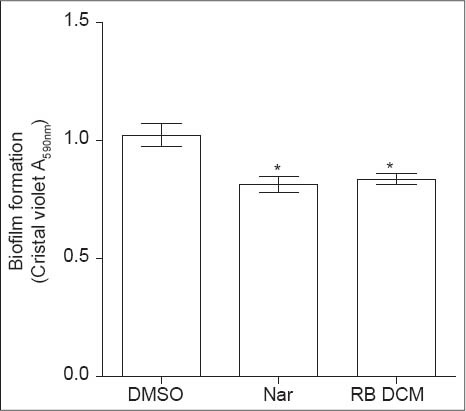
Effect of root barks dichloromethane (RBDCM) extract in biofilm formation by Pseudomonas aeruginosa PAO1. After 24 h of static incubation, biofilm biomass was quantified by using crystal violet staining. RBDCM extract was tested at 100 μg/ml. Dimethyl sulfoxide (1% [vol/vol], fi nal concentration) was used as solvent control and naringenin (Nar: 4 mM, final concentration) as quorum sensing inhibitory control. *Signifi cance at P < 0.05. All experiments were performed in six replicates
DISCUSSION
Few studies have already been reported the anti-QS effects of plants traditionally used in the treatment of infectious diseases [23-26]. C. gilletii belongs to the family of Boraginaceae and it is used in Congolese traditional medicine. Previously we have shown direct and indirect antimicrobial activities against pathogenic microorganisms [27]. To the best of our knowledge, none of the members of this plant family has been screened so far for inhibitory effects on QS, except for an interfering effect in the Vibrio fischerii bioluminescence [28].
In the present investigation, we have shown that P. aeruginosa PAO1 growth is not affected by any of the tested C. gilletii root barks or leaves extracts (at 100 µg/ml final concentration). Besides, by using a reporter strain coupled to aceA gene to evaluate the effect of the C. gilletii extracts on gene transcription machinery, we have discarded all leaves extracts as well as hexane, ethyl-acetate and methanol root barks extracts. Accordingly, only dichloromethane root barks extract was found to specifically reduce in the same time QS-dependent virulence factors (pyocyanin and elastase) production, QS-regulated genes (lasB and rhlA) expression as well as QS-regulatory genes, suggesting the occurrence of a tissue specific compound(s) in C. gilletii that affect QS machinery in P. aeruginosa PAO1. However, we cannot exclude that other tested extract contain QSI compounds, particularly for leaves methanol extract which inhibits transcription of the QS-regulated lasB and rhlA genes and QS-independent aceA gene. Moreover, inhibition kinetic analysis should be led in order to detect time points in which the highest inhibition level of the QS phenotype and the QS genes could be recorded.
Biofilm formation in P. aeruginosa represents a protective mode of growth which may enhance bacterial survival under conditions of environmental stress [29]. Interestingly, RBDCM extract was found to specifically reduce biofilm formation by P. aeruginosa PAO1, which could be attributed to its QSI propriety. However, Shrout et al. [9] demonstrated that the QS dependence of biofilm formation is nutritionally conditional (i.e., QS systems are needed for biofilm formation in growth media with succinate as the sole carbon source but not glucose). Accordingly, as we used glucose as the sole carbon source, we cannot amputate biofilm reduction in the presence of RBDCM extract to the sole QS systems disruption.
Since root barks of C. gilletii are known to contain phenolic compounds [27], this class of molecules could represent one of the putative active compounds as some of them, catechin [19], naringenin [20] and perbergin [30] have already demonstrated anti-QS effect. However, QSI compounds from C. gilletii can be a new class of chemical structure compared with those flavonoids reported elsewhere and may show a different mechanism of inhibition. Besides, at this stage we do not have sufficient data to speculate the quorum inhibitory mechanism and the transcriptional and/or post-transcriptional level of interference of root barks of C. gilletii.
CONCLUSION
This study highlights anti-virulence propriety of C. gilletii, which could contribute to explain its efficacy in the traditional treatment of infectious diseases caused by P. aeruginosa. Further investigations are needed in order to identify the chemical nature of compound(s) responsible for these observed effects. Isolated compounds will have a greater advantage for human use and search for such compounds may contribute to the prevention of bacterial diseases without the concern of antibiotic resistance. Finally, we must point out that C. gilletii, although belonging to the family of Boraginaceae, one of the most important botanical families of plants producing pyrrolizidine alkaloids (PAs), does not harbor these alkaloids in investigated root barks and leaves samples (detection limit, 2 µg of PAs per gram of plant material) [31], excluding thus toxicological risk due to PAs and ensuring a probable safe use of this plant.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
REFERENCES
- 1.Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67:351–68. doi: 10.2165/00003495-200767030-00003. [DOI] [PubMed] [Google Scholar]
- 2.Pesci EC, Pearson JP, Seed PC, Iglewski BH. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3127–32. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2012;76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta RK, Setia S, Harjai K. Expression of quorum sensing and virulence factors are interlinked in Pseudomonas aeruginosa:An in vitro approach. Am J Biomed Sci. 2011;3:116–25. [Google Scholar]
- 5.Reis RS, Pereira AG, Neves BC, Freire DM. Gene regulation of rhamnolipid production in Pseudomonas aeruginosa –A review. Bioresour Technol. 2011;102:6377–84. doi: 10.1016/j.biortech.2011.03.074. [DOI] [PubMed] [Google Scholar]
- 6.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–8. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 7.Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol. 2002;184:1140–54. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patriquin GM, Banin E, Gilmour C, Tuchman R, Greenberg EP, Poole K. Influence of quorum sensing and iron on twitching motility and biofilm formation in Pseudomonas aeruginosa. J Bacteriol. 2008;190:662–71. doi: 10.1128/JB.01473-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrout JD, Chopp DL, Just CL, Hentzer M, Givskov M, Parsek MR. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol Microbiol. 2006;62:1264–77. doi: 10.1111/j.1365-2958.2006.05421.x. [DOI] [PubMed] [Google Scholar]
- 10.Chrzanowski L, Lawniczak L, Czaczyk K. Why do microorganisms produce rhamnolipids? World J Microbiol Biotechnol. 2012;28:401–19. doi: 10.1007/s11274-011-0854-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davey ME, Caiazza NC, O’Toole GA. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J Bacteriol. 2003;185:1027–36. doi: 10.1128/JB.185.3.1027-1036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soberón-Chávez G, Lépine F, Déziel E. Production of rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol. 2005;68:718–25. doi: 10.1007/s00253-005-0150-3. [DOI] [PubMed] [Google Scholar]
- 13.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol. 1996;21:1137–46. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 14.Medina G, Juárez K, Díaz R, Soberón-Chávez G. Transcriptional regulation of Pseudomonas aeruginosa rhlR, encoding a quorum-sensing regulatory protein. Microbiology. 2003;149:3073–81. doi: 10.1099/mic.0.26282-0. [DOI] [PubMed] [Google Scholar]
- 15.Wade DS, Calfee MW, Rocha ER, Ling EA, Engstrom E, Coleman JP, et al. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. J Bacteriol. 2005;187:4372–80. doi: 10.1128/JB.187.13.4372-4380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKnight SL, Iglewski BH, Pesci EC. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 2000;182:2702–8. doi: 10.1128/jb.182.10.2702-2708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruimy R, Andremont A. Quorum sensing in Pseudomonas aeruginosa:Molecular mechanism, clinical impact, and inhibition. Réanimation. 2004;13:176–84. [Google Scholar]
- 18.Kambu K. Kinshasa: C.R.P; 1990. Eléments de Phytothérapie Comparée:Plantes Médicinales Africaines. [Google Scholar]
- 19.Vandeputte OM, Kiendrebeogo M, Rajaonson S, Diallo B, Mol A, El Jaziri M, et al. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl Environ Microbiol. 2010;76:243–53. doi: 10.1128/AEM.01059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandeputte OM, Kiendrebeogo M, Rasamiravaka T, Stévigny C, Duez P, Rajaonson S, et al. The flavanone naringenin reduces the production of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Microbiology. 2011;157:2120–32. doi: 10.1099/mic.0.049338-0. [DOI] [PubMed] [Google Scholar]
- 21.Ishida T, Ikeda T, Takiguchi N, Kuroda A, Ohtake H, Kato J. Inhibition of quorum sensing in Pseudomonas aeruginosa by N-acyl cyclopentylamides. Appl Environ Microbiol. 2007;73:3183–8. doi: 10.1128/AEM.02233-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müh U, Schuster M, Heim R, Singh A, Olson ER, Greenberg EP. Novel Pseudomonas aeruginosa quorum-sensing inhibitors identified in an ultra-high-throughput screen. Antimicrob Agents Chemother. 2006;50:3674–9. doi: 10.1128/AAC.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasamiravaka T, Jedrzejowski A, Kiendrebeogo M, Rajaonson S, Randriamampionona D, Rabemanantsoa C, et al. Endemic malagasy Dalbergia species inhibit quorum sensing in Pseudomonas aeruginosa PAO1. Microbiology. 2013;159:924–38. doi: 10.1099/mic.0.064378-0. [DOI] [PubMed] [Google Scholar]
- 24.Adonizio A, Kong KF, Mathee K. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother. 2008;52:198–203. doi: 10.1128/AAC.00612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adonizio AL, Downum K, Bennett BC, Mathee K. Anti-quorum sensing activity of medicinal plants in Southern Florida. J Ethnopharmacol. 2006;105:427–35. doi: 10.1016/j.jep.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Tan LY, Yin WF, Chan KG. Silencing quorum sensing through extracts of Melicope lunu-ankenda. Sensors (Basel) 2012;12:4339–51. doi: 10.3390/s120404339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okusa PN, Penge O, Devleeschouwer M, Duez P. Direct and indirect antimicrobial effects and antioxidant activity of Cordia gilletii De Wild (Boraginaceae) J Ethnopharmacol. 2007;112:476–81. doi: 10.1016/j.jep.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Okusa PN, Stévigny C, Devleeschouwer M, Duez P. Optimization of the culture medium used for direct TLC–bioautography. Application to the detection of antimicrobial compounds from Cordia gilletii de wild (boraginaceae) J Planar Chromatogr Mod TLC. 2010;23:245–9. [Google Scholar]
- 29.Webb JS, Givskov M, Kjelleberg S. Bacterial biofilms:Prokaryotic adventures in multicellularity. Curr Opin Microbiol. 2003;6:578–85. doi: 10.1016/j.mib.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Rajaonson S, Vandeputte OM, Vereecke D, Kiendrebeogo M, Ralambofetra E, Stévigny C, et al. Virulence quenching with a prenylated isoflavanone renders the Malagasy legume Dalbergia pervillei resistant to Rhodococcus fascians. Environ Microbiol. 2011;13:1236–52. doi: 10.1111/j.1462-2920.2011.02424.x. [DOI] [PubMed] [Google Scholar]
- 31.Okusa PN, Beuerle T, Stévigny C, Duez P. Absence of pyrrolizidine alkaloids in Cordia gilletii de wild (boraginaceae) Biochem Syst Ecol. 2012;41:1–2. [Google Scholar]


