Abstract
Aim:
The present study investigates synergistic effect of combination treatment of lycopene, quercetin and poloxamer188 in 3-nitropropionic acid (3-NP)-induced Huntington’s disease (HD).
Materials and Methods:
Anxiety and depression were induced in male Wistar rat by intra-peritoneal administration of 3-NP (10 mg/kg) for 14 days. Body weight was assessed on day 1, 7 and 14, whereas locomotion, anxiety and depression were assessed at the end of the experiment.
Results:
Administration of 3-NP induces HD like symptoms and produced a significant decrease in body weight on day 7 (P < 0.01)and day 14 (P < 0.001), further decreased locomotion, time spent and number of entries in light area as well as increased immobility period were observed. The rats treated with lycopene and quercetin alone significantly restore the body weight and locomotion count as well as alleviate anxiety and depression. However, combination treatment of lycopene and quercetin with and without poloxamer 188 produced more significant effect on body weight compared to Huntington control rats, but no significant effect was found between the treated groups. However, significant increased on locomotion, time spent and number of entries in light area and decreased immobility period was observed in the combination treated groups when compared to single drug therapy.
Conclusion:
Combination treatment of lycopene and quercetin with and without poloxamer 188 in HD more effectively alleviate anxiety and depression than single drug therapy.
KEY WORDS: Huntington disease, 3-nitropropionic acid, lycopene, quercetin
INTRODUCTION
Huntington’s disease (HD) is a neurodegenerative disorder characterized by progressive motor dysfunction, chorea, dystonia, emotional disturbances, memory and weight loss [1]. Today about 5-7 people/100,000 are affected by HD and in India pervasiveness of HD is somewhat higher [2]. Incidence of juvenile HD is in between 1% and 9.6% [3]. The symptoms of HD usually develop at the age of 30-50 and severity increases with age [4]. HD is caused by a CAG polyglutamine expansion, which is more than 35, produced malformed Huntington protein, results death of nerve cell in the basal ganglion [5-8]. The malformed protein also affects cellular pathways and hippocampal neurogenesis involved in mood disorders leads to depression and anxiety [9]. In caudate, putamen, and cingulate low glucose metabolism in HD results in dysfunctioning of paralimbic frontal lobes and basal ganglia due to energy impairment. This leads to depression as they are responsible for normal mood regulation [10].
7 (3-NP) is a well-versed experimental model to study HD in various animal models [11]. It produces mitochondrial dysfunction, oxidative stress and inhibits mitochondrial Complex II enzyme results in neuronal cell death due to energy impairment and apoptosis [12-14]. Further 3-NP increases the production of nitric oxide and depresses the spinal reflexes in a time-dependent manner that leads to depression [15]. Increased oxidative stress causes striatal damage and produce anxiety [16].
Currently, no treatment is available for HD, though some symptoms can be managed with medication such as antidepressant, antipsychotic and therapies such as physiotherapy and occupational therapy. Flavonoids (or bioflavonoid) are a class of plant secondary metabolites reported as antioxidant, anti-allergic, anti-cancer, antioxidant, anti-inflammatory and anti-viral [17]. Carotenoids are organic pigments found in the chloroplasts of bacteria and fungi. It decreases the risk of disease particularly certain cancers and eye disease due to its antioxidant potential [18]. Several studies reported synergistic effect of bioflavonoid and carotenoids combinations [19]. Further, lycopene and quercetin in combination with tyrosol were proved synergistic effect in the prevention of macrophage activation [20]. The treatment with lycopene significantly improved memory and restored glutathione system functioning and hence lycopene could be used to manage 3-NP induced behavioral and biochemical alterations [21]. Quercetin improved mitochondrial dysfunctions and antioxidant status and also ameliorate behavioral deficits along with histopathological changes [22]. Poloxamer 188, which is a polymer has ability to repair damaged cell membranes by increasing the lipid packing density as it provides mechanical sealing to neurons [23].
Therefore the present study designed to investigates synergistic effect of combinatorial treatment of lycopene, quercetin and poloxamer 188 on anxiety and depression in 3-NP-induced HD in Wistar rats.
MATERIALS AND METHODS
Drugs and Chemical
Lycopene was obtained as a gift sample from Zedip formulation (Ahemdabad, India). Quercetin and poloxamer 188 were procured from Research Lab Fine Chem. Industries (Mumbai, India). 3-NP was purchased from Sigma Aldrich (USA). All other reagents and chemicals were of analytical grade and purchased from local suppliers of Pune.
Animals
Male Wistar rats (200-250 g) were procured from National Institute of Biosciences, Pune. Rats were randomly placed separately in polypropylene cages with paddy husk as bedding. They were housed in environmentally controlled conditions (24 ± 2°C, 12 h light/12 h dark cycle), with free access to the standard diet (Nutrivet Lab., Pune) ad libitum. All the experimental procedures and protocols used in this study were reviewed and approved (SCOP/Institutional Animal Ethics Committee [IAEC]/2013/14/155) by the IAEC of Sinhgad College of Pharmacy, Pune, constituted under Committee for Purpose of Control and Supervision of Experiments on Animals by Ministry of Environment and Forests, Government of India, New Delhi, India. Ethical guidelines were strictly followed during all the experimental procedures. All efforts were made to minimize animal suffering and reduce the number of animals used.
Experimental Design
After 1 week of acclimatization, male Wistar rats were randomly divided into six groups (n = 6) and received treatment for 14 days. Group I served as control, received vehicle 1% gum acacia (w/v), Group II rats were injected with 3-NP served as Huntington control (HC), Group III-VI were injected with 3-NP and concomitantly treated orally with lycopene (25 mg/kg), quercetin (50 mg/kg), lycopene (25 mg/kg) and quercetin (50 mg/kg), and lycopene (25 mg/kg), quercetin (50 mg/kg) and poloxamer 188 (80 mg/kg) respectively. 3-NP was freshly prepared using distilled water and administered at a dose of 10 mg/kg body weight for 14 days. The lycopene, quercetin and poloxamer 188 were administered as a suspension prepared in 1% gum acacia (w/v) for 14 days. The behavioral observations were taken in between 9:00 A.M. and 11:00 A.M.
Evaluation of Body Weight
As the disease progresses swallowing become more difficult thereby decreased intake of calories results in body weight loss. Body weight was measured on 1st (before treatment), 7th, and 14th day post 3-NP administration using electronic weighing balance (Contech, C7-6K1)[24].
Evaluation of Locomotor Activity
As HD progresses, impairment in motor functions alters muscular movements and reduces the locomotor activity. Animals were placed in actophotometer where beam of light falls on photoelectric cells, and basal activity score was recorded over the period of 5 min that is recorded as no. of beams cut during locomotions [25].
Light and Dark Model
Neuropsychiatric problems are common in HD. Light-dark box is a potent and useful method for screening and detecting anxiolytics activity of a wide range of compounds with various modes of action. The instrument consists of 2 parts, 1/3 with opaque walls and covered (dark compartment), whereas the remaining 2/3 was open and illuminated (light compartment). The door between the two compartments permits rats to move from one side to another. Each rat was released in the light compartment and observed for 5 min. Time spent in light and dark compartment, and no. of entries in light, and dark compartment were recorded [26,27].
Forced Swim Test
Forced swim test is the most commonly used for assessment of depression in animal models. Rats were forced to swim in a cylinder from which they cannot escape. The water was deep enough, so the animal could not touch the bottom with its tail or feet. A depth of 30 cm is commonly recommended with temperature 24-30°C. Animals were observed continuously for 5 min during the swim test, and duration and time of immobility were measured. Any animal that sinks below the surface was removed from the water immediately [28,29].
Statistical Analysis
All the values are expressed as mean ± standard error of the mean (n = 6). The data were analyzed using one-way analysis of variance, followed by Tukey’s multiple comparison tests. P < 0.05 were considered the minimum level of significance.
RESULTS
Body Weight
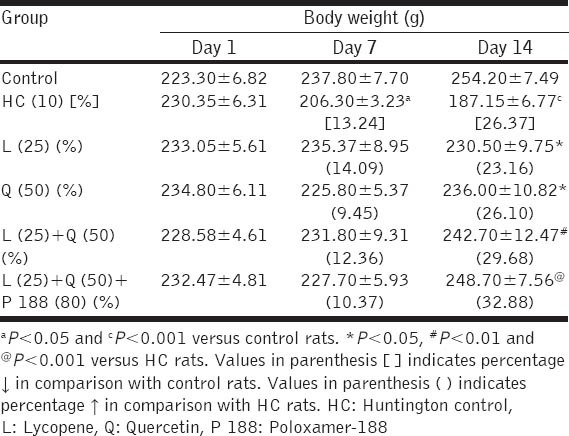
Intra-peritoneal administration of 3-NP for 14 days showed significant decrease in the body weight in HC rats when compared with control rats at day 7 and day 14 (13.24%, P < 0.05 and 26.37%, P < 0.001; respectively). Lycopene (25 mg/kg) and quercetin (50 mg/kg) as well as their combination with and without poloxamer 188 treatments in rats could not produce significant restoration in body weight on day 7. However, on 14th day significant restoration in the body weight was observed in rats treated with lycopene (23.16%) and quercetin (26.10%) when compared with HC rats (P < 0.05 and P < 0.05; respectively). Further, combination treatment of lycopene (25 mg/kg) and quercetin (50 mg/kg) with and without poloxamer 188 found to produce more significant restoration in the body weight compared with HC rats (29.68%; P < 0.01 and 32.88%; P < 0.01; respectively). However, no significant difference was found between the drug-treated groups [Table 1].
Table 1.
Effect of combination treatment of lycopene, quercetin and poloxamer 188 on body weight
Locomotor Activity
Significant decrease in the number of locomotions was observed in 3-NP treated HC rats when compared with control rats (P < 0.001). Rats treated with quercetin (50 mg/kg) along with 3-NP restored the number of locomotion compared with HC rats (P < 0.01). Further, combination treatment of lycopene (25 mg/kg) and quercetin (50 mg/kg) with and without poloxamer188 more significantly restored the number of locomotions as compared to HC rats (P < 0.001 and P < 0.001; respectively). Rats treated with lycopene (25 mg/kg) could not produce significant change in the locomotion counts. However, rats treated with lycopene and quercetin with or without poloxamer showed a significant increase in locomotion count when compared with lycopene (25 mg/kg) treated rats (P < 0.05 and P < 0.01; respectively) [Figure 1].
Figure 1.
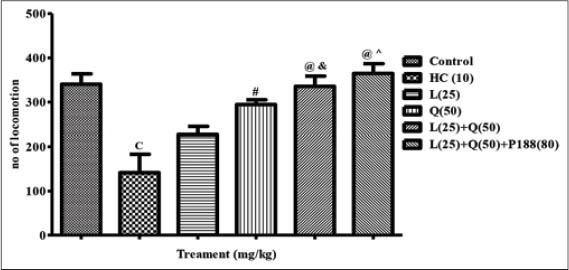
Effect of combination treatment of lycopene, quercetin and poloxamer188 on locomotor activity. cP < 0.001 versus control; #P < 0.01,@P < 0.001 versus HC; &P < 0.05, ^P < 0.01 versus L (25), HC: Huntington control, P: Lycopene, Q: Quercetin, P 188: Poloxamer 188
Light and Dark Model
Significantly decreased duration of time spent in light area and increased in the dark area was observed in HC rats injected with 3-NP for 14 days when compared with control rats (P < 0.001 and P < 0.001; respectively). Rats treated with lycopene and quercetin alone along with 3-NP increased the duration of time spent in light area (P < 0.001 and P < 0.01; respectively) and decreased in dark area (P < 0.001 and P < 0.001; respectively) when compared with HC rats. Further, combination treatment of lycopene (25 mg/kg) and quercetin (50 mg/kg) with and without poloxamer 188 more significantly increased the duration of time spent in light area (P < 0.001 and P < 0.01; respectively) and decrease in duration of time spent in dark area as compared to HC rats (P < 0.001 and P < 0.001; respectively). However the significant difference were observed in the rats treated with lycopene and quercetin combination with and without poloxamer 188 in the duration of time spent in light area (P < 0.001 and P < 0.01; respectively) and dark area (P < 0.001 and P < 0.001; respectively) when compared with quercetin treated rats. However, no significant change was observed in comparison with lycopene.
Further, a significant decrease in the number of entries in light area and increase in the dark area was observed in HC rats when compared with control rats (P < 0.001 and P < 0.001; respectively). Rats treated with lycopene and quercetin alone significantly increased the number of entries in light area (P < 0.001 and P < 0.01; respectively) and decreased in dark area (P < 0.001 and P < 0.001; respectively) when compared with HC rats. Further, combination treatment of lycopene (25 mg/kg) and quercetin (50 mg/kg) with and without poloxamer 188 more significantly increased the number of entries in light area (P < 0.001 and P < 0.001; respectively) and decrease in number of entries in dark area as compared to HC rats (P < 0.001 and P < 0.001; respectively). However, rats treated with lycopene, quercetin and poloxamer 188 combination produced significant decrease in the number of entries in the dark area when compared with lycopene, quercetin and their combination without poloxamer 188 treatment (P < 0.001, P < 0.001 and P < 0.05; respectively) [Figure 2].
Figure 2.
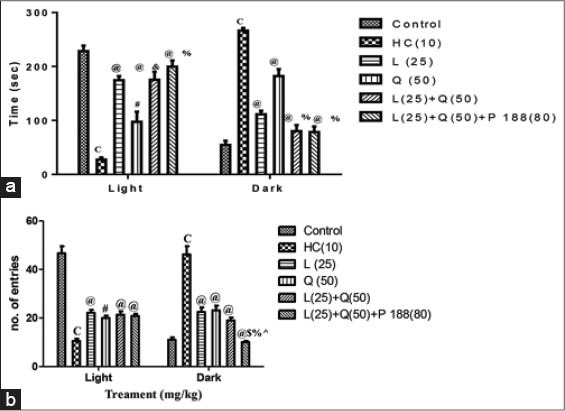
Effect of combination treatment of lycopene, quercetin and poloxamer188 on anxiety (a) time spent in light and dark area and (b) number of entries in light and dark area, cP < 0.001 versus control; #P < 0.01 and @P < 0.001 versus HC; &P < 0.01, %P < 0.001 versus Q (50); $P < 0.001 versus L (25); ^P < 0.05 versus L (25) and Q (50) combination, HC: Huntington control, P: Lycopene, Q: Quercetin, P 188: Poloxamer 188
Forced Swim Test
Significant decrease in the onset of immobility and increase duration of immobility was observed in HC rats when compared with control rats (P < 0.001 and P < 0.001; respectively). Rats treated with lycopene and quercetin alone along with 3-NP increase onset of immobility (P < 0.001 and P < 0.05; respectively) and decrease duration of immobility (P < 0.001 and P < 0.01; respectively) when compared with HC rats. Further, combination treatment of lycopene (25 mg/kg) and quercetin (50 mg/kg) with and without poloxamer 188 along with 3-NP more significantly increased onset of immobility (P < 0.001 and P < 0.001; respectively) and decreased duration of immobility compared with HC rats (P < 0.001 and P < 0.001; respectively). Further the significant difference was noted on the duration of immobility in lycopene and quercetin with or without poloxamer 188 treated rats, but on the onset of immobility significant difference was observed only with poloxamer188 combination when compared with quercetin treated rats (P < 0.001 and P < 0.01 and P < 0.01; respectively) [Figure 3].
Figure 3.
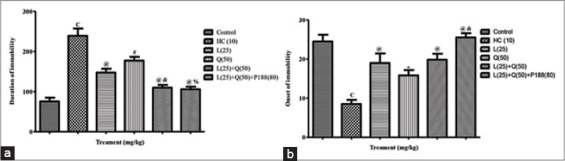
Effect of combination treatment of lycopene, quercetin and poloxamer188 on depression (a) duration of immobility (b) onset of immobility. cP < 0.001 versus control; *P < 0.05, #P < 0.01 and @P < 0.001 versus HC; &P < 0.01, %P < 0.001 versus Q (50) HC: Huntington control, P: Lycopene, Q: Quercetin, P 188: Poloxamer 188
DISCUSSION
HD is a disorder in which nerve cells in certain parts of the brain waste away, or degenerate [30]. HD causes movement, psychiatric and cognitive difficulties [31]. In HD increase in oxidative stress and neuronal loss alters neurotransmitters that regulate mood results in depression and anxiety. In HD, there is a continual life change, which may be one of the sources of anxiety [32]. Strong evidences suggest involvement of energy impairment, excitotoxic processes, and apoptosis worsen the symptoms of HD. Striatum and hippocampus are more affected because nerve cells of the striatum are first to die as HD progresses [33]. HD is not a prevalent within any particular population, races, ethnic groups, sexes. Epidemiology of HD is less as compared to other diseases, but increasing prevalence suggests that there is a new scope for further research on HD [34].
Current medical therapies use pharmaceutical interventions with lifestyle modification to prevent or control HD. Various hypotheses including molecular genetics, oxidative stress, excitotoxicity, metabolic dysfunction, and mitochondrial impairment have been proposed to explain the pathogenesis HD despite to that there is no treatment available fully to stop the progression of the disease [35].
3-NP is known to produces behavioral, biochemical and morphologic changes in animals associated with HD similar to those occurring in human [36]. Intra-peritoneal administration of 3-NP increases oxidative stress, gait impairment and neurodegeneration [37]. Further, it produces depression and anxiety via energy impairment and striatal lesion. It is an irreversible inhibitor of succinic acid dehydrogenase (Complex II) and induces neuronal disorders in rats similar to those in patients with HD [13].
Bioflavonoid and carotenoid are upcoming molecules that can be used for a number of disease with less or no side-effect. They have excellent properties that help to reduce or cure the symptoms of many diseases [38]. Research proved that carotenoids and bioflavonoid have a complementary effect, making them both more effective when administer together rather than separately. The combinations of these are used to treat different diseases such as cancer, diabetes, asthma, coronary diseases, etc. [39-41]. Previous study showed that lycopene has strong antioxidant properties, and quercetin has anti-inflammatory property with the addition of anticancer, anti-diabetes, anti-asthematic activity [42,43]. Lycopene treatment significantly attenuates the impairment in behavioral, biochemical and mitochondrial dysfunction as well as glutathione depletion that cures depression and anxiety [21]. Quercetin significantly decreases the concentration of malondialdehyde (an indicator of lipid peroxidation) and glutathione reductase activity [44]. In addition, poloxamer, 188 which is a polymer has the ability to repair damaged cell membranes by increasing the lipid packing density as it provides mechanical sealing to neurons [23]. Based on this evidences in our investigation we use the combinations of lycopene (caretonoid) and quercetin (bioflavonoid) with and without poloxamer 188 to study synergistic effect in alleviating anxiety and depression.
As HD progresses, there is strong impairment in motor functions that alter muscular movements and reduced locomotor activity [25]. As per the reports intra-peritoneal administration of 3-NP cause muscular atrophy which leads to decrease in locomotion counts. Reduced number of locomotion also indicates central nervous system (CNS)depression which is one of the symptoms of HD. In the present study, 14 days administration of 3-NP produced significant decreased in locomotions that are in agreement of the previous reports. Administration of lycopene and quercetin separately significantly increases the number of locomotion as compared to HC group, which may be due to as they reduce oxidative stress and inflammation. However, combinatorial treatment of quercetin and lycopene with or without poloxamer 188 restored a number of locomotion more significantly; further a significant difference was found as compared to lycopene treated rats, which showed a synergistic effect as they produce anti-oxidant and anti-inflammatory effect together.
3-NP produce CNS depression via oxidative stress and structural defects. Depression is one of the most common psychiatric disorders in the general population. Scientist investigate depression is a common feature of HD. Depression in HD is associated with basal ganglia abnormality and neurodegeneration which can be measured with the help of forced swim test. Due to depression animal will not try to escape a stressful stimulus (water tank) [28]. Administration of 3-NP in rats produced neurodegeneration and exhibit depression. In the present study, lycopene and quercetin significantly minimizes depression induced by 3-NP as compared to HD group. However combinations of these drugs are more effective as compared to individual administration as they work together. Addition of poloxamer 188 to this combination showed more significant effect on onset of immobility. There was more significant difference in the duration of immobility period in the rats treated with lycopene and quercetin with or without poloxamer 188 when compared with quercetin treated rats. On the onset of immobility only the rats treated with lycopene and quercetin with poloxamer 188 showed statistical significant difference when compared with quercetin treated rats.
Anxiety is one of the symptoms of HD. As per previous investigation anxiety in HD may be due to cholinergic hypofunction and increase oxidative stress [26]. 3-NP induces anxiety via increasing oxidative stress in experimental animal, which can be measure with the help of light and dark model as time spend in light or dark model. Light and dark model is one of the best animal models to measure the anxiety in animal. In our study, administration of 3-NP induced anxiety and reduced time spend in light area as compared to control rats. Co-administration of lycopene and quercetin alone along with 3-NP reduced anxiety and significantly increased the duration of time spent in light area. Further more significant reduction in anxiety and increase in the duration of time spent in light area were observed with combination of these drugs with or without poloxamer 188. Combinations of lycopene and quercetin with or without poloxamer188 exhibited significant increase in duration of time spent in light area and decrease in dark area. Further significant increase in time spent in light area and decrease in dark area was observed in rats treated with quercetin when compared with lycopene treated rats.
To sum up, lycopene and quercetin showed significant effect in the management of HD, moreover in our study we observed synergistic effect when lycopene and quercetin administered together with or without poloxamer 188. Further we have observed good results in rats treated with lycopene, quercetin and poloxamer 188 than lycopene and quercetin combination.
CONCLUSION
This study indicates that combination of lycopene and quercetin is an effective nutritional component to alleviate and/or prevent the complications of HD than single drug therapy, and these findings can be used as a basis for future studies.
ACKNOWLEDGMENT
The authors would like acknowledge Prof. M. N Nawale, President, Sinhgad Technical Education Society and Dr. K N Gujar, Principal, Sinhgad College of Pharmacy, Vadgaon (BK), Pune, India, for providing necessary facilities to carry out the present study.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
REFERENCES
- 1.Choudhary S, Kumar P, Malik J. Plants and phytochemicals for Huntington's disease. Pharmacogn Rev. 2013;7:81–91. doi: 10.4103/0973-7847.120505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harper PS. The epidemiology of Huntington's disease. Hum Genet. 1992;89:365–76. doi: 10.1007/BF00194305. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava T, Lal V, Prabhakar S. Juvenile Huntington's disease. Neurol India. 1999;47:340–1. [PubMed] [Google Scholar]
- 4.Wagle AC, Wagle SA, Marková IS, Berrios GE. Psychiatric morbidity in Huntington's disease. Neurol Psychiatry Brain Res. 2000;8:5–16. [Google Scholar]
- 5.Cattaneo E, Rigamonti D, Goffredo D, Zuccato C, Squitieri F, Sipione S. Loss of normal huntingtin function:New developments in Huntington's disease research. Trends Neurosci. 2001;24:182–8. doi: 10.1016/s0166-2236(00)01721-5. [DOI] [PubMed] [Google Scholar]
- 6.Katsuno M, Banno H, Suzuki K, Takeuchi Y, Kawashima M, Tanaka F, et al. Molecular genetics and biomarkers of polyglutamine diseases. Curr Mol Med. 2008;8:221–34. doi: 10.2174/156652408784221298. [DOI] [PubMed] [Google Scholar]
- 7.Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 8.Walker FO. Huntington's disease. Lancet. 2007;369:218–28. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 9.Pla P, Orvoen S, Saudou F, David DJ, Humbert S. Mood disorders in Huntington's disease:From behavior to cellular and molecular mechanisms. Front Behav Neurosci. 2014;8:135. doi: 10.3389/fnbeh.2014.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayberg HS, Starkstein SE, Peyser CE, Brandt J, Dannals RF, Folstein SE. Paralimbic frontal lobe hypometabolism in depression associated with Huntington's disease. Neurology. 1992;42:1791–7. doi: 10.1212/wnl.42.9.1791. [DOI] [PubMed] [Google Scholar]
- 11.Tasset I, Pontes AJ, Hinojosa AJ, de la Torre R, Túnez I. Olive oil reduces oxidative damage in a 3-nitropropionic acid-induced Huntington's disease-like rat model. Nutr Neurosci. 2011;14:106–11. doi: 10.1179/1476830511Y.0000000005. [DOI] [PubMed] [Google Scholar]
- 12.Chang KL, New LS, Mal M, Goh CW, Aw CC, Browne ER, et al. Metabolic profiling of 3-nitropropionic acid early-stage Huntington's disease rat model using gas chromatography time-of-flight mass spectrometry. J Proteome Res. 2011;10:2079–87. doi: 10.1021/pr2000336. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura M, Okimura Y, Fujita H, Yano H, Lee J, Suzaki E, et al. Mechanism of 3-nitropropionic acid-induced membrane permeability transition of isolated mitochondria and its suppression by L-carnitine. Cell Biochem Funct. 2008;26:881–91. doi: 10.1002/cbf.1521. [DOI] [PubMed] [Google Scholar]
- 14.Pang Z, Geddes JW. Mechanisms of cell death induced by the mitochondrial toxin 3-nitropropionic acid:Acute excitotoxic necrosis and delayed apoptosis. J Neurosci. 1997;17:3064–73. doi: 10.1523/JNEUROSCI.17-09-03064.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta R, Deshpande SB. Involvement of nitric oxide in 3-nitropropionic acid-induced depression of spinal reflexes in neonatal rat spinal cord in vitro. Eur J Pharmacol. 2009;617:74–8. doi: 10.1016/j.ejphar.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 16.Teunissen CE, Steinbusch HW, Angevaren M, Appels M, de Bruijn C, Prickaerts J, et al. Behavioural correlates of striatal glial fibrillary acidic protein in the 3-nitropropionic acid rat model:Disturbed walking pattern and spatial orientation. Neuroscience. 2001;105:153–67. doi: 10.1016/s0306-4522(01)00164-6. [DOI] [PubMed] [Google Scholar]
- 17.Michels G, Wätjen W, Niering P, Steffan B, Thi QH, Chovolou Y, et al. Pro-apoptotic effects of the flavonoid luteolin in rat H4IIE cells. Toxicology. 2005;206:337–48. doi: 10.1016/j.tox.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Johnson EJ. The role of carotenoids in human health. Nutr Clin Care. 2002;5:56–65. doi: 10.1046/j.1523-5408.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 19.Garcia M, Vanhoutte P, Pages C, Besson MJ, Brouillet E, Caboche J. The mitochondrial toxin 3-nitropropionic acid induces striatal neurodegeneration via a c-Jun N-terminal kinase/c-Jun module. J Neurosci. 2002;22:2174–84. doi: 10.1523/JNEUROSCI.22-06-02174.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Stefano D, Maiuri MC, Simeon V, Grassia G, Soscia A, Cinelli MP, et al. Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN-gamma. Eur J Pharmacol. 2007;566:192–9. doi: 10.1016/j.ejphar.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 21.Kumar P, Kalonia H, Kumar A. Lycopene modulates nitric oxide pathways against 3-nitropropionic acid-induced neurotoxicity. Life Sci. 2009;85:711–8. doi: 10.1016/j.lfs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Sandhir R, Mehrotra A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid:Implications in Huntington's disease. Biochim Biophys Acta. 2013;1832:421–30. doi: 10.1016/j.bbadis.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Moloughney JG, Weisleder N. Poloxamer 188 (p188) as a membrane resealing reagent in biomedical applications. Recent Pat Biotechnol. 2012;6:200–11. doi: 10.2174/1872208311206030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aziz NA, van der Burg JM, Landwehrmeyer GB, Brundin P, Stijnen T, EHDI Study Group et al. Weight loss in Huntington disease increases with higher CAG repeat number. Neurology. 2008;71:1506–13. doi: 10.1212/01.wnl.0000334276.09729.0e. [DOI] [PubMed] [Google Scholar]
- 25.Bhosale UA, Yegnanarayan R, Pophale PD, Zambare MR, Somani RS. Study of central nervous system depressant and behavioral activity of an ethanol extract of Achyranthes aspera (Agadha) in different animal models. Int J Appl Basic Med Res. 2011;1:104–8. doi: 10.4103/2229-516X.91154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhat S, Joy A. Antianxiety effect of ethanolic extract of leaves of Moringa oleifera in Swiss albino mice. Official J Yenepoya Univ. 2014;2:5–7. [Google Scholar]
- 27.Bilkei-Gorzó A, Gyertyán I, Lévay G. mCPP-induced anxiety in the light-dark box in rats a new method for screening anxiolytic activity. Psychopharmacology (Berl) 1998;136:291–8. doi: 10.1007/s002130050568. [DOI] [PubMed] [Google Scholar]
- 28.Crawley JN. Behavioral Phenotyping of Transgenic and Knockout Mice. 2nd ed. Hoboken, NJ: Wiley; 2007. What's Wrong with My Mouse? [Google Scholar]
- 29.Borsini F, Volterra G, Meli A. Does the behavioral “despair”test measure “despair”? Physiol Behav. 1986;38:385–6. doi: 10.1016/0031-9384(86)90110-1. [DOI] [PubMed] [Google Scholar]
- 30.Paulsen JS, Ready RE, Hamilton JM, Mega MS, Cummings JL. Neuropsychiatric aspects of Huntington's disease. J Neurol Neurosurg Psychiatry. 2001;71:310–4. doi: 10.1136/jnnp.71.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gagnon JF, Petit D, Latreille V, Montplaisir J. Neurobiology of sleep disturbances in neurodegenerative disorders. Curr Pharm Des. 2008;14:3430–45. doi: 10.2174/138161208786549353. [DOI] [PubMed] [Google Scholar]
- 32.Craufurd D, Thompson JC, Snowden JS. Behavioral changes in Huntington Disease. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:219–26. [PubMed] [Google Scholar]
- 33.Barnham KJ, Masters CL, Bush AI. Neurodegenerative and oxidative stress. Nat Rev Drug Discov. 2004;3:205–14. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 34.Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE, et al. A polymorphic DNA marker genetically linked to Huntington's disease. Nature. 1983;306:234–8. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- 35.Paul BD, Sbodio JI, Xu R, Vandiver MS, Cha JY, Snowman AM, et al. Cystathionine γ-lyase deficiency mediates neurodegeneration in Huntington's disease. Nature. 2014;509:96–100. doi: 10.1038/nature13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mealer RG, Subramaniam S, Snyder SH. Rhes deletion is neuroprotective in the 3-nitropropionic acid model of Huntington's disease. J Neurosci. 2013;33:4206–10. doi: 10.1523/JNEUROSCI.3730-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz JB, Henshaw DR, MacGarvey U, Beal MF. Involvement of oxidative stress in 3-nitropropionic acid neurotoxicity. Neurochem Int. 1996;29:167–71. doi: 10.1016/0197-0186(95)00122-0. [DOI] [PubMed] [Google Scholar]
- 38.Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids:Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65:337–53. doi: 10.1016/s0024-3205(99)00120-4. [DOI] [PubMed] [Google Scholar]
- 39.Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke. 2000;31:2301–6. doi: 10.1161/01.str.31.10.2301. [DOI] [PubMed] [Google Scholar]
- 40.Polidori MC, Stahl W, Eichler O, Niestroj I, Sies H. Profiles of antioxidants in human plasma. Free Radic Biol Med. 2001;30:456–62. doi: 10.1016/s0891-5849(00)00345-2. [DOI] [PubMed] [Google Scholar]
- 41.Upaganlawar A, Gandhi H, Balaraman R. Effect of vitamin E alone and in combination with lycopene on biochemical and histopathological alterations in isoproterenol-induced myocardial infarction in rats. J Pharmacol Pharmacother. 2010;1:24–31. doi: 10.4103/0976-500X.64532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basu A, Imrhan V. Tomatoes versus lycopene in oxidative stress and carcinogenesis:Conclusions from clinical trials. Eur J Clin Nutr. 2007;61:295–303. doi: 10.1038/sj.ejcn.1602510. [DOI] [PubMed] [Google Scholar]
- 43.Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1071–7. doi: 10.1152/ajpregu.90925.2008. [DOI] [PubMed] [Google Scholar]
- 44.Choi EJ, Chee KM, Lee BH. Anti- and prooxidant effects of chronic quercetin administration in rats. Eur J Pharmacol. 2003;482:281–5. doi: 10.1016/j.ejphar.2003.09.067. [DOI] [PubMed] [Google Scholar]


