Abstract
Objective:
This study aimed at investigating the genotoxicity and cytotoxicity effect of Tapinanthus globiferus and Zanthoxylum zanthoxyloides to human leukocytes. In addition, the reductive potential and the chemical composition of the two plant extracts were also determined.
Materials and Methods:
Human leukocytes were obtained from healthy volunteer donors. The genotoxicity and cytotoxicity of T. globiferus and Z. zanthoxyloides were assessed using the comet assay and trypan blue exclusion, respectively. The antioxidant activity of the plant extracts was evaluated by the reducing power assay. Furthermore, high-performance liquid chromatography-diode array detector was used to characterize and quantify the constituents of these plants.
Results:
T. globiferus (10-150 µg/mL) was neither genotoxic nor cytotoxic at the concentrations tested, suggesting that it can be consumed safely at relatively high concentrations. However, Z. zanthoxyloides showed cytoxicity and genotoxicity to human leukocytes at the highest concentration tested (150 µg/mL). In addition, the total reducing power of T. globiferus was found higher than Z. zanthoxyloides in potassium ferricyanide reduction. Both plants extract contained flavonoids (rutin and quercetin) and phenolic acids (chlorogenic and caffeic).
Conclusion:
The results obtained support the fact that some caution should be paid regarding the dosage and the frequency of use of Z. zanthoxyloides extract.
KEY WORDS: Cytoxicity, genotoxicity, high performance liquid chromatography-diode array detector, Tapinanthus globiferus, Zanthoxylum zanthoxyloides
INTRODUCTION
Reactive oxygen species (ROS) are oxidizing, highly reactive and unstable molecules are containing oxygen. They produced during normal cellular metabolism as by-products of respiration in the mitochondria. They include hydroxyl radical (OH•), superoxide anion (O2•−), hydrogen peroxide (H2O2) and singlet oxygen [1]. Cumulative evidence suggests that ROS play important roles in signal transduction, sensing of oxygen tension and regulation of functions controlled by oxygen concentration [2]. They are also involved in boosting the immune system [3]. However, ROS can be harmful when its cellular levels exceed the level of cellular antioxidants, which results in oxidative stress. Oxidative stress would eventually cause injury to cellular macromolecules such as membrane lipids, proteins and nucleic acids, thereby affecting the normal functioning of cells.
DNA is one of the major targets of ROS in living cells and tissues. ROS induces DNA mutations that can cause or lead to cancer and age-related disorders [4]. Hydroxyl radicals (OH•), an oxidant obtained from the breakdown of H2O2 is majorly responsible for DNA damage. It reacts with DNA molecule causing DNA protein cross-links, DNA strand breaks and alkali-labile sites [4,5], which may lead to permanent damages that can cause severe biological consequences [6]. Furthermore, Shi et al. [7] revealed that O2•− and H2O2 are capable of inducing strand-breaks and oxidation of DNA bases.
Further, studies have shown that DNA damage can be minimized or prevented by the use of natural antioxidants such as vitamin C, vitamin E, carotenoids, flavonoids, and other polyphenolic compounds, by scavenging or inactivating ROS. Particularly, natural compounds exhibit protective effects when used in oxidative stress-induced DNA damage [8]. Furthermore, plants rich in antioxidants have been shown to protect ROS-induced oxidative DNA damage [9].
Tapinanthus globiferus and Zanthoxylum zanthoxyloides are plants commonly used as folkoric medicine and highly consumed in the Nigeria and Cameroon. T. globiferus known as mistletoe (in English) belongs to the family Loranthaceae. It is a woody, spreading shrub with blackish, smooth stems made rough by the presence of lenticels. It is popularly called “afomo” in South Western Nigeria whereas, Z. zanthoxyloides (family, Rutaceae) is commonly known as candle wood. The root of Z. zanthoxyloides is used as antibacterial toothbrush in South Western Nigeria, and the decoction of its leaves and roots is used to wash wounds for healing. In addition, the bark of the plant is used in the treatment of intestinal worms and edema. Likewise, T. globiferus is commonly consumed for the treatment of hypertension, ulcers, diabetics, weakness of vision, and for promoting muscular relaxation before delivery. Recent studies revealed that the plants exhibit a variety of pharmacological activities including antitrypanosomal [10,11], antimicrobial [12], anti-inflammatory [13] activities, and are rich in antioxidants [14].
Human leukocytes are used to evaluate DNA damage, repair studies and genotoxicity using comet assay because leukocytes are obtained in a relatively non-invasive way and do not require tissue disaggregation [15]. Comet assay is highly sensitive for in vitro genotoxicity test methods on leukocytes [16] and is of particular importance for safety evaluation. For instance, genotoxicity can be a consequence of long-term exposure to very low levels of chemicals and have a hereditary and delayed-onset nature that may lead to major consequences at the population level [17].
Considering the growing interest in the use of medicinal plants to treat and/or prevent various diseases associated with free radicals, there is an urgent need to provide information on toxicity risk-assessment of plants extracts. Therefore, the present study aimed at investigating the possible genotoxic and cytotoxic potential of T. globiferus and Z. zanthoxyloides in human leukocytes. A further attempt was made to determine the reducing potential (conversion of Fe (III) to Fe (II)) of these plants as well as their chemical characterization.
MATERIALS AND METHODS
Chemicals
All chemicals used including solvents were of analytical grade.
Plants Collection and Extraction Procedure
The leaves of T. globiferus and stem bark of Z. zanthoxyloides were obtained from Ogbomoso, Nigeria in 2013 and were identified by Dr. Ogunkunle of the Botany Unit, Department of Pure and Applied Biology (Ladoke Akintola University of Technology, where the specimen was deposited). The dried leaves and stem bark were pulverized into a powdery form, after which 100 g of T. globiferus and 100 g of Z. zanthoxyloides were macerated at room temperature with ethanol (70%) and extracted for 3 days. The combined ethanolic extract of each sample was filtered on the 3rd day and the solvent was fully evaporated under reduced pressure to give a green solid for T. globiferus and yellow solid for Z. zanthoxyloides. The ethanolic extract of T. globiferus was then suspended in water, while, that of Z. zanthoxyloides was suspended in ethanol in order to prepare different concentrations (10-150 µg/mL) used in the experiments.
Quantification of Some Flavonoids and Phenolic Compounds by High Performance Liquid Chromatography-Diode Array Detector (HPLC)
Reverse phase chromatographic analyses were carried out under gradient conditions using C18 column (4.6 mm × 250 mm) packed with 5 µm diameter particles; the mobile phase was water containing 2% acetic acid (A) and methanol (B), and the composition gradient was: 5% of B until 2 min and changed to obtain 25%, 40%, 50%, 60%, 70% and 100% B at 10, 20, 30, 40, 50 and 65 min, respectively, following the method described by Laghari et al. [18] with slight modifications. The extracts of T. globiferus and Z. zanthoxyloides were analyzed, at a concentration of 5 mg/mL. The presence of six phenolics compounds was investigated, namely, gallic, chlorogenic and caffeic acids and the flavonoids quercetin, rutin and kaempferol. Identification of these compounds was performed by comparing their retention time and ultraviolet (UV) absorption spectrum with those of the commercial standards. The flow rate was 0.6 mL/min, injection volume 40 µL and the wavelength were 254 nm for gallic acid, 325 nm for caffeic and chlorogenic acids, and 365 nm for quercetin, rutin and kaempferol. All the samples and mobile phase were filtered through 0.45 µm membrane filter (Millipore) and then degassed by ultrasonic bath prior to use. Stock solutions of standards references were prepared in the HPLC mobile phase at a concentration range of 0.031-0.250 mg/mL for kaempferol, quercetin and rutin; and 0.006-0.250 mg/mL for gallic, caffeic and chlorogenic acids. All chromatography operations were carried out at ambient temperature and in triplicate. The limit of detection (LOD) and limit of quantification (LOQ) were calculated based on the standard deviation of the responses and the slope using three independent analytical curves as defined by ICH [19]. LOD and LOQ were calculated as 3.3 and 10 σ/S, respectively, where σ is the standard deviation of the response and S is the slope of the calibration curve.
Obtension of Human Leukocytes
Heparinized venous blood was obtained from healthy volunteer donors from the Hospital of the Federal University of Santa Maria (UFSM), Santa Maria, RS, Brazil (age 25 ± 10). This work was carried out in accordance with the Guidelines of the Ethical Committee of UFSM and approved by the Institutional Review Board of UFSM (0089.0.248.000-12). Differential erythrocyte sedimentation with dextran was used to separate leukocytes of the blood as previously described [20].
Genotoxicity evaluation of T. globiferus and Z. zanthoxyloides using comet assay
The comet assay was performed under alkaline conditions according to the method of Santos et al. [21]. Briefly, peripheral leukocytes were incubated for 3 h in the absence or presence of plant extract, at different concentrations (10-150 µg/mL). Hydrogen peroxide (100 µM) was used as a positive control, while water was used as negative control (NC). After incubation and electrophoresis, one hundred cells per sample were randomly selected and visually scored according to tail length into five classes: (1) Class 0: Undamaged, without a tail; (2) Class 1: With a tail shorter than the diameter of the head (nucleus); (3) Class 2: With a tail length 1-2 times the diameter of the head; (4) Class 3: With a tail longer than 2 times the diameter of the head and (5) Class 4: Comets with no heads. Comets with no heads and images with nearly all DNA in the tail or with a very wide tail were excluded from the evaluation because they probably represent dead cells. DNA damage was presented as DNA damage index (DI) and it is based on the length of migration. The DI was calculated from cells in different damage classes as follows: DI = n1 + 2n2 + 3n3 + 4n4. Where, n1-n4 represents the number of cells with level 1-4 of damage. The slides were analyzed under blind conditions by at least two individuals.
Cytotoxicity evaluation of T. globiferus and Z. zanthoxyloides by trypan blue
The toxic effects of T. globiferus and Z. zanthoxyloides toward leukocytes were determined as described by Mischell and Shiingi [22] with slight modifications. Briefly, 2.5 µL of different concentrations of the extracts (10-150 µg/mL) was added to leukocytes suspension (497.5 µL) and incubated in the presence or absence of hydrogen peroxide (2 mM) + azide (1 mM), for 3 h at 37°C in a water bath. Hydrogen peroxide (2 mM) + azide (1 mM) was used as a positive control whereas distilled water was used as NC. After the incubation, a volume of 50 µL of leukocytes suspension was mixed with 50 µL of 0.4% trypan blue solution and left for 5 min. The cell viability was determined microscopically (×400 magnification) using a hemocytometer and was calculated as the number of living cells (i.e., those not stained with trypan blue) divided by the total number of cells multiplied by 100.
Reducing Power Assay
The Fe3+ reducing power of the extracts was determined according to a modified method of Mathew and Abraham [23]. Various concentrations of T. globiferus and Z. zanthoxyloides (10-150 µg/mL) (200 µL) were mixed with 625 µL of potassium phosphate buffer solution (0.2 M, pH 6.6) and 625 µL of potassium ferricyanide (1%, w/v), followed by incubation at 50°C for 20 min. The reaction was stopped by adding 625 µL of trichloroacetic acid solution (10%, w/v) and then centrifuged at 5000 ×g for 10 min. A known volume (625 µL) of the upper layer solution (obtained after centrifugation) was taken in another test tube and mixed with 625 µL of distilled water, then, 250 µL of ferric chloride solution (0.1%, w/v) was added and mixed well. The absorbance was measured at 700 nm in a spectrophotometer. The blank was prepared by the same procedure without plant extracts. Ascorbic acid (10-150 µg/mL) was used as a positive control.
Statistical Analysis
Values were expressed as mean ± standard error of the mean. One-way ANOVA, followed by Benferroni post-test was used to evaluate the differences among the groups. The results were considered as statistically significant for P < 0.05.
RESULTS
Phytochemical Constituents
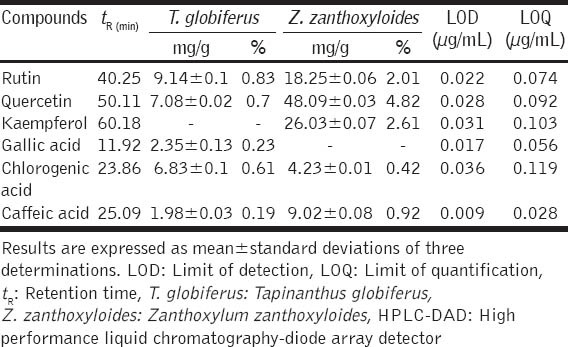
The HPLC analysis was used to identify and quantify the presence or absence of phenolic acids and flavonoids from the leaf extract and stem bark of T. globiferus, and Z. zanthoxyloides respectively. The results of HPLC profile indicate that both plant extracts contain chlorogenic and caffeic acids, rutin and quercetin [Figure 1]. However, gallic acid, present in the leaf extract of T. globiferus, was absent in the stem bark of Z. zanthoxyloides. Similarly, kaempferol, absent in the leaf extract of T. globiferus, was present in the stem bark of Z. zanthoxyloides [Figure 1 and Table 1]. These compounds were identified by comparing their retention times and UV spectra to that of authentic standards analyzed under identical analytical conditions. Quantitative HPLC analysis showed that the rutin was the major component in T. globiferus (9.14 ± 0.1 mg/g) while caffeic acid was the minor (1.98 ± 0.03 mg/g). However, the major component found in Z. zanthoxyloides was quercetin (48.09 ± 0.03 mg/g), while chlorogenic acid (4.23 ± 0.01 mg/g) was the minor [Table 1].
Figure 1.
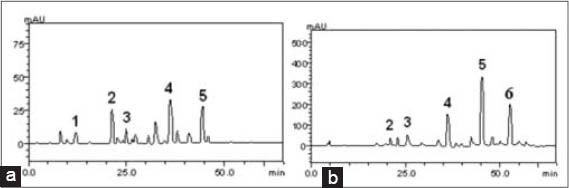
High performance liquid chromatography (HPLC) profile of the leaf extract of Tapinanthus globiferus (a) and Zanthoxylum zanthoxyloides stem bark extracts (b). Gallic acid (peak 1), chlorogenic acid (peak 2), caffeic acid (peak 3), rutin (peak 4), quercetin (peak 5) and kaempferol (peak 6). The chromatography peaks were confirmed by comparing its retention time with those of reference standards and by diode array detector spectra (200-500 nm). Calibration curve for gallic acid: Y = 11611x + 1468.8 (r = 0.9999); chlorogenic acid: Y = 14762x + 1257.5 (r = 0.9997); caffeic acid: Y = 11526x + 1293.1 (r = 0.9995); rutin: Y = 13035x − 1045.9 (r = 0.9998); quercetin: Y = 15105x − 1192.3 (r =0.9998) and kaempferol: Y = 15223x − 1303.9 (r = 0.9999). All chromatography operations were carried out at ambient temperature and in triplicate
Table 1.
Qualitative and quantitative analyses of some flavonoids and phenolic compounds from the leaf extract of T. globiferus and Z. zanthoxyloides stem bark extract by HPLC-DAD
Effects of T. globiferus and Z. zanthoxyloides on DNA Damage
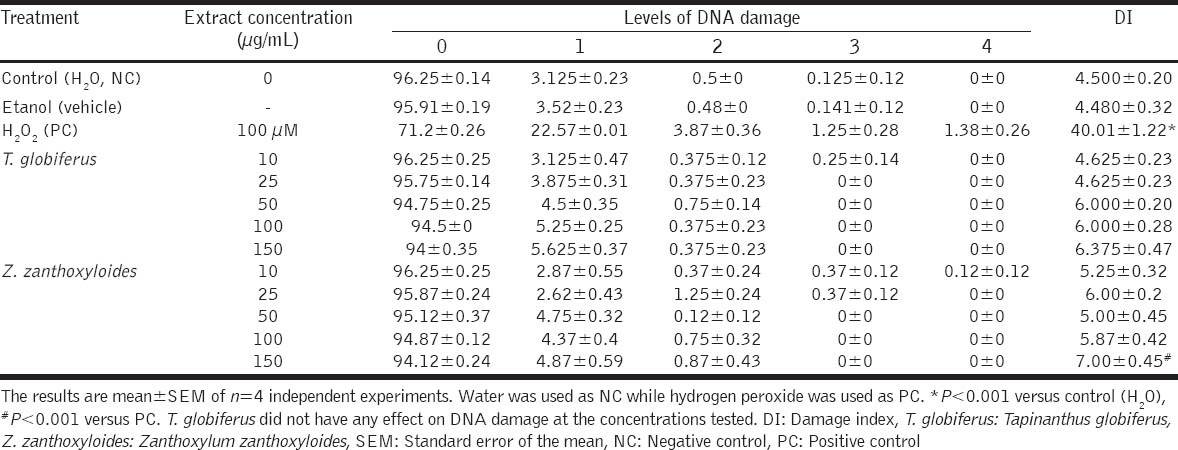
Table 2 shows the comet assay results obtained after exposure of human leukocytes to various concentrations (10-150 µg/mL) of T. globiferus and Z. zanthoxyloides. H2O2 (positive control) induced a significant increase in DNA migration when compared to NC (P < 0.001), as evidenced by the DI [Table 2]. Ethanol used as a vehicle for Z. zanthoxyloides did not have any effect on DNA migration in comparison with the NC (P > 0.05). There was no significant difference in the DI when the cells were treated with T. globiferus (10-150 µg/mL) when compared to NC (P > 0.05). However, a statistically significant increase in DNA DI was observed at 150 µg/mL of Z. zanthoxyloides. Generally, when the human leukocytes were exposed to both plant extracts (10-150 µg/mL), the majority of leukocytes examined on slides were undamaged (Class 0). Few leukocytes showed minor DNA damage (Class 1) and very few showed a large amount of DNA damage (Class 2-4) [Table 2].
Table 2.
Effect of T. globiferus and Z. zanthoxyloides on human leukocytes
Effects of T. globiferus and Z. zanthoxyloides on Leukocytes Viability
In order to assess the toxicity of T. globiferus and Z. zanthoxyloides on human leukocytes, cellular viability was evaluated following exposure, by using the trypan blue assay dye exclusion method. The H2O2 + azide were used to inhibit catalase activity in leukocytes and consequently detect the toxicity induced by H2O2. H2O2 + azide used as positive control, caused a significant decrease in cell viability (approximately 48% decrease) when compared to control [Figure 2a and b; P < 0.05]. T. globiferus at all the concentrations tested did not have any effect on cell viability [Figure 2a], whereas, Z. zanthoxyloides at the highest concentration (150 µg/mL) exhibited a significantly decrease [Figure 2b] when compared to control (P < 0.05). It should be noted that 150 µg/mL of Z. zanthoxyloides concentration was genotoxic and cytotoxic to human leukocytes.
Figure 2.
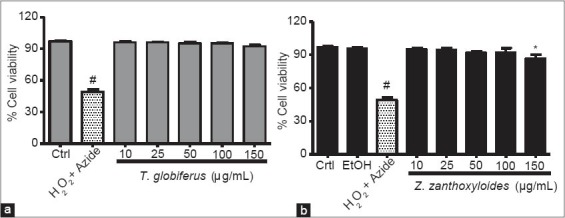
Survival of leukocytes treated with Tapinanthus globiferus (a) and Zanthoxylum zanthoxyloides (b) for 3 h. Results are expressed as mean ± standard error of the mean, n = 4. H2O2 (2 mM) + azide (1 mM) was used as positive control. #P < 0.001 versus control (Ctrl), *P < 0.05 versus Ctrl. T. globiferus was not cytotoxic to leukocytes at the concentrations tested, while, Z. zanthoxyloides does at the highest concentration
Reducing Power Potential of T. globiferus and Z. zanthoxyloides
As depicted in Figure 3, T. globiferus and Z. zanthoxyloides showed increased absorbance with increased concentrations, which indicates increased ferric reducing power. However, the reducing potential of both extracts was lower than that of ascorbic acid used as standard antioxidant. The reducing power of the extracts and ascorbic acid decreased in the order ascorbic acid > T. globiferus > Z. zanthoxyloides.
Figure 3.
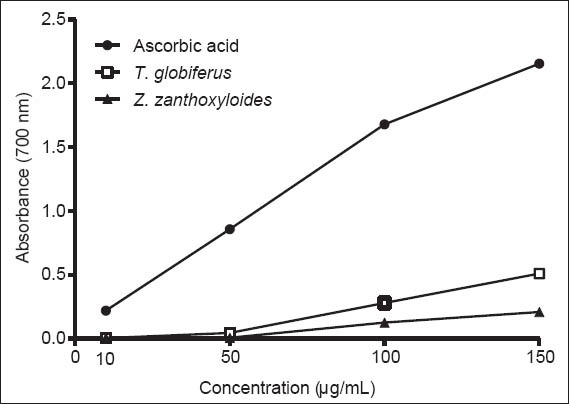
Reductive ability of the leaves extract of Tapinanthus globiferus and Zanthoxylum zanthoxyloides stem bark extract versus ascorbic acid. Values expressed in absorbance are the mean ± standard error of the mean of n = 4 performed in duplicates
DISCUSSION
Although medicinal plants are regarded as safe, there is increasing evidence that plant extracts and/or their chemical constituents can have toxic effects [24]. Therefore, the toxicity evaluation of plant extracts used in folk medicine is highly recommended. In the present study, the genotoxicity and cytotoxicity effects of T. globiferus and Z. zanthoxyloides were investigated in human leukocytes, as well as their reducing potential. The results demonstrated that T. globiferus was neither genotoxic nor cytotoxic to human leukocytes at all the concentrations tested. However, Z. zanthoxyloides was genotoxic and cytotoxic at the highest concentration tested (150 µg/mL). These results indicate that the use of T. globiferus at relatively high concentrations could be regarded as safe. The genotoxicity and cytotoxicity effects of Z. zanthoxyloides at the highest concentration tested leads to DNA damage, an indication of the presence of chemical constituents which interacted with DNA, leading to damage. Another explanation could be a synergistic interaction of compounds within the plant extracts resulting in the observed damage to DNA [25]. Although the comet assay has been criticized for the agarose concentration [15,26], it has become the most popular method for measuring DNA damage of various sorts, including oxidative damage inflicted by ROS [16,26].
Natural antioxidants found in plants and vegetables are extensively studied for their ability to protect the organism and cells from the deleterious effects induced by oxidative stress [27-29]. In previous studies, T. globiferus and Z. zanthoxyloides have shown antioxidant activity by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging and iron chelating activities [30,31]. In this study, the reductive potential of T. globiferus and Z. zanthoxyloides was determined on the basis that this assay has a different mechanism of action in relation to DPPH and iron chelating assays. In addition, reducing power of a compound is associated with antioxidant activity and may serve as a significant reflection of its potential antioxidant capacity [20,32]. This assay is based on the reduction of Fe3+/ferricyanide complex to the Fe2+ form in the presence of antioxidant. The reduction is observed by the change of the yellow test solution to green or blue color depending on the reducing power of antioxidant samples. In addition, a higher absorbance indicates a higher ferric reducing power. Here, T. globiferus and Z. zanthoxyloides showed increased ferric reducing power with an increased concentration as ascorbic acid, indicating that both plant extracts have antioxidant activity. In the agreement to this, Amarowicz and Troszynska [33] demonstrated a direct relationship between reducing power and antioxidant activity. Consequently, the reducing power of these plant extracts may be associated with the antioxidant activity of phenolic acids and flavonoids found in these extracts.
CONCLUSION
The safety evaluation of T. globiferus and Z. zanthoxyloides revealed that T. globiferus (10-150 µg/mL) was neither genotoxic nor cytotoxic to human leukocytes following 3 h exposure. This indicates that its popular use in infusion might be considered safe for consumption. In contrast, Z. zanthoxyloides at the highest concentration tested (150 µg/mL) showed genotoxicity and cytotoxicity effects, therefore not safe for consumption. Both plants showed antioxidant activity as evidenced by their reducing power potential, which can be attributed at least, in part, to their flavonoid and phenolic contents.
ACKNOWLEDGMENTS
This work was supported by grant from The Word Academy of Sciences (TWAS) for the advancement of science in developing countries and the Alexander von Humboldt Foundation (AvH). The financial support of CAPES, CNPq, FAPERGS, FAPERGS-PRONEX-CNPq is also acknowledged.
Footnotes
Source of Support: The financial support of CAPES, CNPq, FAPERGS, FAPERGS-PRONEX-CNPq is also acknowledged,
Conflict of Interest: None declared
REFERENCES
- 1.Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int 2013. 2013 doi: 10.1155/2013/942916. 942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sylvester JT. Hypoxic pulmonary vasoconstriction:A radical view. Circ Res. 2001;88:1228–30. doi: 10.1161/hh1201.093167. [DOI] [PubMed] [Google Scholar]
- 3.Paiva CN, Bozza MT. Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Signal. 2014;20:1000–37. doi: 10.1089/ars.2013.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 5.Kawanishi S, Hiraku Y, Murata M, Oikawa S. The role of metals in site-specific DNA damage with reference to carcinogenesis. Free Radic Biol Med. 2002;32:822–32. doi: 10.1016/s0891-5849(02)00779-7. [DOI] [PubMed] [Google Scholar]
- 6.Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS) –Induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011;711:167–73. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Shi H, Hudson LG, Liu KJ. Oxidative stress and apoptosis in metal ion-induced carcinogenesis. Free Radic Biol Med. 2004;37:582–93. doi: 10.1016/j.freeradbiomed.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Plazar J, Filipic M, Groothuis GM. Antigenotoxic effect of xanthohumol in rat liver slices. Toxicol In Vitro. 2008;22:318–27. doi: 10.1016/j.tiv.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Del Bo C, Riso P, Campolo J, Møller P, Loft S, Klimis-Zacas D, et al. A single portion of blueberry (Vaccinium corymbosum L) improves protection against DNA damage but not vascular function in healthy male volunteers. Nutr Res. 2013;33:220–7. doi: 10.1016/j.nutres.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Mann A, Ifarajimi OR, Adewoye AT, Ukam C, Udeme EE, Okorie II, et al. In vivo antitrypanosomal effects of some ethnomedicinal plants from Nupeland of north central Nigeria. Afr J Tradit Complement Altern Med. 2011;8:15–21. doi: 10.4314/ajtcam.v8i1.60486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abedo JA, Jonah OA, Abdullahi RS, Mazadu MR, Idris HY, Muhammed H, et al. Evaluation of trypanosomal activity of Tapinanthus globiferus and Gongronema latifolium on Trypanosoma congolense. Biosci Res. 2013;10:20–8. [Google Scholar]
- 12.Misra LN, Wouatsa NA, Kumar S, Venkatesh Kumar R, Tchoumbougnang F. Antibacterial, cytotoxic activities and chemical composition of fruits of two Cameroonian Zanthoxylum species. J Ethnopharmacol. 2013;148:74–80. doi: 10.1016/j.jep.2013.03.069. [DOI] [PubMed] [Google Scholar]
- 13.Adekunle AS, Oluba A, Babatola LJ, Kamdem JP, Adesokan A. Antiatherogenic, hypolipidemic and anti-inflammatory benefits of black tea and Zanthozylum zanthoxyloides. Br J Med Med Res. 2014;4:1923–37. [Google Scholar]
- 14.Vyry Wouatsa NA, Misra LN, Venkatesh Kumar R, Darokar MP, Tchoumbougnang F. Zantholic acid, a new monoterpenoid from Zanthoxylum zanthoxyloides. Nat Prod Res. 2013;27:1994–8. doi: 10.1080/14786419.2013.811662. [DOI] [PubMed] [Google Scholar]
- 15.Collins AR. The comet assay for DNA damage and repair:Principles, applications, and limitations. Mol Biotechnol. 2004;26:249–61. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- 16.Dhawan A, Bajpayee M, Parmar D. Comet assay:A reliable tool for the assessment of DNA damage in different models. Cell Biol Toxicol. 2009;25:5–32. doi: 10.1007/s10565-008-9072-z. [DOI] [PubMed] [Google Scholar]
- 17.Llorente MT, Parra JM, Sánchez-Fortún S, Castaño A. Cytotoxicity and genotoxicity of sewage treatment plant effluents in rainbow trout cells (RTG-2) Water Res. 2012;46:6351–8. doi: 10.1016/j.watres.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 18.Laghari AH, Memon S, Nelofar A, Khan KM, Yasmin A. Determination of free phenolic acids and antioxidant activity of methanolic extracts obtained from fruits and leaves of Chenopodium album. Food Chem. 2011;126:1850–5. doi: 10.1016/j.foodchem.2010.11.165. [DOI] [PubMed] [Google Scholar]
- 19.ICH. Text on validation of analytical procedures:Methodology:Q2 (R1) 2005. [Last accessed on 2012 Sep 24]. Available from: http://www.ich.org .
- 20.Kamdem JP, Adeniran A, Boligon AA, Klimaczewski CV, Olalekan EO, et al. Antioxidant activity, genotoxicity and cytotoxicity evaluation of lemon balm (Melissa officinalis L) ethanolic extract:Its potential role in neuroprotection. Ind Crops Prod. 2013;51:26–34. [Google Scholar]
- 21.Santos DB, Schiar VP, Ribeiro MC, Schwab RS, Meinerz DF, Allebrandt J, et al. Genotoxicity of organoselenium compounds in human leukocytes in vitro. Mutat Res. 2009;676:21–6. doi: 10.1016/j.mrgentox.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Mischell BB, Shiingi SM. New York: WH Freeman Company; 1980. Selected Methods in Cellular Immunology. [Google Scholar]
- 23.Mathew S, Abraham TE. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models. Food Chem. 2006;94:520–8. [Google Scholar]
- 24.Ouedraogo M, Baudoux T, Stévigny C, Nortier J, Colet JM, Efferth T, et al. Review of current and “omics”methods for assessing the toxicity (genotoxicity, teratogenicity and nephrotoxicity) of herbal medicines and mushrooms. J Ethnopharmacol. 2012;140:492–512. doi: 10.1016/j.jep.2012.01.059. [DOI] [PubMed] [Google Scholar]
- 25.Matic S, Stanic S, Bogojevic D, Vidakovic M, Grdovic N, Dinic S, et al. Methanol extract from the stem of Cotinus coggygria Scop. and its major bioactive phytochemical constituent myricetin modulate pyrogallol-induced DNA damage and liver injury. Mutat Res. 2013;755:81–9. doi: 10.1016/j.mrgentox.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Collins AR. Measuring oxidative damage to DNA and its repair with the comet assay. Biochim Biophys Acta. 2014;1840:794–800. doi: 10.1016/j.bbagen.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 27.Kamdem JP, Waczuk EP, Kade IJ, Wagner C, Boligon AA, Athayde ML, et al. Catuaba (Trichilia catigua) prevents against oxidative damage induced by in vitro ischemia-reperfusion in rat hippocampal slices. Neurochem Res. 2012;37:2826–35. doi: 10.1007/s11064-012-0876-0. [DOI] [PubMed] [Google Scholar]
- 28.Elekofehinti OO, Kamdem JP, Boligon AA, Athayde ML, Lopes SR, Waczuk EP, et al. African eggplant (Solanum anguivi Lam) fruits with bioactive polyphenolic compounds exert in vitro antioxidant properties and inhibit Ca2+ induced mitochondrial swelling. Asian Pac J Trop Biomed. 2013;3:757–66. doi: 10.1016/S2221-1691(13)60152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbosa Filho VM, Waczuk EP, Kamdem JP, Abolaji AO, Lacerda SR, Martin da Costa JC, et al. Phytochemical constituents, antioxidant activity, cytotoxicity and osmotic fragility effects of Caju (Anacardium microcarpum) Ind Crops Prod. 2014;55:280–8. [Google Scholar]
- 30.Adekunle AS, Aline AB, Afolabi OK, Rocha JB. Determination of free phenolic acids, flavonoid contents and antioxidant capacity of ethanolic extracts obtained from leaves of mistletoe (Tapinanthus globiferus) Asian J Pharm Clin Res. 2012;5:36–41. [Google Scholar]
- 31.Adekunle AS, Kamdem J, Rocha JB. Antioxidant activity and HPLC analysis of Zanthoxylum zanthoxyloides. Rep Opin. 2012;4:6–13. [Google Scholar]
- 32.Klimaczewski CV, Saraiva RA, Roos DH, Boligon A, Athayde MA, Kamdem JP, et al. Antioxidant activity of Peumus boldus extract and alkaloid boldine against damage induced by Fe(II)-citrate in rat liver mitochondria in vitro. Ind Crops Prod. 2014;54:240–7. [Google Scholar]
- 33.Amarowicz R, Troszynska A. Antioxidant and antiradical activity of extracts of phenolic compounds from red bean. Czech J Food Sci. 2004;22:206–8. [Google Scholar]


