Abstract
Clinically useful predictors of weight gain could be used to reduce the epidemic of post-kidney transplant obesity and resulting co-morbidities. The purpose of this study was to identify predictors of weight gain at 12-months following kidney transplant in a cohort of 96 recipients. Demographic, clinical and environmental data were obtained at transplant and 12-months. Descriptive, correlational, and Bayesian network analysis were used to identify predictors. For the 52 (55.9%) recipients who gained weight, the average amount gained was 9.18 ± 6.59 kg. From the 15 baseline factors that met inclusion criteria, Bayesian network modeling identified 4 baseline predictors for weight gain: younger age, higher carbohydrate consumption, higher trunk fat percentage, and higher perception of mental health quality of life. Three are modifiable through either pre- or immediate post-transplant clinical intervention programs.
Keywords: kidney transplant, obesity, activity, diet, weight gain, Bayesian network modeling
Introduction
Weight gain following kidney transplantation is an epidemic in the United States (1) and worldwide (2). We (3) and others (1, 4) have found that kidney transplant recipients experience an average weight gain between 5 and 10 kilograms (kg), which is linked to decreased patient and graft survival (2). Specifically, weight gain after transplantation has been associated with post-transplant hypertension (2), diabetes (2), dyslipidemia (5, 6), and ischemic heart disease (2), which may explain why cardiovascular disease is the leading cause of death following kidney transplantation (7, 8).
Improved appetite and fewer dietary restrictions combined with continued limited physical activity likely play a role in post-transplant weight gain. The few studies that have explored these factors indicate that race (1, 9) and gender (1, 5, 10) are also associated with weight gain. Even steroid-free protocols do not reduce the risk of obesity (4, 11). Therefore, it is more likely that weight gain in renal transplant recipients is a result of a combination of demographic, clinical, and environmental factors.
Recently machine learning using Bayesian Modeling has been used to develop a predictive model of kidney graft survival (12) based on pre-transplant factors. The purpose of our study was to prospectively examine demographic, clinical, and environmental risk factors and use Bayesian analysis to determine a predictive model of weight gain post-transplant. We sought to identify how these factors, considered together as a network, could predict weight gain and the nature of the increased weight mass (i.e., fat or lean). Long-term goals include the prevention and treatment of obesity in transplant recipients using this model.
Patients and Methods
Between 2008 and 2011 at a single mid-south transplant center in the United States, all persons receiving kidney transplantation who met inclusion criteria were informed of the study and provided consent information prior to or at time of kidney transplantation. All study procedures and methods had Institutional Review Board approval. Inclusion criteria included persons 18 years of age or older with the ability to read and understand English. Recipients were excluded if they were receiving steroid therapy prior to the time of transplantation in order to control for the effect of steroid use on baseline weight. Data from 96 kidney transplant recipients (57% male, 65% Black, mean age ± SD of 50.8 ± 12.6 years [range 19–74]) were included in data analysis (Table 1).
Table 1.
Demographic Characteristics at baseline (n=96)
| Characteristic | Frequency (Percent) |
|---|---|
| Male Gender | 55 (58) |
| Race | |
| African-American | 62 (65) |
| Caucasian | 30 (31) |
| Other | 4 (4) |
| Age, years | Mean = 50.83 (SD=12.55) |
| Diabetes | 24 (25) |
| Hypertension | 88 (92) |
| Donor Type—Deceased | 75 (78) |
Note: SD=standard deviation
Medications prescribed for immunosuppression and comorbidities were administered according to the center’s standardized protocol. This protocol-based practice resulted in little variation among subjects in medication regimens. All transplant recipients received prednisone at the time of transplant; 75% received 20 milligrams (mg) per day and 84% received 5 mg per day at 12 months. Almost all (97%) of participants received mycophenolate mofetil at baseline with dosages ranging from 1000–2000 mg daily, with little change at 12 months after transplant. In addition, at baseline 93% received tacrolimus ranging from 2 mg to 12 mg daily and by 12 months, 95% received 2mg to 20 mg of tacrolimus daily.
Measures including demographic data (i.e., gender, age, ethnicity/race, household income), clinical data (i.e., weight, body mass index [BMI; kg/m2], whole body densitometry [DXA], medications), and environmental data (i.e., dietary intake, physical activity, health status, psychosocial) were obtained at baseline, and 12-months following transplantation. Demographic and clinical data were obtained from patient charts. Psychosocial factors, dietary intake and physical activity recall data were elicited by trained research assistants.
Dietary Intake
The Nutrition Data System for Research, considered the “gold standard” for dietary analysis, was used to record dietary information and convert it into micro and macro-nutrients. A 24-hour dietary intake was recorded for two weekdays and one weekend day to account for different eating patterns during the weekend. Interviews were obtained after the first two weeks following transplantation because this period would likely reflect the return to regular eating patterns.
Physical Activity
Physical activity recall was elicited by telephone interview using the well-established Seven Day Physical Activity Report (13). Based on participants’ response, five subsets of physical activity (sleep, light, moderate, hard, and very hard), kilocalories, and number of days of moderate activity were determined.
Depression
The 20 item Center for Epidemiologic Studies for Depression Scale (CES-D) was used to assess depression levels (14). A score of 16 or greater identifies individuals at risk for clinical depression.
Quality of Life
Quality of life was assessed using the Short Form 36 version 2 (SF36v2), a 36-item multidimensional health survey yielding eight subscale scores and two composite scores for physical and mental health (15). The Mental Composite Score (MCS) and Physical Composite Scores (PCS) are the most commonly reported measures of quality of life and are reported in this study. Higher scores indicate fewer physical limitations (PCS) and better mental / emotional health (MCS).
Whole body densitometry (DXA)
DXA measures, including total body and trunk mass (fat and lean), were obtained at hospital discharge (baseline) and again one year later. A DXA–certified research nurse performed measurements using the Hologic Discovery A (Bedford, MA) software version 8.3. Individuals who were too large to fit within the limits of the scan region were scanned with correct placement of the right arm and part of the left arm out of region of interest. Right arm values were then substituted for left arm values.
Statistical Analysis and Interpretation
Descriptive statistics were used to characterize the sample at baseline and 12-months post-transplant. Correlational and linear regression analyses estimated associations between baseline measures and percent change in weight ((12-month -baseline weight)/baseline weight).
The Bayesian Network Webserver (16) was used to create a model linking predictor variables measured at baseline with percent change in weight 12-months post-transplant. The best explanatory network is established by performing an exhaustive search over all possible network structures, scoring the networks using a Bayesian score metric (17), and performing model averaging of high scoring networks (18). We initially selected 15 predictor baseline variables that had a conceptual or clinically relevant association with weight gain or had a statistically suggestive (p < 0.20) association with percent change in weight based on univariate analysis. These 15 candidate variables were included in the initial model, however 4 were removed for various reasons (see rationale in Results). The final model contained a total of 12 variables, 3 of which were categorical (race, diabetes status, and hyperlipidemia status). We used the following settings when learning the network structure: maximum number of parents=9, number of networks to include in model averaging=1000, and model averaging edge selection threshold=0.5. Structural constraints were added to prevent variables from being the parent of factors that predated the study period (i.e., age, gender, race, diabetes, and hyperlipidemia).
To examine the robustness of structure learning, we resampled from the data used to learn the network structure by randomly selecting, without replacement, 45 of the 52 patients and performed structure learning using these resampled data sets with the same method that was used for the complete data set. Twelve of the 21 edges present in the original model (Figure 1), including the four edges directly connecting explanatory factors to percent change in weight, were present in at least 90% of the resample structures (i.e., the structures learned using the resampled datasets). An additional 4 edges were present in over 75% of the resample structures. The edges that were present in less than 75% of the resample structures linked race and whole body total percent fat (present in 45% of resample structures), mental composite score and whole body total percent fat (40%), mental composite score and total fat grams (40%), hyperlipidemia and total carbohydrate grams (55%), and diabetes and total carbohydrate grams (60%). No edges that were not already included in the structure learned from the original complete dataset were present in more than 40% of the resample structures.
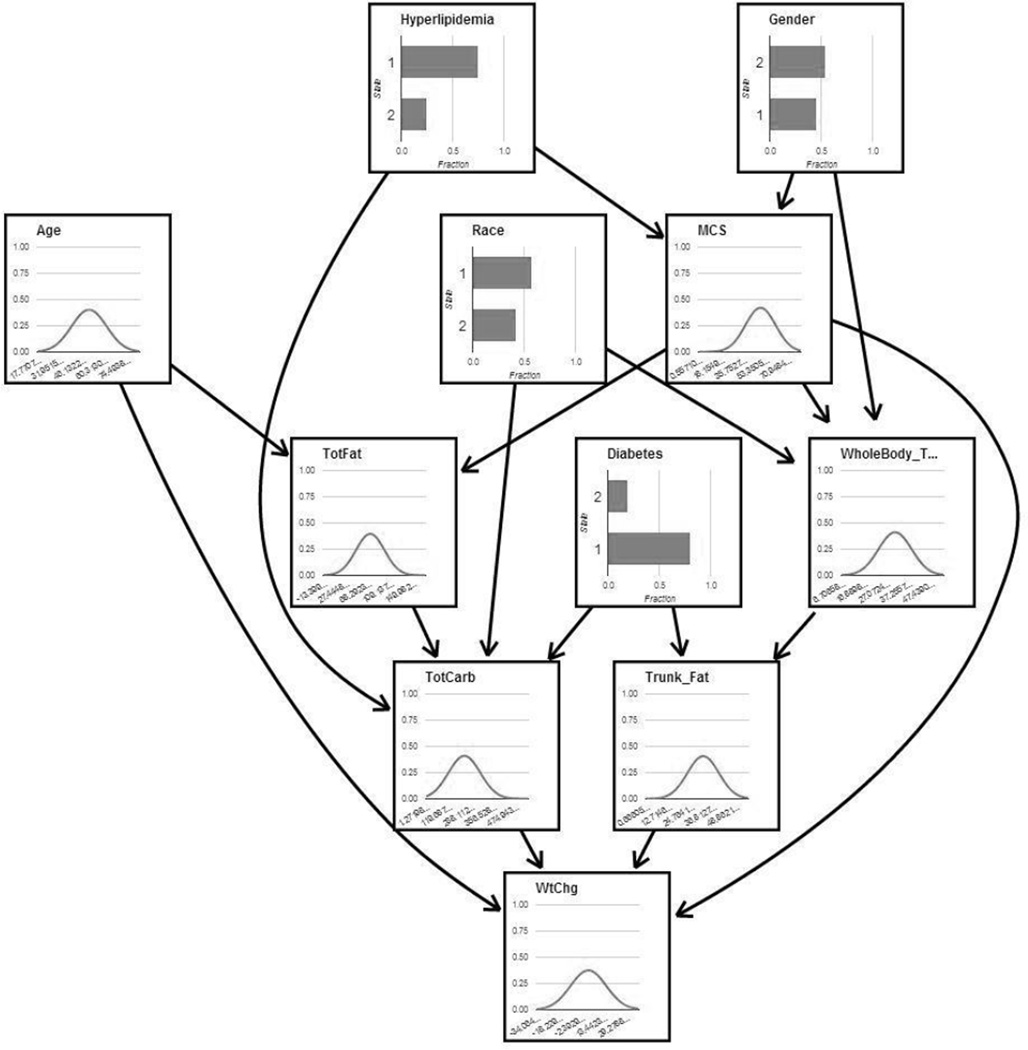
Figure 1.
Bayesian Network Model of weight change following kidney transplantation. Categorical variables are shown as bar charts showing the fraction of individuals for each variable (Diabetes and Hyperlipidemia: 1=no disease, 2=yes; Gender: 1=male, 2=female; Race: 1=white, 2=black). Continuous variables are shown as line charts of the Gaussian distributions that best fit the data. TotFat=Total Fat Gram Intake, MCS= Mental Composite Score of the Short Form 36 version 2, WholeBody_T= Whole Body Total Fat Percent, TotCarb= Total Carbohydrate Gram Intake, Trunk_Fat= Trunk Fat Percent, WtChg= Percent Change in Weight.
We tested the accuracy of the Bayesian network model's predictions using two methods. First, we performed leave-one-out cross validation of the 52 patient data set that was used to learn the network structure. Each patient was selected to be a test case, while the remaining patients were used for parameter learning of the Bayesian network. The values of test case variables were used as evidence in the network to predict the percent change in weight. Second, we used data from 44 patients who had incomplete data (i.e., the patient had missing data for one or more network variables) as a test data set. Predictions for patients with incomplete data were made using available variables as evidence in the network model learned using the 52 patients with complete data (see Figure 2 for associated weight-changes). The accuracy of the predictions of the model was assessed by calculating Pearson’s correlation coefficient and the c-index using the predicted percent change in weight and the observed value of the percent change in weight for a given patient. The c-index is a variant of the area under the receiver operator characteristic that ranges from 0.5 for a random predictor to 1 for a perfect predictor. To calculate the c-index, the model was used to predict the probability that each patient would gain weight 12-months after transplant, given the values of other network variables for that patient. Then, the predicted weight-gain probabilities of all patients who did gain weight with the predicted weight-gain probabilities of all patients who did not gain weight were compared in a pairwise fashion. The c-index was then calculated using the following equation (19): (number of concordant pairs + 0.5*number of ties)/(total number of pairs), where a concordant pair was a pair in which the predicted probability of the patient who did gain weight was higher than the predicted probability of the patient who did not gain weight.
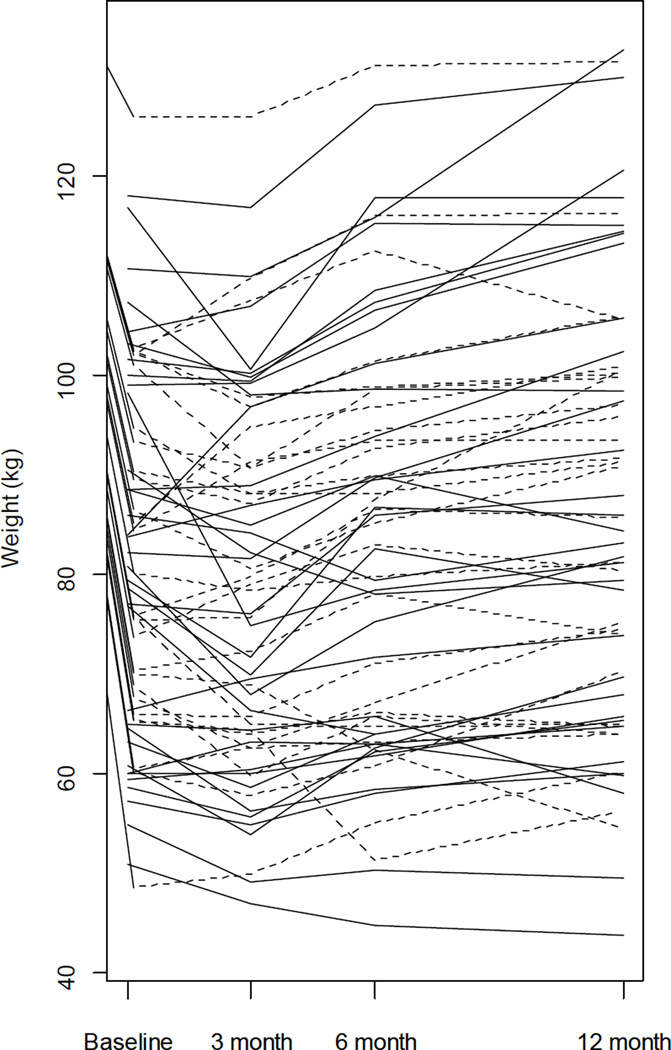
Figure 2.
Trace plot of participant weight. Solid lines: Participants with complete covariate information (n = 52) used to fit Bayesian model. Dashed lines: Participants with incomplete covariate information (n = 44) used as a test data set, see text for details. Note that only baseline and 12 month weights are used for model building.
Results
From baseline to 12 months post-transplant, the kidney transplant recipients experienced a weight change from 81.98 kg ± 1.81 vs. 84.30 kg ± 2.17 (P ≤ 0.01,) (Table 2). Similarly, there were significant changes in BMI (28.10 ± 0.48 vs. 28.93 ± 0.64, P ≤ 0.01). This indicates a great deal of variation exists in weight and it is not normally distributed. Interestingly, for the 52 (55.9%) recipients who gained weight, the average amount gained was 9.18 kg. In addition, 43 recipients (45%) had greater than 5% weight increase from baseline and 30 recipients (31%) had over a 10% increase in weight. Figure 2 shows the actual weight changes of the 96 individual participants.
Table 2.
Weight status and dietary intake of study sample (n=96) at kidney transplantation (baseline) and 12-months post-transplant
| Baseline | 12 Months | ||
|---|---|---|---|
| Weight (kg) | |||
| Mean (SE) | 81.98 (1.81)* | 84.30 (2.17)* | |
| Range | 48.63–126.00 | 43.91 – 133.64 | |
| BMI (kg/m2) | |||
| Mean (SE) | 28.10 (0.48)* | 28.93 (0.64)* | |
| Range | 18.43 – 43.26 | 16.59– 50.25 | |
| Fat (g) | |||
| Mean (SE) | 68.03 (2.62) | 67.30 (3.01) | |
| Range | 12.52–146.34 | 21.82–57.70 | |
| CHO (g) | |||
| Mean (SE) | 193.26 (8.05) | 173.38 (9.05) | |
| Range | 56.62 – 452.80 | 17.27 – 63.71 | |
| Protein (g) | |||
| Mean (SE) | 70.78 ± 2.25 | 68.20 ± 2.67 | |
| Range | 22.50 – 133.24 | 0.30 – 29.04 | |
| Kilocalorie | |||
| Mean (SE) | 1653.14 (57.65) | 1558.56 (67.31) | |
| Range | 718.67 – 3454.92 | 511.86 – 4247.45 | |
Note: Note: p<.01 between timepoints with like symbols. SE=Standard Error. BMI=Body mass index, CHO=carbohydrates, SE=Standard Error.
Age (r=−0.27, P=0.008) and income (r=0.28, P = 0.047) were independently associated with percent change in weight.. Percent change in weight for African-Americans was 3.94±12.09 while it was 1.16±11.52 for Caucasians (P=0.3367). Gender differences in percent change in weight were 1.58±11.19 vs 3.95±12.60 respectively for men and women (P=0.2995). It should be noted that race and gender failed to reach the p<0.2 cut-point required for inclusion in our Bayesian modeling. However, because these factors have been significant in prior studies, these variables were retained for consideration in Bayesian network modeling.
The percent change in weight for the 21 individuals with hyperlipidemia at baseline was −2.50±12.66 compared to 4.08±11.23 for those without hyperlipidemia (P=0.0236); indicating that in the presence of a diagnosis/treatment of hyperlipidemia, weight decreases. Interestingly, HDL levels were found to be inversely associated with percent change in weight (r=−0.3636, P=0.0525, n=29); indicating that as HDL level increases (is in more normal ranges) weight decreases. Because hyperlipidemia met the cut-point for inclusion in modeling, the decision was made to include it in the preliminary stages of our modeling, keeping in mind the potential influence of these inconsistent findings.
The percent change in weight for the 23 individuals with a diagnosis of diabetes (1.02±10.19) was not significantly different (P=0.4613) than that for the 71 individuals without diabetes (3.1266±12.32). However, because diabetes is a significant comorbidity in this population and is linked with obesity in the general population, the decision was made to include it in the preliminary stages of our modeling.
The mean CES-D (depression) score was 17.95 ± 5.34 at baseline, indicating depression, with similar values reported at 12 months (17.01 ± 5.15), but was not significantly associated with percent change in weight. However, the baseline mental composite score of the SF-36v2 was associated with increased weight gain at 12 months (r=0.167, P=0.20) and was included in the Bayesian network analysis.
Significant changes in food intake only occurred in macro nutrients (Table 2). Nutrients that were found to be associated with an increase in weight at one year following kidney transplantation included the consumption of baseline kilocalories of energy (kcals) (r=0.29, P=.005), total fat (r=0.23, P=0.026), total carbohydrates (r=0.30, P=0.004), and total protein (r=0.21, P=0.047) (Table 3) and were included in the Bayesian analysis.
Table 3.
Relationship of percent change in weight (baseline to 12-months post-transplant) to baseline measures
| % Weight Change | ||||
|---|---|---|---|---|
| r | P | n | ||
| Age | −0.2726 | 0.0079 | 94 | |
| Income | 0.2796 | 0.0469 | 51 | |
| CESD | −0.1304 | 0.2967 | 66 | |
| Physical Activity | ||||
| Days of Activity | 0.0193 | 0.8573 | 89 | |
| Sleep | −0.1342 | 0.2126 | 88 | |
| Energy Expenditure | 0.0764 | 0.4742 | 90 | |
| DXA Measures | ||||
| Trunk % Fat | 0.2035 | 0.0780 | 76 | |
| Trunk Mass | 0.1895 | 0.1010 | 76 | |
| Whole Body Total Fat | 0.2019 | 0.0802 | 76 | |
| Whole Body Lean | 0.0445 | 0.7023 | 76 | |
| Whole Body Total Mass | 0.1349 | 0.2453 | 76 | |
| Whole Body Total % Fat | 0.1757 | 0.1290 | 76 | |
| Dietary Intake | ||||
| Kcal Intake | 0.2967 | 0.0045 | 90 | |
| Total Fat Intake | 0.2343 | 0.0263 | 90 | |
| Percent Fat Intake | −0.0375 | 0.7257 | 90 | |
| Total Carbohydrate Intake | 0.3016 | 0.0039 | 90 | |
| Percent Carbohydrate Intake | 0.0945 | 0.3759 | 90 | |
| Total Protein Intake | 0.2098 | 0.0472 | 90 | |
| Percent Protein Intake | 0.0864 | 0.4184 | 90 | |
| Short Form 36 version 2 | ||||
| Mental Composite Score | 0.1676 | 0.2006 | 60 | |
| Physical Composite Score | −0.0057 | 0.9655 | 60 | |
Note: % = percent, CESD= Center for Epidemiologic Studies for Depression Scale, DXA= whole body densitometry, kcal=kilocalorie.
DXA indicated significant changes in body mass distribution from baseline to 12 months post-transplant, with only changes in lean mass (trunk and whole body) failing significance (Table 4). Correlational analyses also identified relationships between percent change in weight and trunk fat (r=0.1895, P=0.1010, n=76), percent trunk fat (r=0.2035, P=0.0780, n=76), whole body fat (r=0.2019, P=0.0802, n=76), and percent whole body fat (r=0.1757, P=0.1290, n=76) and were included in the Bayesian analysis.
Table 4.
DXA Measures of Lean, Fat, and Mass at Baseline and 12-Months Post-Transplant
| Baseline (n=77) | Twelve Months (n=67) | P–Value | ||
|---|---|---|---|---|
| Trunk Fat (kg) | ||||
| Mean (SE) | 12.30 (.66) | 16.15 (.89) | <.0001 | |
| Range | 2.02 – 26.18 | 2.80 – 30.52 | ||
| Trunk Lean (kg) | ||||
| Mean (SE) | 27.80 (.76) | 27.39 (.79) | 0.107 | |
| Range | 15.58 – 42.80 | 16.66 – 41.07 | ||
| Trunk Mass (kg) | ||||
| Mean (SE) | 40.10 (1.18) | 43.58 (1.48) | 0.021 | |
| Range | 23.75 – 68.04 | 22.34 – 71.59 | ||
| Trunk Fat (%) | ||||
| Mean (SE) | 29.68 (1.11) | 35.65 (1.21) | 0.0004 | |
| Range | 8.10– 48.80 | 9.90 – 51.40 | ||
| Whole Body Total Fat (kg) | ||||
| Mean (SE) | 23.64 (1.07) | 29.95 (1.44) | <.0001 | |
| Range | 4.58 – 44.30 | 6.97 – 51.31 | ||
| Whole Body Total Lean (kg) | ||||
| Mean (SE) | 55.61 (1.51) | 55.71 (1.58) | 0.691 | |
| Range | 31.74 – 79.70 | 32.79 – 81.89 | ||
| Whole Body Total Mass (kg) | ||||
| Mean (SE) | 79.28 (2.07) | 85.80 (2.57) | 0.004 | |
| Range | 47.98 – 120.69 | 43.81 – 130.55 | ||
| Whole Body Total Fat (%) | ||||
| Mean (SE) | 29.42 (1.02) | 34.17 (1.07) | 0.0006 | |
| Range | 9.6 – 50.2 | 10.8 – 51.9 | ||
Note: DXA= whole body densitometry, SE=standard error.
There were no differences in physical activity, from baseline to 12 months when measured in days/week of activity, (2.13 + 0.25 vs. 2.84 + 0.28; P=NS); however, there was a small increase when calculated in kilocalories expended/day (32.56 ± 0.19 to 33.61 ± 0.38; P≤0.04). There was also a significant change in minutes /week of sleep which decreased from baseline of (3526.59±.23) to 12 months (3341.94±67.46, P≤0.05), but this has questionable clinical significance. Neither physical activity nor sleep was found to be associated with weight gain and were not included in the Bayesian analysis.
Bayesian Network Model
A Bayesian network is a graphical model in which a set of random variables are connected by arcs that represent conditional dependencies between the variables. The arcs in the network point from a parent variable to a child variable, indicating that the value of the parent influences the probability function of the value of the child. The 15 baseline variables chosen for the model reflected categories of demographic (age, gender, race, income), nutritional intake (Kcals consumed, total fat consumed, total carbohydrates consumed, total protein consumed), co-morbidities (hyperlipidemia, diabetes, mental composite score) and body mass distribution (trunk fat, percent trunk fat, whole body fat, percent whole body fat). We removed 4 of these variables (income, total protein consumed, trunk fat, and whole body fat) from future consideration when creating the network model based on the following considerations: (a) Income data was severely skewed with few individuals reporting incomes over $29,000/year. (b) Inclusion of 4 separate dietary measures was considered redundant. Therefore, total protein consumption was deleted from further consideration, because this element should contribute to weight gain less than fat and carbohydrate consumption; and changes in protein consumption were most likely a function of changes in the other food elements. (c) The 4 measures of body mass distribution actually presented two different measures (percent and mass) for each two different areas (trunk and whole body). We, therefore, selected percent of trunk and whole body fat to include in the network because they more equitably account for differences in height among the study participants. (d) Finally, total energy consumed (kcal) was included during structure learning of the network; however, because kcal did not influence any network variables (i.e., had no “children” in the network), it was removed for clarity.
The Bayesian network model (Figure 1) shows the influence of baseline variables on weight change 12-months post-transplant. The desired outcome variable (percent change in weight) was not required to be a terminal node in the network with no children, but the highest scoring network structures learned from the data placed percent change in weight in this position. The network model identifies four baseline variables (age, total carbohydrates consumed, mental composite score, and percent trunk fat) that directly influence percent weight change, with other variables having an indirect influence. For example, total fat consumed is not directly linked to percent change in weight in the network, but may have an influence through its association with total carbohydrates consumed. Specifically, the percent change in weight at 12-months is predicted to increase by ~1% for each 2 year decrease in baseline age, 25 gram increase in baseline carbohydrates consumption, 2% increase in the baseline percentage of trunk fat, and 2.5 unit increase in baseline mental composite score.
Discussion
The goal of this study was to identify which factors predicted weight gain at 12 months following transplant. Over half of the recipients (55.9%) gained weight with the average amount 9.18 kg. A significant increase was found in fat mass and not in lean muscle mass. Bayesian network modeling identified four significant predictors for weight gain: younger age, higher carbohydrate consumption, higher trunk fat percentage, and higher perception of mental health quality of life. Physical activity was not found to increase during the post-transplant period.
There was a small, albeit statistically significant, increase in weight from pre-transplant to one year post-transplant for the entire group. While large increases in weight as a group may not be present, a clinically significant portion of recipients (25.8%) gained weight sufficient to increase their status to the next higher BMI category. In our study, 31% of participants gained more than 10% of their baseline weight by 12-months post-transplant. This increase represents a significant portion of the transplant population whose recovery and well-being are compromised.
The Bayesian network model learned from the data included weight change as a terminal node in the network, which suggests that risk factors used in the model influence weight change. Specifically, higher baseline trunk fat percentage, total amount of carbohydrates consumed, and higher mental composite scores predicted increases in weight. Interestingly, the model indicated that the best dietary predictor of percent change in weight was total carbohydrates, not total calories consumed, which indicates the importance of introducing a healthy lifestyle early in the post-transplant year.
Other environmental risk factors had an indirect influence on percent weight change. For example, whole body percent fat influences weight change because it is associated with trunk fat percent, and total fat consumed influences weight change because it is associated with total carbohydrates consumed. While our Bayesian network model compares favorably with previous attempts to model transplant outcomes from baseline measures (12), the predictive power of the model was not outstanding, indicating that that other factors, perhaps genetics, may play a role.
The negligible change in physical activity is a disappointing observation. Essentially, not one transplant recipient became more active following their transplant surgery. A possible explanation is that patients have a fear of injuring the transplanted kidney in addition to health problems related and unrelated to the transplant (20). Also, our previous findings identified that a lack of motivation for physical activity was reported by 62% (21). However, Painter et al. (22) have shown that exercise capacity can increase following transplantation. But another small study showed that exercise capacity only increased up to 16 months post-transplant, after which it remained stable or declined (23). Another possibility is that recipients were depressed throughout the study and were thus less active. Our findings suggest that there may be a window of opportunity during the first year for transplant recipients to increase their exercise capacity, and subsequently decrease morbidity. Sustained and supportive efforts by clinicians are needed to reassure the recipient that the transplanted kidney will not be harmed and to increase motivation. This observation provides an opportunity for continued intervention.
It appears that the removal of pre-transplant protein restriction did not result in increased protein intake. Rather, protein and carbohydrate intake declined while fat intake increased. It is important to note that these dietary changes took place within the first three months following transplant surgery (data not shown). Our previous findings (20) and anecdotal observations suggest that recipients increase their food intake immediately following surgery, perhaps as a result of increased appetite from steroids and relaxation of dietary restrictions. These early changes (within 1–2 weeks of transplantation) may provide a previously overlooked opportunity for a lifestyle intervention that could avert the formation of unhealthy dietary practice.
Our transplant recipients experienced several comorbid conditions including diabetes, hypertension, and depression that required a complicated medication regimen. These factors were not significantly associated with weight gain in our study; however, they complicate the delivery of clinical interventions. It is likely that interventions will need to make use of an individualized or tailored approach.
It is clear from our body composition data and others (22, 24) that recipients experience a significant gain in fat mass, not lean muscle mass. Our findings indicate that fat mass increases occurred both in the trunk and whole body; and greater baseline body fat indicates a greater likelihood that the individual would gain more weight during the first year following transplant surgery. This is particularly disturbing in light of findings showing that transplant recipients are arriving at transplant increasingly overweight (25).
Overall, our findings support the need to encourage intervention in the immediate post-transplant period. Given the complex challenges that transplant recipients face, use of an individualized approach such as motivational interviewing would likely be of benefit. Anecdotal observations suggest that if weight gain could be prevented for the first 3–6 months, then continued and significant weight gain could be attenuated.
Acknowledgement
The authors would like to acknowledge the National Institutes of Health/National Institute of Nursing Research for funding R01NR009270 and 3R01NR009270-03S2. Our research associates Robin Bloodworth and Deborah Gibson were instrumental in recruiting and retaining our subjects and managing our database.
Footnotes
Disclaimer: Data were collected while AK Cashion was a Professor at the University of Tennessee Health Science Center.
References
- 1.Clunk JM, Lin CY, Curtis JJ. Variables affecting weight gain in renal transplant recipients. Am J Kidney Dis. 2001;38(2):349. doi: 10.1053/ajkd.2001.26100. [DOI] [PubMed] [Google Scholar]
- 2.el-Agroudy AE, Wafa EW, Gheith OE, Shehab el-Dein AB, Ghoneim MA. Weight gain after renal transplantation is a risk factor for patient and graft outcome. Transplantation. 2004;77(9):1381. doi: 10.1097/01.tp.0000120949.86038.62. [DOI] [PubMed] [Google Scholar]
- 3.Cashion AK, Sanchez ZV, Cowan PA, Hathaway DK, Lo Costello A, Gaber AO. Changes in weight during the first year after kidney transplantation. Prog Transplant. 2007;17(1):40. doi: 10.1177/152692480701700106. [DOI] [PubMed] [Google Scholar]
- 4.Elster EA, Leeser DB, Morrissette C, Pepek JM, Quiko A, Hale DA, et al. Obesity following kidney transplantation and steroid avoidance immunosuppression. Clin Transplant. 2008;22(3):354. doi: 10.1111/j.1399-0012.2008.00792.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson CP, Gallagher-Lepak S, Zhu YR, Porth C, Kelber S, Roza AM, et al. Factors influencing weight gain after renal transplantation. Transplantation. 1993;56(4):822. doi: 10.1097/00007890-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Nazemian F, Naghibi M. Weight-gain-related factors in renal transplantation. Experimental and clinical transplantation. 2005;3(1):329. [PubMed] [Google Scholar]
- 7.Baum CL. Weight gain and cardiovascular risk after organ transplantation. JPEN J Parenter Enteral Nutr. 2001;25(3):114. doi: 10.1177/0148607101025003114. [DOI] [PubMed] [Google Scholar]
- 8.Briggs JD. Causes of death after renal transplantation. Nephrol Dial Transplant. 2001;16(8):1545. doi: 10.1093/ndt/16.8.1545. [DOI] [PubMed] [Google Scholar]
- 9.Baum CL, Thielke K, Westin E, Kogan E, Cicalese L, Benedetti E. Predictors of weight gain and cardiovascular risk in a cohort of racially diverse kidney transplant recipients. Nutrition. 2002;18(2):139. doi: 10.1016/s0899-9007(01)00723-7. [DOI] [PubMed] [Google Scholar]
- 10.El Haggan W, Vendrely B, Chauveau P, Barthe N, Castaing F, Berger F, et al. Early evolution of nutritional status and body composition after kidney transplantation. Am J Kidney Dis. 2002;40(3):629. doi: 10.1053/ajkd.2002.34926. [DOI] [PubMed] [Google Scholar]
- 11.van den Ham EC, Kooman JP, Christiaans MH, Nieman FH, van Hooff JP. Weight changes after renal transplantation: a comparison between patients on 5-mg maintenance steroid therapy and those on steroid-free immunosuppressive therapy. Transpl Int. 2003;16(5):300. doi: 10.1007/s00147-002-0502-1. [DOI] [PubMed] [Google Scholar]
- 12.Brown TS, Elster EA, Stevens K, Graybill JC, Gillern S, Phinney S, et al. Bayesian modeling of pretransplant variables accurately predicts kidney graft survival. American journal of nephrology. 2012;36(6):561. doi: 10.1159/000345552. [DOI] [PubMed] [Google Scholar]
- 13.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, et al. Physical activity assessment methodology in the Five-City Project. American journal of epidemiology. 1985;121(1):91. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 14.McDowell I, Newell C. Measuring health: A guide to rating scales and questionnaires. 2nd ed. New York: Oxford University Press; 1996. [Google Scholar]
- 15.Ware JE, Jr, Kosinski M, Dewey JE. How to score version two of the SF-36 health survey. Lincoln, RI: Quality Metric, Inc.; 2000. [Google Scholar]
- 16.Ziebarth JD, Bhattacharya A, Cui Y. Bayesian Network Webserver: a comprehensive tool for biological network modeling. Bioinformatics. 2013;29(21):2801. doi: 10.1093/bioinformatics/btt472. [DOI] [PubMed] [Google Scholar]
- 17.Dethlefsen C, Boettcher SG. deal: a package for learning bayesian networks. Journal of Statistical Software. 2003;8(20):1. [Google Scholar]
- 18.Tian J, He R, Ram L. Bayesian model averaging using the k-best Bayesian network structures. In: Grünwald P, Spirtes P, editors. Proceedings of the 26th Conference on Uncertainty in Artificial Intelligence, UAI, 2010, 978-0-9749039-6-5. AUAI Press; 2010. [Google Scholar]
- 19.Tripepi G, Jager KJ, Dekker FW, Zoccali C. Statistical methods for the assessment of prognostic biomarkers (Part I): discrimination. Nephrol Dial Transplant. 2010;25(5):1399. doi: 10.1093/ndt/gfq018. [DOI] [PubMed] [Google Scholar]
- 20.Stanfill A, Bloodworth R, Cashion A. Lessons learned: experiences of gaining weight by kidney transplant recipients. Prog Transplant. 2012;22(1):71. doi: 10.7182/pit2012986. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez ZV, Cashion AK, Cowan PA, Jacob SR, Wicks MN, Velasquez-Mieyer P. Perceived barriers and facilitators to physical activity in kidney transplant recipients. Prog Transplant. 2007;17(4):324. doi: 10.1177/152692480701700411. [DOI] [PubMed] [Google Scholar]
- 22.Painter PL, Hector L, Ray K, Lynes L, Dibble S, Paul SM, et al. A randomized trial of exercise training after renal transplantation. Transplantation. 2002;74(1):42. doi: 10.1097/00007890-200207150-00008. [DOI] [PubMed] [Google Scholar]
- 23.Zakliczynski M, Spiechowicz U, Krynicka A, Trybunia D, Pyka L, Wiecek A, et al. Fluctuations of exercise capacity in patients after kidney transplantation. Transplant Proc. 2009;41(1):184. doi: 10.1016/j.transproceed.2008.10.071. [DOI] [PubMed] [Google Scholar]
- 24.Ulivieri FM, Piodi LP, Aroldi A, Cesana BM. Effect of kidney transplantation on bone mass and body composition in males. Transplantation. 2002;73(4):612. doi: 10.1097/00007890-200202270-00024. [DOI] [PubMed] [Google Scholar]
- 25.Friedman AN, Miskulin DC, Rosenberg IH, Levey AS. Demographics and trends in overweight and obesity in patients at time of kidney transplantation. Am J Kidney Dis. 2003;41(2):480. doi: 10.1053/ajkd.2003.50059. [DOI] [PubMed] [Google Scholar]