Abstract
Movements are variable. Recent findings in smooth pursuit eye movements provide an explanation for motor variation in terms of the organization of the brain’s sensory-motor pathways. Variation in sensory estimation is propagated through sensory-motor circuits and ultimately causes motor variation. The sensory origin of motor variation creates trial-by-trial correlations among the responses of neurons at each level of the sensory motor circuit, and between neural and behavioral responses. We suggest that motor variation is a compromise between multiple competing constraints. The brain strives for motor behavior that is ‘good enough’ in the face of constraints that tend to promote variation.
Introduction
Movements are variable. Why? It is tempting to answer that movement varies because neural spike trains are noisy. But it also is possible that the source of motor variation is principled. Variation may arise through a compromise among several competing constraints, because of a limitation on neural processing imposed by the architecture of neural circuits, as a means to optimize some other goals of motor activity — or all of the above.
Spike trains of neurons are stochastic in the sense that spike timing is quite variable. Repetition of the same stimulus leads to approximately equal values of the mean and variance of spike count [1]. It is tempting to think of the stochastic variation in spike counts as ‘noise’. The premise of our paper is that some of the variation is ‘signal’. There are correlations in spiking across neurons even at peripheral sensory levels; the existence of convergence and divergence in neural circuits allows those correlations to be propagated through a circuit and to control variation in motor output. Thus, we describe the trial-by-trial fluctuation in neural responses (and behavior) as ‘variation’ instead of ‘noise’ as a reminder that the variation may be ‘signal’.
Behavioral analysis
The relevance of variation to the neural mechanisms of movement came into clear view when Harris and Wolpert [2•] explained plausible control strategies designed to minimize the variance of the endpoint of the movement. They proposed that the control signals of motoneurons are noisy, and that the noise is proportional to the amplitude of the signal the motoneurons send to muscles. Their theory provided a plausible explanation for the trajectories of saccadic eye movements and reaching arm movements. It also explained why the brain chooses stereotyped movement trajectories when an infinite number of trajectories are possible [3]. Their theory implies, but does not require, that motor variation originate in the final motor pathways.
Smooth pursuit eye movements have provided an excellent behavior for a deeper understanding of signal, noise, and variation in neural sensory-motor processing. Smooth pursuit occurs when a human or non-human primate tracks a small target that is moving smoothly at relatively slow speeds [4,5]. We can track a car as it moves across the horizon, but not a baseball as it races from pitcher to catcher.
On the basis of an analysis of pursuit eye movements, Osborne et al. [6••] proposed that sensory processing leads to errors in estimating the parameters of target motion, and that the motor system follows the erroneous estimates loyally, giving rise to trial-by-trial variation in the initiation of pursuit. They observed that the first 100 ms of a pursuit eye movement is quite variable, and showed that >90% of the variation could be accounted for in terms of mis-estimates of the parameters of the sensory stimulus: target speed, target direction, and time of onset of target motion. For example, suppose that a target moves at 20°/s in the up and right direction (1:30 on the clock, or 45° in polar coordinates). To track the target correctly, the brain must estimate the speed and direction of target motion. Osborne et al. [6••] suggested that those estimates vary from trial-to-trial, with estimates for speed ranging from about 17 to 23°/s and for direction from about 42 to 48°.
A second component of motor variation emerges late in the sensory-motor pathway. The visually driven initiation of pursuit is followed by a later ‘steady-state’ response that is driven by corollary discharge of motor commands [7] as well as by visual motion signals [8]. A theoretically based analysis of recordings from the cerebellum and brainstem demonstrate that much of the variation in the steady-state response arises late in the sensory-motor circuit, accumulates as a function of time, and scales with the magnitude of the eye movement [9,10], as predicted by Harris and Wolpert [2•].
Thus, a single framework has emerged that covers arm movements, saccadic eye movements, and smooth pursuit. At least for pursuit [6••] and saccades [11], variation in estimates of sensory parameters drives much of the variation in the first 100 ms of the movement. For longer-duration movements, motor circuitry creates variation as the movement evolves. In pursuit eye movements, the motor component of variation fits the framework of ‘signal-dependent noise’ [2•,10]. The situation with saccades may provide a way to understand the relationship between sensory versus motor sources of noise. Sensory noise could create errors in specification of saccade amplitude [11], while signal-dependent motor noise may dictate a control strategy that leads to their smooth and stereotyped trajectories [2•].
Neural correlates of movement variation
One of the most frequent observations in recordings of neural activity is that spike trains are highly variable, even across repetitions of the same sensory stimulus [12] or of nominally identical movements [13]. Trial-by-trial variation in neural responses appears both in the spike counts across large or small analysis windows and in the intervals between successive spikes [1,14]. The existence of trial-by-trial variation in neural and motor responses raises the question of whether the two are related. A priori, we might guess that the trial-by-trial variation in the spiking patterns of an individual neuron is truly independent noise. If this were true, then the large numbers of neurons at each level of a mammalian sensory-motor system should allow the noise to be averaged away [15], and trial-by-trial variation in neural spike trains should not be related to motor variation.
Recordings from neurons during pursuit contradict the common wisdom that trial-by-trial neural variation is simply noise that can be eliminated easily. The evidence that neural variation is partly a signal comes from analysis of trial-by-trial ‘neuron–behavior’ correlations between the spike trains of individual neurons and the kinematic parameters of eye movement behavior. For pursuit, neuron–behavior correlations appear in area MT [16•,17], the smooth eye movement region of the frontal eye fields [18], the cerebellar floccular complex [9], and several types of neurons in the brain-stem [10].
Neuron–behavior correlations imply that some of the variation in the firing of one neuron is being transmitted all the way to the final output. This could occur if the neuron has a powerful influence on the output, or more probably because the trial-by-trial variation in the firing of the neuron under study is a proxy for correlated variation in many neurons that together control the output. Thus, trial-by-trial variation in neural responses can be more than just single-neuron noise — it can be a signal that propagates through the system.
There are clear parallels between our data on pursuit eye movements and others’ data on perceptual judgments. The trial-by-trial variation in the activity of single cortical sensory neurons predicts, albeit weakly, perceptual behavior [19,20]. Thus, a general principle is that one spike train in one neuron at any of multiple levels of a sensory-motor circuit predicts something about the variation in an impending movement.
Shared variation in neural populations: a cause of motor variation
For multiple repetitions of the same sensory stimulus, the variation of neural responses in the sensory cortex has two components [21•]. The same appears to be true at multiple levels of the sensory-motor circuit for pursuit eye movements [10,22•].
The first component is independent variability that is largely private to individual neurons and causes fluctuations in the timing of action potentials. Independent variability can be reduced by averaging across the members of a neural population at downstream sites, where multiple neurons converge onto single post-synaptic targets.
The second component is shared and appears on multiple or even most neurons in a given population. The shared component of variation could arise from top-down influences such as attention or other state-dependent variation [21•], or from correlated variation that is present in sensory representations [23,24]. In the visual system, correlated variation in the responses of motion selective neurons could arise even in the retina [25]. The correlated, shared component of variation is difficult or even impossible to remove by averaging. As a result, it propagates along sensory-motor circuits and ultimately causes trial-by-trial variation in motor behavior.
In smooth pursuit eye movements, the shared component of variation is expressed as trial-by-trial ‘noise’ correlations in the number of spikes discharged by pairs of neurons in extrastriate area MT [23,24]. The magnitude of the correlation varies for different pairs of neurons, and is generally larger if the pairs share preferred stimulus features such as receptive field location, preferred direction, and preferred speed. This particular ‘structure’ in the neuron–neuron correlations is precisely the kind of variation that affects behavioral output because it cannot be removed by simply averaging the activity of large populations of similarly tuned neurons [26]. Averaging over N neurons results in an output with variance that is roughly 1/N times the average variance of the input neurons, plus an order 1 contribution given by the average covariance.
Structured neuron–neuron correlations lead to neuron–behavior correlations, because a positive fluctuation in any one neuron is highly predictive of a positive fluctuation in the average across many similar neurons. The magnitude of neuron–behavior correlation is proportional to how strongly the neuron under study is correlated with related neurons that prefer the same stimulus [17], and inversely related to how much independent variation is carried by the neuron under study and how much variation is added ‘downstream’ [18]. The analysis of the general case is slightly more complicated [27•], but the intuition that positive correlations between neurons with similar preferred stimuli lead to high neuron–behavior correlations is roughly true and holds even when the population of neurons is decoded optimally.
MT-pursuit correlations are relatively weak because of the large independent variation in MT spike trains. As signals proceed into the system from area MT to cerebellum to brainstem to motoneurons, the independent variability related to interspike interval variability decreases. Less of the neurons’ fluctuations in spiking are independent of behavior; more of the fluctuations are correlated across neurons and therefore drive variation in the behavior. Neuron–behavior correlations increase.
A unifying framework
Our findings in pursuit eye movements suggest the unifying framework outlined in Figure 1. The framework has four concepts.
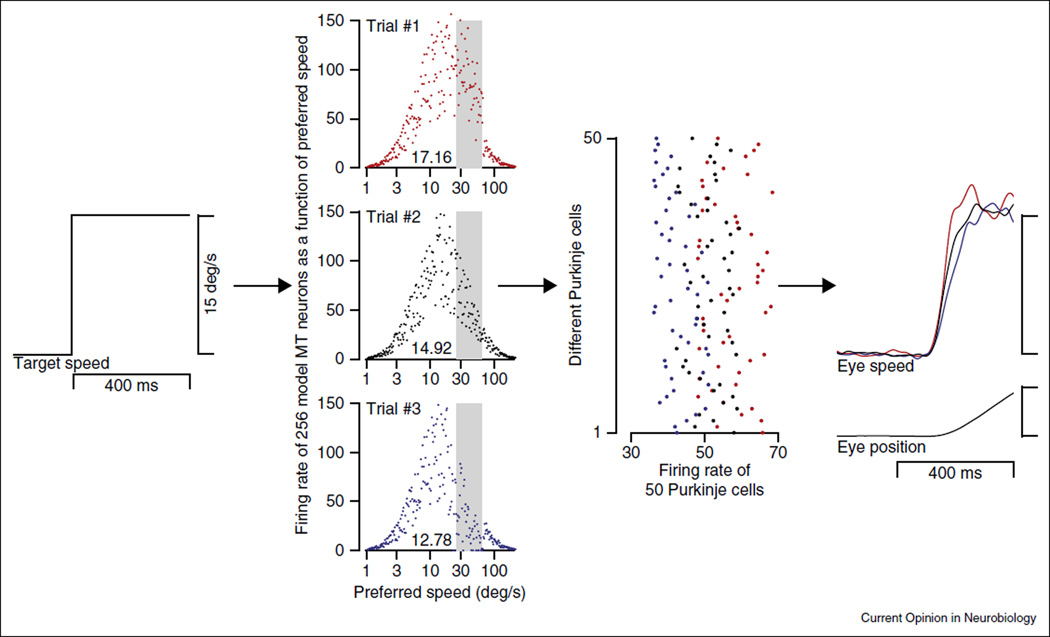
Figure 1.
Different repetitions of the same target motion lead to different sensory representations, motor commands, and motor outputs. Here, the first column shows a step of target speed of 15°/s. The second column summarizes three responses of a single population of neurons in extrastriate area MT by plotting each neuron’s response as a function of its preferred speed. The neurons with preferred speeds near target speed show the largest responses, and the responses are quite variable across neurons. The numbers at the bottom of each graph indicate the estimate of target speed obtained by using a vector averaging decoder to find the center of mass of the population response. The gray bar indicates neurons that show correlated variation in spike counts. The third column represents the motor system with the discharge on three trials of a population of 50 model floccular Purkinje cells represented by the dots distributed along the y-axis. The firing rate across the population (x-axis) was low, medium, and high for the blue, black, and red trials, driven by the estimates of target speed from the corresponding model MT population responses. Within each trial, the population of Purkinje cells showed some degree of variation in firing rate because of the irregularity of the interspike intervals (CV approximately 0.4). Because of the different estimates of target speed, the firing rate of the Purkinje cell population varies systematically, leading to the different trajectories of eye speed demonstrated in the fourth column.
Concept #1: Noise in sensory processing creates shared variation in populations of sensory neurons
The shared variation leads to a redundant code characterized by positive neuron–neuron correlations between similarly tuned neurons in the sensory cortex [23,24]. In Figure 1, the three graphs in the second column plot the firing rates of a population of model MT neurons as a function of each neuron’s preferred speed, for three repetitions of the same target motion. Shared variation causes the activity of neurons in the gray shaded regions to fluctuate up and down together. In reality, groups of neurons with other preferred speeds also will fluctuate together, but in Figure 1 we have allowed only those in the gray shaded region to do so. The correlated variations make a large population of MT neurons act as if they were a small population [15].
Concept #2: A decoding computation estimates target speed by finding the center of mass of the population responses [28,29]
The observed relationship between actual MT population responses and pursuit behavior supports the idea that the sensory decoder for pursuit is akin to ‘vector averaging’ [29–31]. In Figure 1, application of vector averaging estimates target speed from the three population responses as 12.78, 14.92, and 17.16°/s, even though the actual target speed was 15°/s.
Concept #3: The motor system follows loyally the variable estimates of target speed obtained from sensory cortex
Analysis of our recording experiments implies that little of the variation in the initiation of pursuit arises downstream from the cerebellum [9], or even from the smooth eye movement region of the frontal eye fields [18] or area MT [16•]. We suggest that the variable estimates of sensory parameters are distributed to most or all of the neurons in successive downstream populations. As a result, the decoded estimates of target speed and direction create a shared component of neural variation that propagates through the sensory-motor circuit to Purkinje cells of the cerebellum (3rd column of Figure 1) and creates different initial trajectories of eye velocity (4th column of Figure 1).
Concept #4: The statistics of neural responses are controlled mainly by correlated sensory variation
At each level, including sensory cortex, the variation in neural spike counts is correlated with variation in motor behavior, and neural variation is correlated across the population. As signals proceed deeper into the motor system, neural discharges become much more regular so that the amount of ‘independent’ variation decreases and, as a result, the magnitude of neuron–behavior correlations increases. Indeed, by the time the signals reach the cerebellum, a single extra spike in one Purkinje cell predicts on average 0.5 standard deviations of the pursuit behavior [22•].
Theory versus reality
We have proposed that shared variation in sensory representations causes sensory-driven variation in motor behavior, even when independent variation would be removed by averaging because huge populations of neurons are involved. Motor variation is created by correlations across the sensory neuron population, and increasing the population size above 50–100 neurons cannot reduce behavioral variation [15,24].
Simple models of neural activity based on the observed patterns of correlations in MT seem to substantiate our proposal. Information about the stimulus saturates when the size of the population exceeds 50–100 neurons [32]. The information contained in a population response is meaningful for our arguments because it defines limits on precision. If information saturates as a function of population size, then the precision of sensory estimates should be finite, as it is [6••,24], and motor variation and significant neuron–behavior correlations are inevitable.
Yet, two recent theoretical papers raise some concerns about our proposal [27•,33•]. First, noise correlations between neurons with similar preferred speeds do not limit the information contained in model population responses [27•]. Second, the ‘information’ present in a population response can, under some circumstances, increase without bound as population size increases. For example, information does not saturate in realistic model sensory populations with tuning curves that have heterogeneous amplitudes [33•].
Theory and reality diverge when we realize that measures of information assume optimal decoders, while the decoders used in the brain may be decidedly suboptimal. We think that the brain uses decoders that average the responses across neurons with similar preferred stimuli. Vector averaging is an example [30,31]. Vector averaging is close to optimal for decoding a well-behaved population response [28]. But, it averages across neurons with similar preferred stimuli in a way that loses information and therefore would limit the precision of estimates of speed and direction under conditions of shared variation [26]. We suggest that suboptimal averaging in the brain lies at the heart of the differences between theoretical analyses and the realities of our data.
We can be confident of the existence but not the location of suboptimal inference in the sensory-motor circuit for pursuit. Suboptimal inference between the retina and area MT may be the cause of the shared variation that is expressed in MT as structured neuron–neuron correlations that limit behavioral precision [24]. However, even if the information content in MT is unlimited, the MT population decoder must be suboptimal, at least in the sense that it averages activity across neurons with similar preferred stimuli. Otherwise, we cannot explain our observation that behavioral precision is limited [6••] even though MT neurons have tuning curves with heterogeneous amplitudes [33•].
We are comfortable with the idea that the brain performs suboptimal inference. Even though behavioral performance seems to obey optimal frameworks [34–36], we do not think that the brain performs perfectly optimal computations. We assume that sensory areas are not calculating information, but rather are doing what they can do easily within the constraints of neural architecture and broad task demands: estimating or approximating relevant features such as the speed and direction of target motion. Thus, suboptimal inference, potentially at early stages of sensory processing, may be a major cause of motor variation [37••].
Why is there motor variation?
Is motor variation a feature or is it just noise? Does it have a function that is crucial to the operation of sensory-motor circuits? Is it a by-product of fundamental constraints on neural processing? Could the nervous system eliminate motor variation if it wanted to?
Studies in birdsong provide a tangible example where the system seems to exploit variation to explore the parameter space of movement in the service of learning. By adapting to retain those behaviors that lead to more favorable outcomes, the system can resist the degradation that might come with damage or aging [38]. This situation easily could apply to all movement systems, suggesting that movement variation, within limits, may be advantageous for the organism.
The organization of sensory-motor circuits may not pro-duce perfect behavior, but it may be ‘optimal’ in other important ways. We imagine that the brain cannot reduce motor variation without relaxing other constraints. For example, less motor variation may require longer reaction times, or complex neural computations with expanded numbers of neurons requiring larger brains and more energy consumption. Inherent limitations on the degree of motor variation might explain why professional athletes cannot perform their skills perfectly [39], even when doing so would have enormous financial benefit. In principle, it might be possible to prevent the shared component of neural variation from propagating through a sensory-motor circuit, but in practice the ‘solutions’ might create new problems.
We imagine that the brain computes the representation of visual motion through suboptimal estimates rather than through optimal, precise calculations [40•]. Sensory estimates might be more accurate if the problem were more constrained, for example if the system were confronted with only a limited set of moving stimuli. However, the nearly infinite variety of stimulus forms and trajectories in the world may force the brain to use suboptimal inference as a compromise between limited neural resources and more veridical representations of motion [37••]. For example, the brain may have chosen to allow tolerable variation in sensory estimates to limit the number of neurons that are involved in a single task, and thus limit the brain’s size and energetic demands. Perhaps ‘good enough’ (rather than perfect) is the brain’s goal, and maybe that is almost always good enough.
Acknowledgements
We thank Jeff Beck for many helpful discussions and Greg Field and Mati Joshua for comments on an early version of the paper. Research that led to this review was supported by the Howard Hughes Medical Institute, the Sloan and Swartz Foundations, and the NIH (EY03878 and EY017210).
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest or competing financial interests
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Softky WR, Koch C. The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J Neurosci. 1993;13:334–350. doi: 10.1523/JNEUROSCI.13-01-00334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394:780–784. doi: 10.1038/29528. Theoretical analysis showed that the detailed trajectories of arm and saccadic eye movements emerge seamlessly from the assumptions that first, the motor command signals are noisy and second, the magnitude of the noise scales with the amplitude of the movement command. This paper was the first to draw the attention of the field to the existence and importance of motor variation.
- 3.Atkeson CG, Hollerbach JM. Kinematic features of unrestrained vertical arm movements. J Neurosci. 1985;5:2318–2330. doi: 10.1523/JNEUROSCI.05-09-02318.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol. 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lisberger SG, Westbrook LE. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neurosci. 1985;5:1662–1673. doi: 10.1523/JNEUROSCI.05-06-01662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Osborne LC, Lisberger SG, Bialek W. A sensory source for motor variation. Nature. 2005;437:412–416. doi: 10.1038/nature03961. The visually driven first 100 ms of smooth pursuit eye movements is quite variable. A theoretically driven analysis shows that more than 90% of the motor variation can be described in terms of sensory-estimation errors for target speed, target direction, and the time of target motion onset. This paper led to the conclusion that most of motor variation arises from noise in sensory representations.
- 7.Lisberger SG. Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience. 2009;162:763–776. doi: 10.1016/j.neuroscience.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldreich D, Krauzlis RJ, Lisberger SG. Effect of changing feedback delay on spontaneous oscillations in smooth pursuit eye movements of monkeys. J Neurophysiol. 1992;67:625–638. doi: 10.1152/jn.1992.67.3.625. [DOI] [PubMed] [Google Scholar]
- 9.Medina JF, Lisberger SG. Variation, signal, and noise in cerebellar sensory-motor processing for smooth pursuit eye movements. J Neurosci. 2007;27:6832–6842. doi: 10.1523/JNEUROSCI.1323-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshua M, Lisberger SG. A framework for using signal, noise, and variation to determine whether the brain controls movement synergies or single muscles. J Neurophysiol. 2014;111:733–734. doi: 10.1152/jn.00510.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Beers RJ. The sources of variability in saccadic eye movements. J Neurosci. 2007;27:8757–8770. doi: 10.1523/JNEUROSCI.2311-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Churchland MM, Yu BM, Cunningham JP, Sugrue LP, Cohen MR, Corrado GS, et al. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat Neurosci. 2010;13:369–378. doi: 10.1038/nn.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Churchland MM, Asafar A, Shenoy KV. A central source of movement variability. Neuron. 2006;52:1085–1096. doi: 10.1016/j.neuron.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bair W, Koch C, Newsome W, Britten K. Power spectrum analysis of bursting cells in area MT in the behaving monkey. J Neurosci. 1994;14:2870–2892. doi: 10.1523/JNEUROSCI.14-05-02870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shadlen MN, Britten KH, Newsome WT, Movshon JA. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J Neurosci. 1996;16:1486–1510. doi: 10.1523/JNEUROSCI.16-04-01486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hohl SS, Chaisanguanthum KS, Lisberger SG. Sensory population decoding for visually guided movements. Neuron. 2013;79:167–179. doi: 10.1016/j.neuron.2013.05.026. Trial-by-trial variation in the neural responses in extrastriate area MT is correlated positively with trial-by-trial variations in the initiation of pursuit. This finding provides strong support for the sensory origin of motor variation in smooth pursuit eye movements, and supports the proposal that motor variation is a by-product of suboptimal inference.
- 17.Lee J, Lisberger SG. Gamma synchrony predicts neuron–neuron correlations and correlations with motor behavior in extrastriate visual area MT. J Neurosci. 2013;33:19677–19694. doi: 10.1523/JNEUROSCI.3478-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoppik D, Nagel KI, Lisberger SG. Cortical mechanisms of smooth eye movements revealed by dynamic covariations of neural and behavioral responses. Neuron. 2008;58:248–260. doi: 10.1016/j.neuron.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- 20.Gu Y, Angelaki DE, DeAngelis GC. Contribution of correlated noise and selective decoding to choice probability measurements in extrastriate visual cortex. eLife. 2014 doi: 10.7554/eLife.02670. http://dx.doi.org/10.7554/eLife.02670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goris RL, Movshon JA, Simoncelli EP. Partitioning neural variability. Nat Neurosci. 2014;17:858–865. doi: 10.1038/nn.3711. Thorough quantitative analysis shows that the responses of neurons in the cortical visual pathways have two separate components of variation. One component is simply independent noise, but a second component is correlated across neurons and over time. The paper suggests that the second (shared) component arises from arousal, attention, and adaptation. However, the same principles hold and the same analysis would work in the sensory-motor pathways for pursuit eye movements, where the shared component of variation probably arises from sensory processing.
- 22. Chaisanguanthum KS, Joshua M, Medina JF, Bialek WB, Lisberger SG. The neural code for motor control in the cerebellum and oculomotor brainstem. eNeuro. 2014 doi: 10.1523/ENEURO.0004-14.2014. http://dx.doi.org/10.1523/ENEURO.0004-14.2014. A single extra action potential in one cerebellar Purkinje cell is informative of about the impending smooth pursuit eye movement. This paper argues that the information in single spikes is a direct consequence of the sensory origin of motor noise, which leads to correlations across neurons within a single population and correlations between the trial-by-trial variations in neural responses and behaviour.
- 23.Bair W, Zohary E, Newsome WT. Correlated firing in macaque visual area MT: time scales and relationship to behavior. J Neurosci. 2001;21:1676–1697. doi: 10.1523/JNEUROSCI.21-05-01676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Lisberger SG. Noise correlations in cortical area MT and their potential impact on trial-by-trial variation in the direction and speed of smooth pursuit eye movements. J Neurophysiol. 2009;101:3012–3030. doi: 10.1152/jn.00010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ala-Laurila P, Greschner M, Chichilniski EJ, Rieke F. Cone photoreceptor contributions to noise and correlations in the retinal output. Nat Neurosci. 2011;14:1309–1316. doi: 10.1038/nn.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zohary E, Shadlen MN, Newsome WT. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature. 1994;370:140–143. doi: 10.1038/370140a0. [DOI] [PubMed] [Google Scholar]
- 27. Moreno-Bote R, Beck J, Kanitscheider I, Pitkow X, Latham P, Pouget A. Information-limiting correlations. Nat Neurosci. 2014;17:1410–1417. doi: 10.1038/nn.3807. Theoretical treatment shows that in large networks, the magnitude of noise correlations between neurons with similar preferred stimuli does not necessarily limit the information in a population response. Instead, a particular structure called ‘differential correlations’ limits information in a population response. Differential correlations can be quite small and masked by larger correlations that arise from other sources. It is not clear whether differential correlations in MT actually contribute to limits on behavioral precision in pursuit. A decoder that averages across neurons with similar preferred stimuli would create limits on precision given the structured, positive neuron–neuron correlations known to exist in MT, with or without differential correlations.
- 28.Salinas E, Abbott LF. Vector reconstruction from firing rates. J Comput Neurosci. 1994;1:89–107. doi: 10.1007/BF00962720. [DOI] [PubMed] [Google Scholar]
- 29.Groh JM. Converting neural signals from place codes to rate codes. Biol Cybern. 2001;85:159–165. doi: 10.1007/s004220100249. [DOI] [PubMed] [Google Scholar]
- 30.Churchland MM, Lisberger SG. Shifts in the population response in the middle temporal visual area parallel perceptual and motor illusions produced by apparent motion. J Neurosci. 2001;21:9387–9402. doi: 10.1523/JNEUROSCI.21-23-09387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priebe NJ, Lisberger SG. Estimating target speed from the population response in visual area MT. J Neurosci. 2004;24:1907–1916. doi: 10.1523/JNEUROSCI.4233-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbott LF, Dayan P. The effect of correlated variability on the accuracy of a population code. Neural Comput. 1999;11:91–101. doi: 10.1162/089976699300016827. [DOI] [PubMed] [Google Scholar]
- 33. Ecker AS, Berens P, Tolias AA, Bethge M. The effect of noise correlations in populations of diversely tuned neurons. J Neurosci. 2011;31:14272–14283. doi: 10.1523/JNEUROSCI.2539-11.2011. The information in a population code is limited by neuron–neuron noise correlations when the tuning curves of the neurons are homogenous, but not when the amplitude of the tuning curves is more realistically heterogeneous. This is different from our framework, but mainly because of the use of information as a behavioral endpoint. The behavior predicted by a decoder that averages across neurons with similar preferred stimuli, such as the vector averaging computation used by smooth pursuit eye movements [29,30], would still show limited precision because of structured neuron–neuron noise correlations.
- 34.Kording KP, Wolpert DM. Bayesian integration in sensorymotor learning. Nature. 2004;427:244–247. doi: 10.1038/nature02169. [DOI] [PubMed] [Google Scholar]
- 35.Knill DC. Robust cue integration: a Bayesian model and evidence from cue-conflict studies with stereoscopic and figure cues to slant. J Vis. 2007;7:5–24. doi: 10.1167/7.7.5. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Lee J, Lisberger SG. The interaction of Bayesian priors and sensory data and its neural circuit implementation in visually-guided movement. J Neurosci. 2012;32:17632–17645. doi: 10.1523/JNEUROSCI.1163-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beck JM, Ma WJ, Pitkow X, Latham PE, Pouget A. Not noisy, just wrong: the role of suboptimal inference in behavioral variability. Neuron. 2012;74:30–39. doi: 10.1016/j.neuron.2012.03.016. Theoretical analysis and carefully contrived examples make the case that variation in the output of a system is not just noise. Instead, this paper proposes that variation results from using suboptimal neural computations to estimate parameters from neural population codes. It may not be possible to find optimal computations that will work well across the broad range of tasks faced the brain.
- 38.Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of ‘crystalized’ adult birdsong. Nature. 2007;450:1240–1245. doi: 10.1038/nature06390. [DOI] [PubMed] [Google Scholar]
- 39.Chaisanguanthum JS, Chen HH, Sabes PN. Motor variability arises from a slow random walk in neural state. J Neurosci. 2014;34:12071–12080. doi: 10.1523/JNEUROSCI.3001-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chaisanguanthum KS, Lisberger SG. A neurally-efficient implementation of sensory population decoding. J Neurosci. 2011;31:4868–4877. doi: 10.1523/JNEUROSCI.6776-10.2011. Sensory decoding for smooth pursuit eye movements relies on a population vector sum that is normalized by the total activity across the sensory population [29,30]. Here, a theoretical analysis shows how the computation could be performed by estimates, and that the normalization step could be performed by a post-synaptic cellular depression mechanism. It argues that sensory decoding is a process of estimation rather than exact calculation, and that the resulting estimate errors are small relative to the size of the variation contained in the overall population response.