Abstract
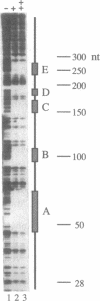
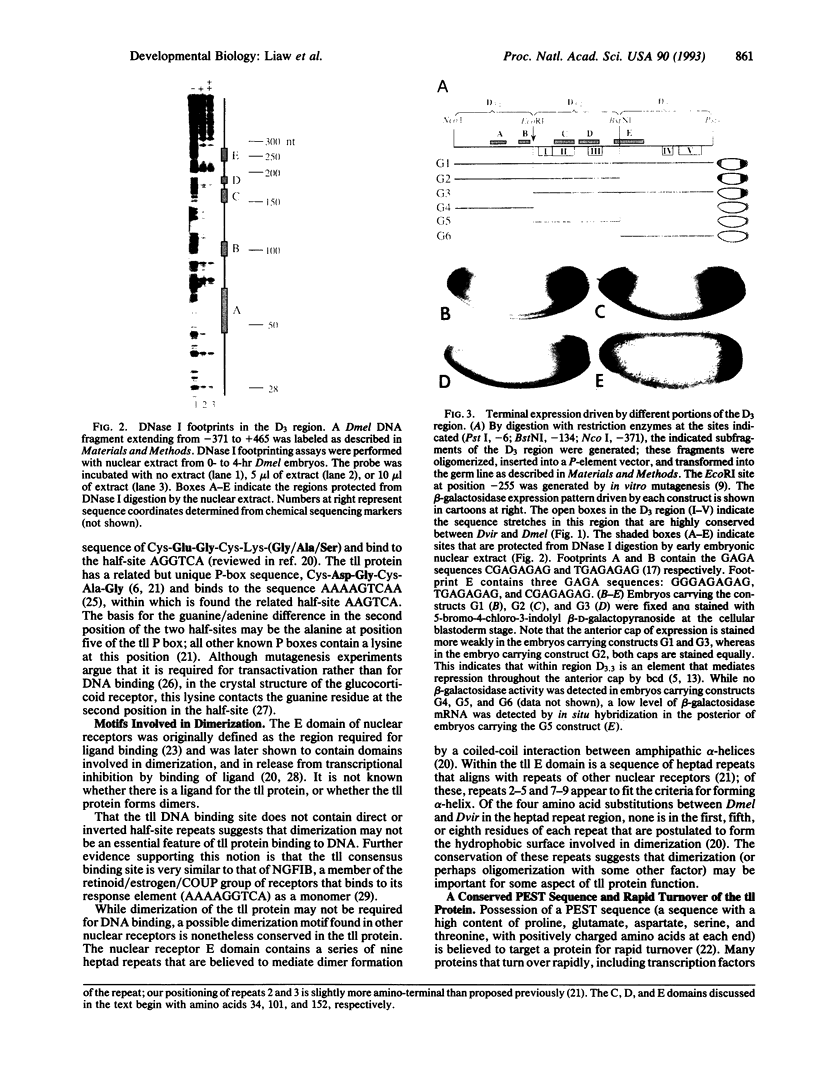
At the poles of the Drosophila embryo, cell fate is established by a pathway that begins with the activation of a membrane-associated tyrosine kinase (the torso gene product); this then leads to activation of a serine/threonine kinase (Drosophila Raf-1). Activated Raf-1 then leads, by an undefined mechanism, to the transcriptional activation of the tailless (tll) gene; the tll gene product, itself a transcription factor, subsequently regulates the expression of an array of target genes. To further define this pathway, we have utilized sequence comparison between Drosophila melanogaster and Drosophila virilis to identify conserved elements in the tll promoter region. As assessed by DNase I footprinting and promoter dissection experiments, two of these elements are potential regulatory targets of Raf-1-activated transcription factors. Sequence comparison also reveals that the unique residues in the DNA-binding domain of the tll protein, the next component in the pathway, are conserved. One of these residues, the alanine after the last cysteine in the first zinc finger, may be responsible for part of the difference between the tll protein DNA binding site and the closely related half-site of the retinoid/estrogen receptors. Consistent with the rapid turnover of the tll protein, it contains a PEST sequence (rich in proline, glutamate and aspartate, serine, and threonine) that is also conserved.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baniahmad A., Köhne A. C., Renkawitz R. A transferable silencing domain is present in the thyroid hormone receptor, in the v-erbA oncogene product and in the retinoic acid receptor. EMBO J. 1992 Mar;11(3):1015–1023. doi: 10.1002/j.1460-2075.1992.tb05140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Tjian R. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell. 1988 Jun 3;53(5):699–711. doi: 10.1016/0092-8674(88)90088-8. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Samuels H. H. Interactions among a subfamily of nuclear hormone receptors: the regulatory zipper model. Mol Endocrinol. 1990 Sep;4(9):1293–1301. doi: 10.1210/mend-4-9-1293. [DOI] [PubMed] [Google Scholar]
- Fortini M. E., Rubin G. M. Analysis of cis-acting requirements of the Rh3 and Rh4 genes reveals a bipartite organization to rhodopsin promoters in Drosophila melanogaster. Genes Dev. 1990 Mar;4(3):444–463. doi: 10.1101/gad.4.3.444. [DOI] [PubMed] [Google Scholar]
- Hartley J. L., Gregori T. J. Cloning multiple copies of a DNA segment. Gene. 1981 May;13(4):347–353. doi: 10.1016/0378-1119(81)90014-7. [DOI] [PubMed] [Google Scholar]
- Jamal S., Ziff E. Transactivation of c-fos and beta-actin genes by raf as a step in early response to transmembrane signals. Nature. 1990 Mar 29;344(6265):463–466. doi: 10.1038/344463a0. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Krust A., Green S., Argos P., Kumar V., Walter P., Bornert J. M., Chambon P. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986 May;5(5):891–897. doi: 10.1002/j.1460-2075.1986.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Wood K., Mamon H., Haser W., Roberts T. Raf-1: a kinase currently without a cause but not lacking in effects. Cell. 1991 Feb 8;64(3):479–482. doi: 10.1016/0092-8674(91)90228-q. [DOI] [PubMed] [Google Scholar]
- Luisi B. F., Xu W. X., Otwinowski Z., Freedman L. P., Yamamoto K. R., Sigler P. B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991 Aug 8;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- Mahoney P. A., Lengyel J. A. The zygotic segmentation mutant tailless alters the blastoderm fate map of the Drosophila embryo. Dev Biol. 1987 Aug;122(2):464–470. doi: 10.1016/0012-1606(87)90310-1. [DOI] [PubMed] [Google Scholar]
- Neufeld T. P., Carthew R. W., Rubin G. M. Evolution of gene position: chromosomal arrangement and sequence comparison of the Drosophila melanogaster and Drosophila virilis sina and Rh4 genes. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10203–10207. doi: 10.1073/pnas.88.22.10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro A. E., Hollenberg S. M., Evans R. M. Transcriptional inhibition by a glucocorticoid receptor-beta-galactosidase fusion protein. Cell. 1988 Dec 23;55(6):1109–1114. doi: 10.1016/0092-8674(88)90255-3. [DOI] [PubMed] [Google Scholar]
- Pankratz M. J., Busch M., Hoch M., Seifert E., Jäckle H. Spatial control of the gap gene knirps in the Drosophila embryo by posterior morphogen system. Science. 1992 Feb 21;255(5047):986–989. doi: 10.1126/science.1546296. [DOI] [PubMed] [Google Scholar]
- Pawson T., Bernstein A. Receptor tyrosine kinases: genetic evidence for their role in Drosophila and mouse development. Trends Genet. 1990 Nov;6(11):350–356. doi: 10.1016/0168-9525(90)90276-c. [DOI] [PubMed] [Google Scholar]
- Pignoni F., Baldarelli R. M., Steingrímsson E., Diaz R. J., Patapoutian A., Merriam J. R., Lengyel J. A. The Drosophila gene tailless is expressed at the embryonic termini and is a member of the steroid receptor superfamily. Cell. 1990 Jul 13;62(1):151–163. doi: 10.1016/0092-8674(90)90249-e. [DOI] [PubMed] [Google Scholar]
- Pignoni F., Steingrímsson E., Lengyel J. A. bicoid and the terminal system activate tailless expression in the early Drosophila embryo. Development. 1992 May;115(1):239–251. doi: 10.1242/dev.115.1.239. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. Regulation of enzyme levels by proteolysis: the role of pest regions. Adv Enzyme Regul. 1988;27:135–151. doi: 10.1016/0065-2571(88)90014-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller W. C., Poole S. J., Kornberg T. In vitro transcription of the Drosophila engrailed gene. Genes Dev. 1988 Jan;2(1):68–81. doi: 10.1101/gad.2.1.68. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Nüsslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992 Jan 24;68(2):201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Staden R. Computer methods to locate signals in nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):505–519. doi: 10.1093/nar/12.1part2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrímsson E., Pignoni F., Liaw G. J., Lengyel J. A. Dual role of the Drosophila pattern gene tailless in embryonic termini. Science. 1991 Oct 18;254(5030):418–421. doi: 10.1126/science.1925599. [DOI] [PubMed] [Google Scholar]
- Umesono K., Evans R. M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989 Jun 30;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Wilson T. E., Paulsen R. E., Padgett K. A., Milbrandt J. Participation of non-zinc finger residues in DNA binding by two nuclear orphan receptors. Science. 1992 Apr 3;256(5053):107–110. doi: 10.1126/science.1314418. [DOI] [PubMed] [Google Scholar]