Regorafenib is an oral multikinase inhibitor licensed for use in gastrointestinal cancers. In clinical trials, regorafenib showed a consistent toxicity profile, including clinically significant hand–foot skin reaction (HFSR). Treatment modifications and symptomatic measures, as recommended in this review, can be used to manage HFSR and help patients to continue treatment at an optimal dose.
Keywords: regorafenib, hand–foot skin reaction, hand–foot syndrome, palmar–plantar erythrodysesthesia, treatment modifications, symptom management
Abstract
Background
Regorafenib is an orally available, small-molecule multikinase inhibitor with international marketing authorizations for use in colorectal cancer and gastrointestinal stromal tumors. In clinical trials, regorafenib showed a consistent and predictable adverse-event profile, with hand–foot skin reaction (HFSR) among the most clinically significant toxicities. This review summarizes the clinical characteristics of regorafenib-related HFSR and provides practical advice on HFSR management to enable health care professionals to recognize, pre-empt, and effectively manage the symptoms, thereby allowing patients to remain on active therapy for as long as possible.
Design
This review is based on a systematic literature search of the PubMed database (using synonyms of HFSR, regorafenib, and skin toxicities associated with targeted therapies or cytotoxic chemotherapy). However, as this search identified very few articles, the authors also use their clinical experience as oncologists and dermatologists managing patients with treatment-related HFSR to provide recommendations on recognition and management of HFSR in regorafenib-treated patients.
Results
Regorafenib-related HFSR is similar to that seen with other multikinase inhibitors (e.g. sorafenib, sunitinib, cabozantinib, axitinib, and pazopanib) but differs from the hand–foot syndrome seen with cytotoxic chemotherapies (e.g. fluoropyrimidines, anthracyclines, and taxanes). There have been no controlled trials of symptomatic management of regorafenib-related HFSR, and limited good-quality evidence from randomized clinical trials of effective interventions for HFSR associated with other targeted therapies. Recommendations on prevention and management of regorafenib-related HFSR in this review are therefore based on the expert opinion of the authors (dermatologists and oncologists with expertise in the management of treatment-related skin toxicities and oncologists involved in clinical trials of regorafenib) and tried-and-tested empirical experience with other multikinase inhibitors and cytotoxic chemotherapies.
Conclusions
As recommended in this review, treatment modifications and supportive measures to prevent, reduce, and manage HFSR can allow patients to continue regorafenib at the optimal dose to derive benefit from treatment.
introduction
Regorafenib is an orally available, small-molecule multikinase inhibitor that blocks the activity of a variety of protein kinases involved in the regulation of tumor angiogenesis [vascular endothelial growth factor (VEGF) receptor-1, -2, and -3 and TIE-2], oncogenesis (KIT, RET, RAF-1, and wild-type and V600E-mutant BRAF), and the tumor microenvironment [platelet-derived growth factor receptor (PDGFR) and fibroblast growth factor receptor] [1]. Preclinical evidence of antitumor activity [1, 2] has been confirmed in clinical trials covering a range of advanced solid tumors, including hepatocellular carcinoma, gastrointestinal stromal tumors (GIST), and colorectal cancer [3–11]. Regorafenib met its primary end points (overall survival and progression-free survival, respectively) in phase III placebo-controlled trials in patients with treatment-refractory metastatic colorectal cancer [10, 11] and advanced pretreated GIST [9] and as a result now has international marketing authorizations for use in these settings.
Across all of these clinical trials, regorafenib showed a consistent and predictable adverse-event profile, with skin toxicities such as hand–foot skin reaction (HFSR) among the most clinically significant (see supplementary Table S1, available at Annals of Oncology online) [3–11]. In a meta-analysis of regorafenib trials, HFSR occurred at a rate of 61% overall and 20% at grade 3, with higher overall rates in patients with renal-cell carcinoma (71%) and GIST (60%) than in those with colorectal cancer (47%) [12]. HFSR is also seen with varying frequencies in patients treated with other multikinase inhibitors. A meta-analysis of clinical trials of sorafenib indicated an overall HFSR rate of 34% and a rate of grade 3 HFSR of 9% [13]. In a similar meta-analysis of sunitinib trials, the rates were 19% overall and 6% for grade 3 HFSR [14]. Although therapeutic response to other targeted agents (including sunitinib and sorafenib) has been correlated with HFSR occurrence [15–18], it is unknown whether this is the case for regorafenib.
The variation in incidence of HFSR across tumor types and among the different multikinase inhibitors is presumed to reflect the different molecular pathways involved and the variation in degree of target kinase inhibition between agents. It is speculated that combined inhibition of different receptors (e.g. VEGF receptor and PDGFR) may be required to trigger the dermatologic symptoms. Agents that target only one of these pathways (e.g. imatinib, which targets PDGFR, and bevacizumab, which targets VEGF) are rarely associated with HFSR [13], while the combination of bevacizumab and sorafenib increases the HFSR incidence over that seen with sorafenib alone [19]. Blockade of both pathways could alter microvascular structure or disrupt endothelial and vascular repair mechanisms, resulting in persistent damage to vessels and fibroblasts at areas of frequent trauma or friction (such as the palms of the hands, soles of the feet, and elbows) [12]. Vascular competence is important for tissue repair, and thus vascular damage can impair the skin's ability to recover from day-to-day wear and tear. In addition, regorafenib uniquely inhibits the endothelium-specific TIE-2 receptor, thus affecting angiopoietin pathways, which are responsible for vascular remodeling and are implicated in pathologic inflammation. TIE-2 inhibition may therefore also have a role in the increased risk of HFSR in regorafenib recipients [12].
HFSR can be troublesome for patients, affecting their ability to carry out everyday activities and get on with their lives. It is therefore important that health care professionals are able to recognize and manage the symptoms in order to reduce the impact of HFSR on patients. To this end, the aim of this review is to summarize the key pathophysiological features of regorafenib-associated HFSR and to identify and recommend appropriate approaches (including supportive interventions and treatment modifications) to manage HFSR in patients receiving regorafenib.
methods
This review has been developed as an academic collaboration between dermatologists and oncologists with expertise in the management of drug-related skin toxicities and oncologists involved in the clinical trials of regorafenib in colorectal cancer. The authors reviewed published articles identified by a literature search of the PubMed database using synonyms for regorafenib and HFSR management, as well as a general search for articles on any skin toxicities associated with anticancer therapies (including capecitabine/5-fluorouracil and kinase inhibitors). The full search strings are listed in the supplementary Appendix, available at Annals of Oncology online. The search, which was conducted in October 2014, was not restricted to any language, article type, or publication period, to avoid excluding potentially relevant articles. The literature review was supplemented by the authors' own knowledge of the therapy area.
results
The regorafenib literature search identified a total of 167 articles, including primary trial reports and reviews, opinion pieces, and case studies. To date, no clinical studies of interventions to prevent or manage regorafenib-related HFSR have been published. Eight articles have provided advice on the management of regorafenib-related HFSR, in each case as part of a larger review of the management of toxicities associated with either regorafenib alone [20–25] or all targeted therapies in colorectal cancer [26, 27].
In general, there is little high-quality evidence from randomized clinical trials to support specific interventions for HFSR related to any kinase inhibitor, with most recommendations being based on empirical experience and expert opinion [28–39]. Table 1 lists approaches that have been investigated in mostly small trials or individual cases of patients receiving other kinase inhibitors (most frequently sorafenib) [40–52]. Most of these HFSR management options involve topical interventions, such as corticosteroids, keratolytics, moisturizers, or phototherapy.
Table 1.
Management approaches for hand–foot skin reaction (HFSR) associated with licensed kinase inhibitors
| Reference | Kinase inhibitor | Intervention | Outcome |
|---|---|---|---|
| Clinical studies | |||
| [40] | Sorafenib | 10% urea-based cream three times daily + best supportive care (n = 439) or best supportive care alone excluding all creams (n = 432), starting on day 1 of sorafenib treatment, for up to 12 weeks |
|
| [41] | Sorafenib | Hydrocolloid dressing containing ceramide (group A, n = 17) versus 10% urea cream (group B, n = 16) for grade 1 HFSR on the soles of the feet |
|
| [42] | Sunitinib, sorafenib, or axitinib | Topical heparin-containing ointment, shock absorbers, and skin moisturizers (n = 26) |
|
| [43] | Sorafenib | Vitamin E 300 mg/day (n = 10) |
|
| [44] | Sorafenib or gefitinib | Taohongsiwu (traditional Chinese medicine; n = 60) versus oral vitamin B6 (n = 32) |
|
| Case reports | |||
| [45] | Sorafenib | Topical clobetasol, cetirizine tablets, cold sponging |
|
| [46] | BRAF inhibitors (vemurafenib or dabrafenib) | Topical steroids and keratolytics |
|
| [47] | Sorafenib | Narrow-band ultraviolet B phototherapy |
|
| [48] | Sunitinib or imatinib | Topical psoralen + ultraviolet A therapy (±methoxsalen or prednisone) |
|
| [49] | Sorafenib | Topical corticosteroids, podiatric management, and thermal water gel |
|
| [50] | Sorafenib | Topical clobetasol propionate ointment |
|
| [51] | Sorafenib | Topical prednicarbate ointment, fusidic acid cream and moisturizer (dexpanthenol) |
|
| [52] | Dabrafenib | Pregabalin |
|
discussion
In the absence of high-quality evidence from randomized clinical trials or detailed advice on regorafenib-specific HFSR management, the following discussion offers recommendations from the authors' extensive clinical experience, supplemented by evidence on the management of HFSR associated with other small-molecule kinase inhibitors and chemotherapy.
regorafenib-related HFSR pathophysiology
Symptoms affecting the hands and feet following initiation of different systemic anticancer treatments have been reported under a variety of names, including HFSR, hand–foot syndrome, palmar–plantar erythrodysesthesia, acral erythema, toxic erythema of chemotherapy, and Burgdorf reaction [36]. The first report, in 1974, was in relation to mitotane therapy for hypernephroma [53]. Since then, variations of the syndrome (or, more probably, syndromes) have been described in association with many cytotoxic and targeted agents, especially 5-fluorouracil and capecitabine, doxorubicin and pegylated liposomal doxorubicin, docetaxel, cytarabine, sorafenib, sunitinib, and vemurafenib [34, 36, 54].
The features of HFSR seen with regorafenib are akin to those reported for other targeted agents and differ in important aspects (which may have implications for management) from the syndrome that clinicians may be familiar with in association with traditional cytotoxic therapies such as capecitabine (Table 2) [55]. For clarity, the terminology referring to the two forms is commonly distinguished, with HFSR referring specifically to symptoms related to targeted therapy and hand–foot syndrome being reserved for symptoms related to traditional chemotherapy; this distinction is used in the present review. In HFSR, following a prodromal phase of dysesthesia (described as a sensation developing from tingling to burning over a few days), patients develop bilateral, painful, sharply demarcated, asymmetric erythema and large, tense blisters evolving into callus-like hyperkeratosis. Pain may be out of proportion to the clinical appearance of the lesions. Symptoms typically occur at pressure-bearing points such as the palms of the hands, soles of the feet (especially the heels and metatarsal head area), elbows, and amputation sites, while they are unlikely to be seen on the dorsal hand or foot; they may also develop at other areas of friction, such as the finger tips (e.g. from frequent use of mobile devices), sides of the feet, and spaces between fingers and toes [34, 36, 49, 56, 57]. The symptoms are typically more localized, and edema is less likely, in patients receiving multikinase inhibitors than in those receiving cytotoxic agents [26, 58]. With traditional cytotoxic agents, the hands are likely to be affected more than the feet, while, for multikinase inhibitors, the feet may be more likely to be affected [36].
Table 2.
Differing features of hand–foot syndrome and hand–foot skin reaction (created based on author knowledge and using [55])
| Hand–foot syndrome | Hand–foot skin reaction |
|---|---|
| Associated with traditional cytotoxic chemotherapies, including cytarabine, anthracyclines, fluoropyrimidines, and taxanes | Associated with multikinase inhibitors (e.g. sorafenib, sunitinib, and regorafenib) and BRAF inhibitors (e.g. vemurafenib and dabrafenib) |
| Onset weeks to months after starting treatment | Onset days to weeks after starting treatment |
| Mechanism unclear, but doxorubicin-induced symptoms may be caused by concentration of cytostatic in skin via eccrine sweat ducts | With multikinase inhibitors, mechanism may be insufficient repair to frictional trauma due to inhibition of PDGFR and VEGFR With BRAF inhibitors, mechanism may be paradoxical hyperproliferation |
Characterized by:
|
Characterized by:
|
| Symmetrical, diffuse distribution | Localized at pressure points |
PDGFR, platelet-derived growth factor receptor; VEGFR, vascular endothelial growth factor receptor.
The difference in appearance of HFSR due to targeted therapies compared with the hand–foot syndrome seen in patients receiving cytotoxic chemotherapy may reflect different underlying pathological processes. For example, there is evidence to show that doxorubicin-associated hand–foot syndrome may be a direct toxic effect resulting from transport of the active agent to the skin surface via sweat [59–61], whereas no such effect has been found in patients receiving sorafenib [62] and evidence is inconclusive for sunitinib [63, 64]. In contrast, as described earlier, multikinase inhibitors may play a role in disrupting the natural balance of vascular and epidermal trauma and repair at sites of pressure and friction through effects on a variety of molecular signaling pathways [19, 65].
Time-to-event analyses in phase III trials of regorafenib confirmed clinical experience that, as with sorafenib and sunitinib [66], HFSR tends to occur shortly after the start of treatment (median time to first occurrence was 15 days in the phase III study in colorectal cancer [67]), and the severity is also likely to peak early (medium time to maximum severity was 22 days in that study [67]). In contrast, the occurrence of capecitabine-induced hand–foot syndrome is typically more delayed (median time to first presentation was 72–79 days [68, 69]).
With appropriate intervention, including prespecified dose reductions described in this review, patients in the regorafenib clinical trials were largely able to continue treatment, with few patients (0%–1.4%) stopping treatment permanently because of HFSR. In clinical practice, however, with a wider range of patients than are eligible to take part in clinical trials, the rate of permanent discontinuation due to HFSR is often higher, emphasizing the need for optimal recognition, prevention, and management of this clinically significant syndrome.
impact of HFSR
While HFSR is not considered to be a life-threatening event (although the potential risk of superinfection of the damaged skin should not be ignored [55]), the location of the lesions can have a substantial impact on patients by preventing them from walking or carrying out daily tasks. No studies have yet directly assessed the impact of regorafenib-related HFSR on patients' quality of life. However, cutaneous toxicities in general and HFSR in particular have been shown to decrease quality of life and impair social functioning in patients receiving other targeted therapies, such as sorafenib and sunitinib [70–73]. In some cases, particularly if the patient does not report symptoms early enough or there is no appropriate and effective management plan in place, there is a risk that the HFSR may be so severe that the patient is unable or unwilling to continue anticancer therapy. In contrast, it has been shown that appropriate and effective intervention to manage cutaneous toxicities can improve quality of life [44, 74, 75].
No data on the economic cost of treating regorafenib-related HFSR have been reported. However, an analysis of the cost of managing cutaneous toxicities associated with sorafenib and sunitinib for cancer at a single US dermatology department found that sorafenib-related HFSR was the most costly cutaneous toxicity to manage, accounting for a median medication cost of $968 per patient [76]. While this US cost analysis cannot be directly extrapolated to European health care systems, it seems likely that appropriate, timely, and effective intervention to pre-empt and manage regorafenib-related HFSR would reduce the burden of symptoms on patients and limit the cost of managing those symptoms by preventing them from becoming chronic and severe.
prevention and management of regorafenib-related HFSR
The goals of HFSR management are to reduce the risk of HFSR developing and to alleviate symptoms of established HFSR, to enable patients to maintain their quality of life and continue to receive effective anticancer therapy.
Although no study has been undertaken to identify patients most likely to be at risk of regorafenib-related HFSR, Dranitsaris et al. have developed and validated a prediction index for sorafenib-related HFSR, based on female sex, poor performance status (Eastern Cooperative Oncology Group 2 or lower), lung or liver metastases at baseline, two or more organs involved, baseline white blood cell count above 5.5 × 109 cells/l, and week of therapy (up to week 8, with highest scores assigned to weeks 3–7) [77]. In the absence of clinical research into risk factors specifically related to regorafenib, these sorafenib-associated factors may be of assistance in prompting a heightened index of suspicion of HFSR risk in patients receiving regorafenib. It is currently unknown whether specific genetic polymorphisms may exist, as they do for sorafenib and sunitinib, that may increase a patient's risk of high-grade toxicity [78].
Before starting treatment with regorafenib, it is important to establish a baseline against which to compare any incipient symptoms. Patients should have a full-body examination by an appropriately experienced health care professional, supported if feasible by a formal assessment of quality of life (using an established tool such as the Skindex [79] or Dermatology Life Quality Index [80], or a specific HFSR tool such as the HFS-14 [81] once validated in patients receiving multikinase inhibitors). Clinical examination should pay special attention to the hands and feet to identify predisposing factors, such as hyperkeratosis, eczema, or fungal disease. Any identified risk factors should be treated, ideally before starting regorafenib therapy, for example with manicures or pedicures to remove hyperkeratotic skin or referral to a podiatrist or dermatologist (particularly for those with comorbidities, such as diabetes). For patients with evidence of abnormal weight bearing, mechanical support and correction should be considered. Patients should be advised to use alcohol-free moisturizers liberally and to avoid hot water (e.g. dishwashing or hot baths and showers), because heat may exacerbate symptoms. Patients should also avoid constrictive footwear and reduce friction on the skin when applying lotion, during massages, or in the process of everyday tasks, such as typing. Vigorous exercise or activities that place undue stress on the hands and feet (such as heavy lifting or long walks) should be avoided, especially during the first month, to reduce the risk of blistering. Patients should use padded insoles in their shoes throughout treatment to reduce pressure on the feet, and they should be advised to wear thick cotton gloves or socks to prevent injury and keep palms and soles dry [32, 34, 37, 39].
Once they start treatment, patients should be monitored frequently for signs of incipient HFSR, for example during the first week of treatment and then every 1–2 weeks during the first two cycles and every 4–6 weeks thereafter [20, 23, 25]. This surveillance schedule reflects the time profile of new HFSR cases reported in the phase III CORRECT study, with most cases developing in the first couple of cycles [67]. This pattern is also seen in clinical practice and has prompted some clinicians to explore the use of a reduced starting dose, with the potential to escalate to the full 160 mg dose if patients are able to tolerate treatment [82, 83]. To date, however, such experience is restricted to single centers and small case series, and a reduced starting dose of regorafenib has not been evaluated in a controlled clinical trial.
If symptoms do develop, the patient should be managed by a multidisciplinary team [32, 37] including oncologist, dermatologist, podiatrist, primary-care physician, and nurse. It is important to attempt to identify the cause of the symptoms, ruling out alternative causes, such as erythema multiforme, fungal infections, other types of drug reactions, or persisting sensory neuropathy following cytotoxic chemotherapy.
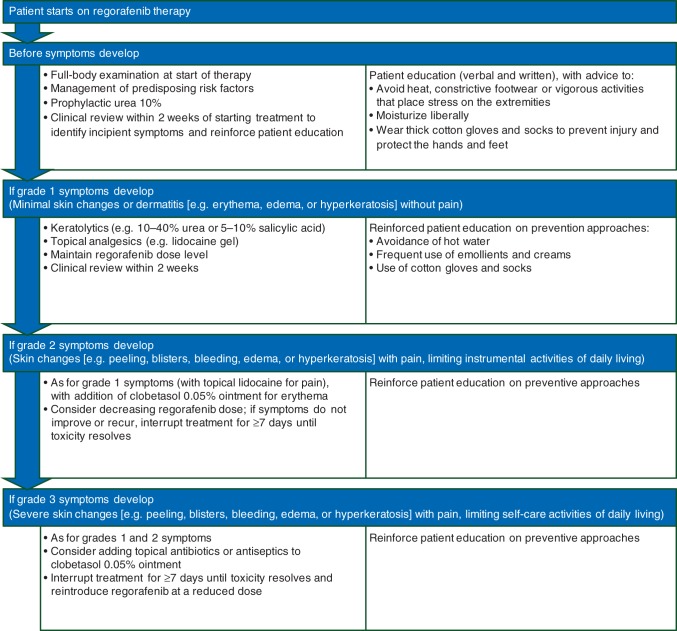
The management approach depends on the severity of the symptoms, graded using the National Cancer Institute's Common Terminology Criteria for Adverse Events version 4 [84] (Figure 1). Options include physical protection of the affected area and symptomatic interventions (Table 3), as well as consideration of regorafenib treatment modifications (Table 4).
Figure 1.
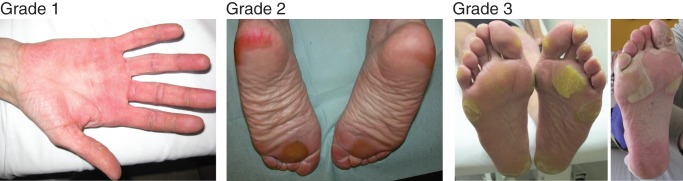
Palmar–plantar erythrodysesthesia syndrome, grades 1–3, according to the National Cancer Institute Common Terminology Criteria for Adverse events, version 4 [84]. Grade 1: numbness, dysesthesia, paresthesia, tingling, painless swelling, erythema, or discomfort of the hands or feet that does not disrupt the patient's normal activities. Grade 2: painful erythema and swelling of the hands or feet and/or discomfort that affects the patient's instrumental activities of daily living. Grade 3: moist desquamation, ulceration, blistering, or severe pain of the hands or feet, or severe discomfort that causes the patient to be unable to work or perform self-care activities of daily living. Note that, as discussed in the text, hand–foot skin reaction due to small-molecule kinase inhibitors may have a different manifestation, so the descriptions here may not match symptoms experienced by patients receiving regorafenib. Photos provided by Siegfried Segaert and Eric Van Cutsem.
Table 3.
Recommended supportive measures to prevent or manage hand–foot skin reaction
| Purpose | Intervention | Timing |
|---|---|---|
| Control of calluses | Check condition of hands and feet Suggest a manicure/pedicure to remove hyperkeratotic skin Recommend pumice stone use for callus or ‘rough spot’ removal |
Before initiating treatment with regorafenib |
| Avoid pressure points Avoid items that rub, pinch, or create friction |
During regorafenib treatment | |
| Moisturizers | Nonurea-based creams or ointments | Apply liberally when needed, especially after hand washing |
| Keratolytic creams | 10%–40% urea-based 5%–10% salicylic acid |
Use sparingly and only to affected (hyperkeratotic) areas |
| Pain control | Topical analgesics, e.g. lidocaine 2%–4% | As needed |
| Management of grade 2–3 symptoms | Topical corticosteroids, e.g. clobetasol 0.05% Avoid systemic steroids |
Twice daily |
| Cushioning/protection of tender areas | Use socks/gloves to cover moisturized areas Wear well-padded footwear Use insole cushions or inserts (e.g. silicon, gel) Foot soaks with tepid water and Epsom salts |
As needed |
Table 4.
Regorafenib dose modifications to manage hand–foot skin reaction as outlined in the phase III clinical trial protocols [9–11]
| Hand–foot skin reaction grade | Occurrence | Suggested dose modificationa |
|---|---|---|
| Grade 1: numbness, dysesthesia, paresthesia, tingling, painless swelling, erythema, or discomfort of the hands or feet that does not disrupt the patient's normal activities | Any | Maintain dose level and institute immediate supportive measures for symptomatic relief |
| Grade 2: painful erythema and swelling of the hands or feet and/or discomfort that affects the patient's normal activities | First occurrence | Consider decreasing dose by one dose level and institute immediate supportive measures. If no improvement, interrupt therapy for a minimum of 7 days, until toxicity resolves to grade 0–1b |
| No improvement within 7 days or second occurrence | Interrupt therapy until toxicity resolves to grade 0–1. When resuming treatment, treat at reduced dose levelb | |
| Third occurrence | Interrupt therapy until toxicity resolves to grade 0–1. When resuming treatment, decrease dose by one additional dose levelb,c | |
| Fourth occurrence | Discontinue therapy | |
| Grade 3: moist desquamation, ulceration, blistering, or severe pain of the hands or feet, or severe discomfort that causes the patient to be unable to work or perform activities of daily living | First occurrence | Institute immediate supportive measures. Interrupt therapy for a minimum of 7 days until toxicity resolves to grade 0–1. When resuming treatment, decrease dose by one dose levelb |
| Second occurrence | Institute immediate supportive measures. Interrupt therapy for a minimum of 7 days until toxicity resolves to grade 0–1. When resuming treatment, decrease dose by one additional dose levelb,c | |
| Third occurrence | Discontinue treatment permanently |
aDose level 0 (standard dose) = 160 mg orally once daily (4 × 40 mg tablets of regorafenib); dose level −1 = 120 mg orally once daily (3 × 40 mg tablets); dose level −2 = 80 mg orally once daily (2 × 40 mg tablets). A more conservative dose modification approach, if medically indicated, is acceptable.
bIf toxicity returns to grade 0–1 after dose reduction, dose re-escalation is permitted at the discretion of the investigator.
cPatients requiring more than two dose reductions (i.e. to <80 mg) should go off protocol therapy.
Grade 1 HFSR is characterized by minimal skin changes or dermatitis (e.g. erythema, edema, or hyperkeratosis) without pain [84]. Health care professionals should reinforce patient education about preventing symptoms and institute supportive measures such as control of hyperkeratotic areas and moisturizers. Patients should be advised to avoid hot water and to use emollients and creams frequently to maintain moisture and prevent loss of dermal integrity. If disease is present at an unexpected site, unusual sources of friction in the area should be identified, such as shoelaces rubbing on the anterior ankle. Keratolytics, such as 10%–40% urea or 5%–10% salicylic acid, may be indicated for hyperkeratotic lesions, while topical analgesics (e.g. lidocaine gel) can help to relieve pain. Oozing lesions may be treated with baths containing an antiseptic, such as potassium permanganate, chlorhexidine gluconate, or diluted bleach. Patients should be advised to wear cotton gloves and socks, including at night, to prevent further injury, to help retain moisture, and to increase penetration of topical medications. The dose of regorafenib does not need to be modified at this level of toxicity (see Table 4). A 2-week follow-up in the clinic is recommended, when special attention should be paid to the palms of the hands and soles of the feet. Patients with unique skin symptoms or whose HFSR persists despite the above recommendations should be referred to a dermatologist [37, 39].
Grade 2 HFSR manifests with painful skin changes (e.g. peeling, blisters, bleeding, edema, or hyperkeratosis) and limits instrumental activities of daily living [84]. The goal of management is to control hyperkeratosis, cushion callused areas, moisturize skin, and relieve discomfort. Treatment should be as for grade 1 toxicity (including creams containing urea or salicylic acid for hyperkeratotic areas and topical analgesics for pain), with the addition of clobetasol 0.05% ointment or foam twice daily for erythematous areas. Patients should be assessed for bleeding risk and kidney function before systemic pain medications (e.g. nonsteroidal anti-inflammatory drugs, opioids, or GABA agonists such as gabapentin or pregabalin) are used [37, 39]. If necessary, the regorafenib dose can be reduced by one level for a minimum of 7 days (maximum 28 days), until the HFSR reaches grade 1 or 0, at which point patients can resume treatment at the full starting dose. If symptoms recur on re-exposure to regorafenib, treatment interruption for at least a further 7 days can be considered, with the drug reintroduced at a reduced dose thereafter (see Table 4 for full recommendations on regorafenib treatment modifications).
Grade 3 HFSR is the most severe grade, involving painful skin changes (e.g. peeling, blisters, bleeding, edema, or hyperkeratosis) limiting self-care activities of daily living [84]. The goal of management is to reduce symptoms and prevent further negative impact on the patients' quality of life. Patients should have symptomatic treatment as indicated for grades 1 and 2, with regorafenib treatment interrupted for a minimum of 7 days (maximum 28 days) until the HFSR reaches grade 1 or 0; regorafenib should then be resumed at one dose level lower than the previous dose (see Table 4). If toxicity does not recur, it may be possible to re-escalate back to the full starting daily dose. However, if HFSR recurs on re-exposure to regorafenib, treatment may need to be interrupted again for at least a further 7 days and reintroduced at a further reduced dose (minimum 80 mg/day) or discontinued permanently if supportive measures and treatment modifications do not control symptoms (see Table 4). Additional supportive measures include topical corticosteroids and topical antibiotics. Following control of an acute episode of erythema (with or without blisters), patients may develop hyperkeratotic, tender lesions. In these cases, topical keratolytic creams can be used, for example containing 10%–40% urea or 5%–10% salicylic acid. In addition, antiproliferative agents such as tazarotene 0.1% may be of benefit [37, 39].
patient education
As regorafenib is an oral agent, patients will usually take each dose in their home, rather than under direct supervision of a health care professional. It is therefore critical that patients are educated on the risk of HFSR and can identify the symptoms early, so that they can take appropriate steps to reduce the impact on their daily lives. Nurses play a key role in ensuring that patients understand the information that they are given; a checklist published by De Wit et al. can help to ensure that the patient is given consistent and comprehensive advice [23]. Verbal communications should be supported by written materials that patients can refer to at later times—an illustrated pamphlet on the early signs and symptoms of HFSR can encourage patients to be proactive about pre-empting, detecting, and managing HFSR.
Patients should be reassured that HFSR is not a reason for discontinuing regorafenib if it is managed early and proactively. The health care team should take care to explain the rationale for treatment modifications (i.e. that there is a need to find a balance between tolerability and the most effective dose). Frequent communication between patients and health care professionals is encouraged; clinical assessment (whether face to face or by telephone) during the first week of treatment and at regular intervals thereafter (e.g. every 1–2 weeks during the first two cycles) ensures that symptoms are detected at the earliest possible stage [20, 23]. Such frequent contact has an important role in reinforcing patient education about HFSR prevention and management [39].
conclusions
The multikinase inhibitor regorafenib has proven efficacy in terms of prolonging overall and progression-free survival and is indicated for use in patients with advanced colorectal cancer or GIST. Evidence from clinical trials and real-world experience indicates that there is a high risk that patients will experience HFSR. However, this potentially troublesome adverse event need not prevent patients from receiving effective anticancer treatment with regorafenib, as long as health care professionals and patients understand the risk, take appropriate preventive actions, recognize early symptoms, and institute supportive measures and appropriate dose adjustments as outlined in this review and summarized in Figure 2. The recommendations are based on tried-and-tested empirical experience and the expert opinions of the authors, in the absence of randomized clinical trials of specific symptomatic treatments. Future clinical trials are needed that are designed to formally assess interventions to manage HFSR and other skin toxicities related to multikinase inhibitors such as regorafenib.
Figure 2.
Regorafenib-related hand–foot skin reaction management approaches.
funding
This work was supported by Bayer HealthCare Pharmaceuticals, Whippany, NJ, USA, which provided financial support for editorial assistance by Succinct Medical Communications, but was not otherwise involved in the literature review, content development, or advice provided in this article. No grant numbers apply.
disclosure
FC served on advisory boards for Roche, Bayer, Merck Serono, and Astellas. ML has provided consultancy for Bayer and Onyx. SS has served as a paid speaker for Bayer. EVC has received research funding from Bayer. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank Succinct Medical Communications, Marlow, Bucks, UK, for editorial assistance in the preparation of this manuscript. The authors retained full control over the content and the decision to publish the article.
references
- 1.Wilhelm SM, Dumas J, Adnane L et al. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011; 129: 245–255. [DOI] [PubMed] [Google Scholar]
- 2.Schmieder R, Hoffmann J, Becker M et al. Regorafenib (BAY 73–4506): antitumor and antimetastatic activities in preclinical models of colorectal cancer. Int J Cancer 2014; 135: 1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mross K, Frost A, Steinbild S et al. A phase I dose-escalation study of regorafenib (BAY 73–4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res 2012; 18: 2658–2667. [DOI] [PubMed] [Google Scholar]
- 4.Strumberg D, Scheulen ME, Schultheis B et al. Regorafenib (BAY 73–4506) in advanced colorectal cancer: a phase I study. Br J Cancer 2012; 106: 1722–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunakawa Y, Furuse J, Okusaka T et al. Regorafenib in Japanese patients with solid tumors: phase I study of safety, efficacy, and pharmacokinetics. Invest New Drugs 2014; 32: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisen T, Joensuu H, Nathan PD et al. Regorafenib for patients with previously untreated metastatic or unresectable renal-cell carcinoma: a single-group phase 2 trial. Lancet Oncol 2012; 13: 1055–1062. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Tak WY, Gasbarrini A et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer 2013; 49: 3412–3419. [DOI] [PubMed] [Google Scholar]
- 8.George S, Wang Q, Heinrich MC et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol 2012; 30: 2401–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demetri GD, Reichardt P, Kang YK et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013; 381: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grothey A, Van Cutsem E, Sobrero A et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013; 381: 303–312. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Qin S, Xu R et al. Regorafenib monotherapy in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015; 16: 619–629. [DOI] [PubMed] [Google Scholar]
- 12.Belum VR, Wu S, Lacouture ME. Risk of hand-foot skin reaction with the novel multikinase inhibitor regorafenib: a meta-analysis. Invest New Drugs 2013; 31: 1078–1086. [DOI] [PubMed] [Google Scholar]
- 13.Chu D, Lacouture ME, Fillos T, Wu S. Risk of hand-foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol 2008; 47: 176–186. [DOI] [PubMed] [Google Scholar]
- 14.Chu D, Lacouture ME, Weiner E, Wu S. Risk of hand-foot skin reaction with the multitargeted kinase inhibitor sunitinib in patients with renal cell and non-renal cell carcinoma: a meta-analysis. Clin Genitourin Cancer 2009; 7: 11–19. [DOI] [PubMed] [Google Scholar]
- 15.Jain L, Sissung TM, Danesi R et al. Hypertension and hand-foot skin reactions related to VEGFR2 genotype and improved clinical outcome following bevacizumab and sorafenib. J Exp Clin Cancer Res 2010; 29: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otsuka T, Eguchi Y, Kawazoe S et al. Skin toxicities and survival in advanced hepatocellular carcinoma patients treated with sorafenib. Hepatol Res 2012; 42: 879–886. [DOI] [PubMed] [Google Scholar]
- 17.Poprach A, Pavlik T, Melichar B et al. Skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Ann Oncol 2012; 23: 3137–3143. [DOI] [PubMed] [Google Scholar]
- 18.Nakano K, Komatsu K, Kubo T et al. Hand-foot skin reaction is associated with the clinical outcome in patients with metastatic renal cell carcinoma treated with sorafenib. Jpn J Clin Oncol 2013; 43: 1023–1029. [DOI] [PubMed] [Google Scholar]
- 19.Azad NS, Aragon-Ching JB, Dahut WL et al. Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res 2009; 15: 1411–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grothey A, George S, Van Cutsem E et al. Optimizing treatment outcomes with regorafenib: personalized dosing and other strategies to support patient care. Oncologist 2014; 19: 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell J, Khoukaz T, McNeal D, Brent L. Adverse event management strategies: optimizing treatment with regorafenib in patients with metastatic colorectal cancer. Clin J Oncol Nurs 2014; 18: E19–E25. [DOI] [PubMed] [Google Scholar]
- 22.Khan G, Moss RA, Braiteh F, Saltzman M. Proactive strategies for regorafenib in metastatic colorectal cancer: implications for optimal patient management. Cancer Manag Res 2014; 6: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Wit M, Boers-Doets CB, Saettini A et al. Prevention and management of adverse events related to regorafenib. Support Care Cancer 2014; 22: 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan SL, Ma BB. An update on the safety and efficacy of regorafenib in the treatment of solid cancers. Expert Opin Drug Metab Toxicol 2014; 10: 1607–1614. [DOI] [PubMed] [Google Scholar]
- 25.Sastre J, Argiles G, Benavides M et al. Clinical management of regorafenib in the treatment of patients with advanced colorectal cancer. Clin Transl Oncol 2014; 16: 942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urban C, Anadkat MJ. A review of cutaneous toxicities from targeted therapies in the treatment of colorectal cancers. J Gastrointest Oncol 2013; 4: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belum VR, Cercek A, Sanz-Motilva V, Lacouture ME. Dermatologic adverse events to targeted therapies in lower GI cancers: clinical presentation and management. Curr Treat Options Oncol 2013; 14: 389–404. [DOI] [PubMed] [Google Scholar]
- 28.Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol 2014; 71: 787–794. [DOI] [PubMed] [Google Scholar]
- 29.Macedo LT, Lima JP, dos Santos LV, Sasse AD. Prevention strategies for chemotherapy-induced hand-foot syndrome: a systematic review and meta-analysis of prospective randomised trials. Support Care Cancer 2014; 22: 1585–1593. [DOI] [PubMed] [Google Scholar]
- 30.Burbach GJ, Zuberbier T. Hand-foot syndrome with tyrosine kinase inhibitor therapy: treatment recommendations [article in German]. Urologe A 2013; 52: 1574–1578. [DOI] [PubMed] [Google Scholar]
- 31.Gomez P, Lacouture ME. Clinical presentation and management of hand-foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: experience in breast cancer. Oncologist 2011; 16: 1508–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manchen E, Robert C, Porta C. Management of tyrosine kinase inhibitor-induced hand-foot skin reaction: viewpoints from the medical oncologist, dermatologist, and oncology nurse. J Support Oncol 2011; 9: 13–23. [DOI] [PubMed] [Google Scholar]
- 33.Bednarikova D, Kocak I. Hand-foot syndrome after administration of tyrosinkinase inhibitors [article in Czech]. Klin Onkol 2010; 23: 300–305. [PubMed] [Google Scholar]
- 34.Degen A, Alter M, Schenck F et al. The hand-foot-syndrome associated with medical tumor therapy—classification and management. J Dtsch Dermatol Ges 2010; 8: 652–661. [DOI] [PubMed] [Google Scholar]
- 35.Yang CH, Chuang CK, Hsieh JJ, Chang JW. Targeted therapy and hand-foot skin reaction in advanced renal cell carcinoma. Expert Opin Drug Saf 2010; 9: 459–470. [DOI] [PubMed] [Google Scholar]
- 36.Lipworth AD, Robert C, Zhu AX. Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): focus on sorafenib and sunitinib. Oncology 2009; 77: 257–271. [DOI] [PubMed] [Google Scholar]
- 37.Anderson R, Jatoi A, Robert C et al. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs). Oncologist 2009; 14: 291–302. [DOI] [PubMed] [Google Scholar]
- 38.Milano G, Mortier L, Digue L et al. Hand-foot syndrome and sorafenib [Article in French]. Bull Cancer 2009; 96: 191–197. [DOI] [PubMed] [Google Scholar]
- 39.Lacouture ME, Wu S, Robert C et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist 2008; 13: 1001–1011. [DOI] [PubMed] [Google Scholar]
- 40.Ren ZG, Zhu KS, Yang HY et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol 2015; 33: 894–900. [DOI] [PubMed] [Google Scholar]
- 41.Shinohara N, Nonomura N, Eto M et al. A randomized multicenter phase II trial on the efficacy of a hydrocolloid dressing containing ceramide with a low-friction external surface for hand-foot skin reaction caused by sorafenib in patients with renal cell carcinoma. Ann Oncol 2014; 25: 472–476. [DOI] [PubMed] [Google Scholar]
- 42.Li JR, Yang CR, Cheng CL et al. Efficacy of a protocol including heparin ointment for treatment of multikinase inhibitor-induced hand-foot skin reactions. Support Care Cancer 2013; 21: 907–911. [DOI] [PubMed] [Google Scholar]
- 43.Bozkurt DB, Kara B, Oguz KI et al. Hand-foot syndrome due to sorafenib in hepatocellular carcinoma treated with vitamin E without dose modification; a preliminary clinical study. J BUON 2011; 16: 759–764. [PubMed] [Google Scholar]
- 44.Zhao C, Chen J, Yu B et al. Effect of modified taohongsiwu decoction on patients with chemotherapy-induced hand-foot syndrome. J Tradit Chin Med 2014; 34: 10–14. [DOI] [PubMed] [Google Scholar]
- 45.Sil A, Das NK. Sorafenib-induced hand-foot syndrome in a patient of renal cell carcinoma. Indian J Pharmacol 2014; 46: 334–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanneste L, Wolter P, Van den Oord JJ et al. Cutaneous adverse effects of BRAF inhibitors in metastatic malignant melanoma, a prospective study in 20 patients. J Eur Acad Dermatol Venereol 2014; 29: 61–68. [DOI] [PubMed] [Google Scholar]
- 47.Hung CT, Chiang CP, Wu BY. Sorafenib-induced psoriasis and hand-foot skin reaction responded dramatically to systemic narrowband ultraviolet B phototherapy. J Dermatol 2012; 39: 1076–1077. [DOI] [PubMed] [Google Scholar]
- 48.Bos WE, Nijsten TE, de Jonge MJ, Hamberg AP. Topical psoralen plus UV-A therapy for tyrosine kinase inhibitor-induced hand-foot syndrome. Arch Dermatol 2012; 148: 546–547. [DOI] [PubMed] [Google Scholar]
- 49.Sibaud V, Delord JP, Chevreau C. Sorafenib-induced hand-foot skin reaction: a Koebner phenomenon? Target Oncol 2009; 4: 307–310. [DOI] [PubMed] [Google Scholar]
- 50.Echeverria B, Llombart B, Botella-Estrada R, Guillen C. Palmoplantar cutaneous reaction to sorafenib [article in Spanish]. Actas Dermosifiliogr 2009; 100: 736–737. [PubMed] [Google Scholar]
- 51.Hutten M, Lassay L, Sachs B et al. Successful topical treatment of sorafenib-induced hand-foot skin reaction in a child with hepatocellular carcinoma. Pediatr Dermatol 2009; 26: 349–350. [DOI] [PubMed] [Google Scholar]
- 52.Lilly E, Burke M, Kluger H, Choi J. Pregabalin for the treatment of painful hand-foot skin reaction associated with dabrafenib. JAMA Dermatol 2014; 151: 102–103. [DOI] [PubMed] [Google Scholar]
- 53.Zuehlke RL. Erythematous eruption of the palms and soles associated with mitotane therapy. Dermatologica 1974; 148: 90–92. [DOI] [PubMed] [Google Scholar]
- 54.Boussemart L, Routier E, Mateus C et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol 2013; 24: 1691–1697. [DOI] [PubMed] [Google Scholar]
- 55.Hoesly FJ, Baker SG, Gunawardane ND, Cotliar JA. Capecitabine-induced hand-foot syndrome complicated by pseudomonal superinfection resulting in bacterial sepsis and death: case report and review of the literature. Arch Dermatol 2011; 147: 1418–1423. [DOI] [PubMed] [Google Scholar]
- 56.Lai SE, Kuzel T, Lacouture ME. Hand-foot and stump syndrome to sorafenib. J Clin Oncol 2007; 25: 341–343. [DOI] [PubMed] [Google Scholar]
- 57.Boone SL, Jameson G, Von Hoff D, Lacouture ME. Blackberry-induced hand-foot skin reaction to sunitinib. Invest New Drugs 2009; 27: 389–390. [DOI] [PubMed] [Google Scholar]
- 58.Robert C, Mateus C, Spatz A et al. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol 2009; 60: 299–305. [DOI] [PubMed] [Google Scholar]
- 59.Jacobi U, Waibler E, Schulze P et al. Release of doxorubicin in sweat: first step to induce the palmar-plantar erythrodysesthesia syndrome? Ann Oncol 2005; 16: 1210–1211. [DOI] [PubMed] [Google Scholar]
- 60.Martschick A, Sehouli J, Patzelt A et al. The pathogenetic mechanism of anthracycline-induced palmar-plantar erythrodysesthesia. Anticancer Res 2009; 29: 2307–2313. [PubMed] [Google Scholar]
- 61.Templeton AJ, Ribi K, Surber C et al. Prevention of palmar-plantar erythrodysesthesia with an antiperspirant in breast cancer patients treated with pegylated liposomal doxorubicin (SAKK 92/08). Breast 2014; 23: 244–249. [DOI] [PubMed] [Google Scholar]
- 62.Jain L, Gardner ER, Figg WD et al. Lack of association between excretion of sorafenib in sweat and hand-foot skin reaction. Pharmacotherapy 2010; 30: 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lankheet NA, Huitema AD, Mallo H et al. The effect of seasonal variation and secretion of sunitinib in sweat on the development of hand-foot syndrome. Eur J Clin Pharmacol 2013; 69: 2065–2072. [DOI] [PubMed] [Google Scholar]
- 64.Lankheet NA, Blank CU, Mallo H et al. Determination of sunitinib and its active metabolite N-desethylsunitinib in sweat of a patient. J Anal Toxicol 2011; 35: 558–565. [DOI] [PubMed] [Google Scholar]
- 65.Lacouture ME, Reilly LM, Gerami P, Guitart J. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol 2008; 19: 1955–1961. [DOI] [PubMed] [Google Scholar]
- 66.Lee WJ, Lee JL, Chang SE et al. Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. Br J Dermatol 2009; 161: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 67.Grothey A, Sobrero AF, Siena S et al. Time profile of adverse events (AEs) from regorafenib (REG) treatment for metastatic colorectal cancer (mCRC) in the phase III CORRECT study. J Clin Oncol 2013; 31(Suppl): abstr 3637. [Google Scholar]
- 68.Gomez-Martin C, Sanchez A, Irigoyen A et al. Incidence of hand-foot syndrome with capecitabine in combination with chemotherapy as first-line treatment in patients with advanced and/or metastatic gastric cancer suitable for treatment with a fluoropyrimidine-based regimen. Clin Transl Oncol 2012; 14: 689–697. [DOI] [PubMed] [Google Scholar]
- 69.Gressett SM, Stanford BL, Hardwicke F. Management of hand-foot syndrome induced by capecitabine. J Oncol Pharm Pract 2006; 12: 131–141. [DOI] [PubMed] [Google Scholar]
- 70.Rosen AC, Case EC, Dusza SW et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: a questionnaire study in a dermatology referral clinic. Am J Clin Dermatol 2013; 14: 327–333. [DOI] [PubMed] [Google Scholar]
- 71.McLellan B, Kerr H. Cutaneous toxicities of the multikinase inhibitors sorafenib and sunitinib. Dermatol Ther 2011; 24: 396–400. [DOI] [PubMed] [Google Scholar]
- 72.Charles C, Sultan S, Bungener C et al. Impact of cutaneous toxicities associated with targeted therapies on quality of life. Results of a longitudinal exploratory study [article in French]. Bull Cancer 2013; 100: 213–222. [DOI] [PubMed] [Google Scholar]
- 73.Nardone B, Hensley JR, Kulik L et al. The effect of hand-foot skin reaction associated with the multikinase inhibitors sorafenib and sunitinib on health-related quality of life. J Drugs Dermatol 2012; 11: e61–e65. [PubMed] [Google Scholar]
- 74.Haley AC, Calahan C, Gandhi M et al. Skin care management in cancer patients: an evaluation of quality of life and tolerability. Support Care Cancer 2011; 19: 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dreno B, Bensadoun RJ, Humbert P et al. Algorithm for dermocosmetic use in the management of cutaneous side-effects associated with targeted therapy in oncology. J Eur Acad Dermatol Venereol 2013; 27: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borovicka JH, Calahan C, Gandhi M et al. Economic burden of dermatologic adverse events induced by molecularly targeted cancer agents. Arch Dermatol 2011; 147: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 77.Dranitsaris G, Vincent MD, Yu J et al. Development and validation of a prediction index for hand-foot skin reaction in cancer patients receiving sorafenib. Ann Oncol 2012; 23: 2103–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsuchiya N, Narita S, Inoue T et al. Risk factors for sorafenib-induced high-grade skin rash in Japanese patients with advanced renal cell carcinoma. Anticancer Drugs 2013; 24: 310–314. [DOI] [PubMed] [Google Scholar]
- 79.Chren MM, Lasek RJ, Sahay AP, Sands LP. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg 2001; 5: 105–110. [DOI] [PubMed] [Google Scholar]
- 80.Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc 2004; 9: 169–180. [DOI] [PubMed] [Google Scholar]
- 81.Sibaud V, Dalenc F, Chevreau C et al. HFS-14, a specific quality of life scale developed for patients suffering from hand-foot syndrome. Oncologist 2011; 16: 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DeSimone PA. Nightly dosing of regorafenib. J Clin Oncol 2015; 33(Suppl): abstr 549. [Google Scholar]
- 83.Tabchi S, Ghosn M. Regorafenib: start low and go slow. Target Oncol 2015; 10(3): 445–447. [DOI] [PubMed] [Google Scholar]
- 84.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/About.html (20 April 2015, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.