Breast cancer-specific mortality among older women modestly improved from 2002 to 2009 across all races, but not other-cause mortality in the US population. Racial disparity in mortality persisted, but did not widen in this period. Efforts should be devoted to improving other-cause mortality for all women, with special attention toward decreasing breast cancer mortality for non-Hispanic black women.
Keywords: breast cancer-specific mortality, competing risk, older stage IV breast cancer, other-cause mortality, SEER program, Medicare
Abstract
Background
Research on temporal mortality trends for stage IV breast cancer is limited, especially among older patients by race. We evaluated factors associated with overall, breast cancer-specific and other-cause mortalities using contemporary population data.
Patients and methods
Using the Surveillance, Epidemiology, and End Results–Medicare linked data, we identified older women (≥66 years) with stage IV breast cancer diagnosed in 2002–2009. Overall mortality was estimated by the Kaplan–Meier method, compared by log-rank tests, and modeled by Cox models. Competing risk analysis was used to evaluate breast cancer-specific and other-cause mortalities.
Results
The median overall survival time for non-Hispanic blacks improved from 8.6 months in 2002–2003 to 9.9 months in 2007–2009, whereas that for non-Hispanic whites improved from 12.1 to 14.8 months. In the multivariate model, the risk of breast cancer-specific death for patients diagnosed in 2007–2009 was significantly lower (P = 0.02), whereas the risk of other-cause mortality changed little (P = 0.88) compared with those risks for patients diagnosed in 2002–2003. Non-Hispanic blacks had the higher risk of both mortality types compared with non-Hispanic whites; a diagnosis time–race interaction term was not statistically significant for either cause of death.
Conclusion
Breast cancer-specific mortality among older women modestly improved from 2002 to 2009 across all races, but not other-cause mortality. Racial disparity in mortality persisted, but did not widen in this period. Efforts should be devoted to improving other-cause mortality for all women, with special attention toward decreasing breast cancer mortality for non-Hispanic black women.
introduction
Breast cancer is the most common non-cutaneous cancer in America and the leading cause of cancer deaths for women. Approximately 235 030 new cases of breast cancer were diagnosed in the USA in 2014, 43% of which occurred in women ages 65 years and older [1, 2]. Breast cancer mortality dropped by 34% from 1990 to 2010 due to widespread breast cancer screening and improved therapies that specifically targeted breast cancer [3]; however, the 5-year survival rate for individuals with stage IV breast cancer remains as low as 22%–24% [2].
Research on racial disparities in breast cancer has been given substantial attention over the last two decades [4–6]. Studies have attributed the worse overall mortality observed in black patients to factors such as differences in screening patterns, tumor biology, treatment, and socioeconomic status [7–9]. For patients diagnosed with stage IV breast cancer, Dawood et al. [10] reported that disparity in survival times between black and white patients increased from 1988 to 2003. With therapeutic breakthroughs brought about by targeted agents, such as trastuzumab, in the late 1990s, it is possible that treatment advances offer the opportunity to narrow the black–white gap in survival times. Conversely, if barriers to accessing new treatments are more common among blacks than whites, racial disparities may persist or even worsen. Studies using more contemporary data will provide important insights into this issue.
Unlike younger women with breast cancer, causes of death other than breast cancer are non-ignorable competing risks among older women; therefore, other-cause mortality should be addressed carefully when studying mortality among older patients [11–14]. To estimate the magnitude of racial disparity and determine whether the magnitude has changed over time for women with stage IV breast cancer, we used the Surveillance, Epidemiology, and End Results (SEER)–Medicare data to respectively examine breast cancer-specific mortality and other-cause mortality by race for women diagnosed in 2002–2009. Estimations of overall mortality, breast cancer-specific mortality, and other-cause mortality over time will help investigators to better understand the impact of modern clinical management of breast cancer over the last decade and to identify areas for further health-care improvements regarding racial disparity.
patients and methods
patient cohort
We used data from the SEER–Medicare database, which links the National Cancer Institute's SEER registry with Medicare claims and enrollment files. The SEER–Medicare database connected 94% of patients aged 65 years or older in SEER registries with Medicare files. Using this population-based database, we identified women who were diagnosed with stage IV breast cancer as the first primary cancer between 1 January 2002 and 31 December 2009. Patients with more than one primary cancer were excluded. Stage IV was defined according to the SEER's American Joint Cancer Committee, third edition criteria. Several additional inclusion criteria were implemented: age at breast cancer diagnosis ≥66 years, alive at diagnosis, enrollment in Medicare Part A and Part B but without Health Maintenance Organization (HMO) coverage from 12 months before the diagnosis date through 12 months after the diagnosis date, unless the patient died within 12 months of the diagnosis, but had continuous Medicare Part A and B enrollment and no HMO coverage until death. We restricted our population to women age ≥66 years to allow for at least 1 year of complete Medicare claims to calculate comorbidities. After applying these inclusion criteria, the analytic cohort consisted of data from 5018 women. All data were de-identified and the study was given exemption by MD Anderson Cancer Center's institutional review board.
covariate and outcome measures
The primary outcomes were overall, breast cancer-specific and other-cause mortalities. Survival time was calculated from the date of breast cancer diagnosis to the date of death or right-censored at the follow-up cutoff (31 December 2010). We determined the cause of death via ICD-9 codes.
We extracted from the SEER–Medicare database the following patient information: race, region of residence, age at diagnosis, date of cancer diagnosis, estrogen receptor (ER) status, progesterone receptor (PR) status, and tumor size. We calculated the severity of comorbid conditions from Medicare claims using a modified Charlson comorbidity score [15, 16]. We used Medicare claims to identify the treatments (chemotherapy, radiation, and surgery) received within 12 months of breast cancer diagnosis. The variable of surgery captures only breast-related surgery, but the variable for the radiation is not site-specific and can include distant disease sites. More details on covariate definitions are listed in supplementary Table S1, available at Annals of Oncology online. We did not identify any patterns or bias associated with the missing observations; therefore, data from subjects with unknown or missing information were excluded from our analysis.
statistical analysis
We grouped the patients according to their year of diagnosis: 2002–2003, 2004–2006, and 2007–2009. We compared patient and tumor characteristics across these three time periods using Kruskal–Wallis tests and chi-squared tests for continuous and categorical variables, respectively. We used the Kaplan–Meier method and log-rank tests to estimate the overall mortality and compare the overall mortalities. We used Cox proportional hazard models to evaluate the risk factors associated with overall mortality, and checked the proportional hazards assumption.
As a non-trivial proportion of the patients in the study cohort died from causes other than breast cancer, it is proper to use competing risk methods [13]. We estimated the cumulative incidence functions and used Gray's test to compare the cause-specific mortality [11]. We used Cox regression models, incorporating competing risks, to evaluate the risk factors [12]. We used SAS (v9.3; SAS Institute, Cary, NC, USA) to conduct all analyses.
results
Among the 5018 women with stage IV breast cancer who met the eligibility criteria, the mean age at diagnosis was 77.3 years. Table 1 summarizes patient characteristics by the three time periods of diagnosis. The percentages of unknown tumor size and hormone receptor information decreased with the advancing year of diagnosis. Among patients with known tumor characteristics, patients diagnosed after 2003 had more favorable tumor characteristics such as tumor size. The use of chemotherapy and radiation did not change significantly over the three time periods, whereas the use of surgery decreased over time (P < 0.001). Supplementary Table S2, available at Annals of Oncology online lists patient characteristics by race. The use of chemotherapy, radiation, and surgery were similar among the three groups by race. Non-Hispanic black women had the worst characteristics regarding the Charlson comorbidity score and tumor size.
Table 1.
Distribution of patient characteristics by year of diagnosis using three time periods
| Variable | Level | 2002–2003 N = 1206 |
2004–2006 N = 1945 |
2007–2009 N = 1867 |
P-value |
|---|---|---|---|---|---|
| Age at diagnosis | Mean | 77.3 | 77.4 | 77.3 | 0.87 |
| Race | |||||
| White | 981 (81.3%) | 1513 (77.8%) | 1494 (80.0%) | 0.01 | |
| Black | 146 (12.1%) | 269 (13.8%) | 206 (11.0%) | ||
| Others | 79 (6.6%) | 163 (8.4%) | 167 (8.9%) | ||
| Comorbidity score | |||||
| 0 | 828 (68.7%) | 1294 (66.5%) | 1207 (64.6%) | 0.02 | |
| 1 | 252 (20.9%) | 382 (19.6%) | 406 (21.7%) | ||
| 2+ | 126 (10.4%) | 269 (13.8%) | 254 (13.6%) | ||
| Tumor size (mm) | |||||
| ≤20 | 125 (10.4%) | 325 (16.7%) | 304 (16.3%) | <0.0001 | |
| >20, ≤50 | 335 (27.8%) | 589 (30.3%) | 627 (33.6%) | ||
| >50 | 237 (19.7%) | 418 (21.5%) | 412 (22.1%) | ||
| Unknown | 509 (42.2%) | 613 (31.5%) | 524 (28.1%) | ||
| Region of patient's residence | |||||
| Midwest | 161 (13.3%) | 270 (13.9%) | 262 (14.0%) | 0.95 | |
| Northeast | 325 (26.9%) | 506 (26.0%) | 474 (25.4%) | ||
| South | 291 (24.1%) | 493 (25.3%) | 472 (25.3%) | ||
| West | 429 (35.6%) | 676 (34.8%) | 659 (35.3%) | ||
| Urban/rural residence | |||||
| Big metro | 676 (56.1%) | 1076 (55.3%) | 1036 (55.5%) | 0.23 | |
| Metro | 362 (30.0%) | 556 (28.6%) | 516 (27.6%) | ||
| Other | 168 (13.9%) | 313 (16.1%) | 315 (16.9%) | ||
| ER status | |||||
| Unknown | 459 (38.1%) | 486 (25.0%) | 382 (20.5%) | <0.0001 | |
| Positive | 557 (46.2%) | 1093 (56.2%) | 1136 (60.8%) | ||
| Negative | 190 (15.8%) | 366 (18.8%) | 349 (18.7%) | ||
| PR status | |||||
| Unknown | 490 (40.6%) | 520 (26.7%) | 408 (21.8%) | <0.0001 | |
| Positive | 405 (33.6%) | 830 (42.7%) | 871 (46.7%) | ||
| Negative | 311 (25.8%) | 595 (30.6%) | 588 (31.5%) | ||
| Hormone receptor | |||||
| Negative | 175 (14.5%) | 350 (18.0%) | 327 (17.5%) | <0.0001 | |
| Positive | 570 (47.3%) | 1108 (57.0%) | 1156 (61.9%) | ||
| Unknown | 461 (38.2%) | 487 (25.0%) | 384 (20.6%) | ||
| Chemotherapy | |||||
| No | 723 (60.0%) | 1228 (63.1%) | 1170 (62.7%) | 0.17 | |
| Yes | 483 (40.0%) | 717 (36.9%) | 697 (37.3%) | ||
| Radiation therapy | |||||
| No | 808 (67.0%) | 1293 (66.5%) | 1261 (67.5%) | 0.78 | |
| Yes | 398 (33.0%) | 652 (33.5%) | 606 (32.5%) | ||
| Surgery | |||||
| No | 720 (59.7%) | 1214 (62.4%) | 1272 (68.1%) | <0.001 | |
| Yes | 486 (40.3%) | 731 (37.6%) | 595 (31.9%) | ||
Values are given as n (%).
ER, estrogen receptor; PR, progesterone receptor.
survival outcomes
overall mortality
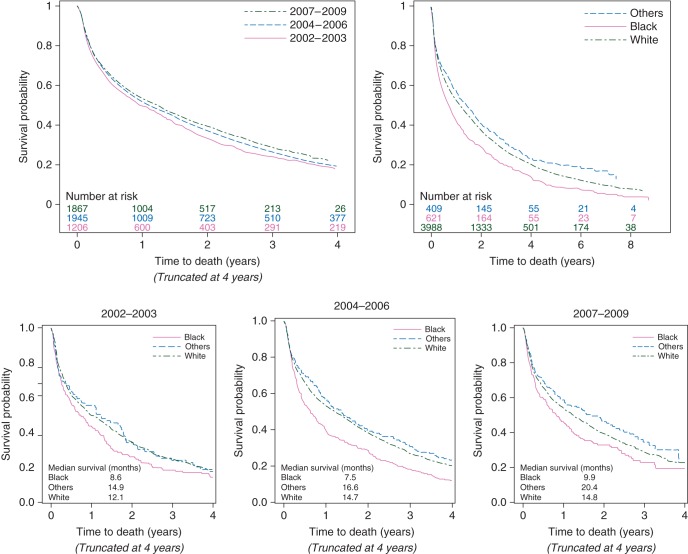
During the median follow-up of 58.1 months, 4043 patients died. Of those deaths, 79% were due to breast cancer, and 21% resulted from other causes. The median overall survival time was 13.2 months for all patients, and 11.6, 13.1, and 14.6 months for the three consecutive diagnosis time periods, respectively. Median breast cancer-specific survival was 19.5 months for all patients, and 17.5, 19.5, and 21.1 months for the three diagnosis time periods, respectively. When examining the temporal changes by race, the median overall survival time for non-Hispanic black women improved from 8.6 months (95% confidence interval [CI] 5.6–13.1) in 2002–2003 to 9.9 months (95% CI 7.1–13.5) in 2007–2009, whereas the median overall survival time for non-Hispanic white women improved from 12.1 months (95% CI 10.2–14.6) to 14.8 months (95% CI 12.8–16.8).
Figure 1 presents the overall mortality distributions by the period of diagnosis and race. The overall mortality significantly improved over the three time periods (P = 0.01) and non-Hispanic black women had the worst overall survival time compared with those of other races (P < 0.0001). Among the three diagnosis time periods, the overall mortality of non-Hispanic blacks was worse for those diagnosed between 2004 and 2006, with a median survival of 7.5 months, which then increased to 9.9 months for those diagnosed between 2007 and 2009. The interaction term between race and the time period of diagnosis was not statistically significant (P = 0.58).
Figure 1.
Overall survival time by diagnosis year in three categories and race: log-rank test by the diagnosis period, P = 0.01; log-rank test by race, P < 0.0001.
Supplementary Table S3, available at Annals of Oncology online presents the results of univariate Cox proportional models for overall mortality, which were used to guide variable selection in the multivariate analyses. The factors independently associated with worse overall mortality were an earlier year of diagnosis, being non-Hispanic black, of an older age, having a higher comorbidity score, living in the Midwest or South, having hormone receptor-negative disease, not receiving chemotherapy, not receiving surgery, and not receiving radiation therapy. Compared with non-Hispanic white women, non-Hispanic black women had a higher risk of overall mortality, whereas patients in the other race group had a lower risk. Supplementary Table S4, available at Annals of Oncology online lists the results of multivariable Cox proportional model for overall mortality.
breast cancer-specific mortality and other-cause mortality
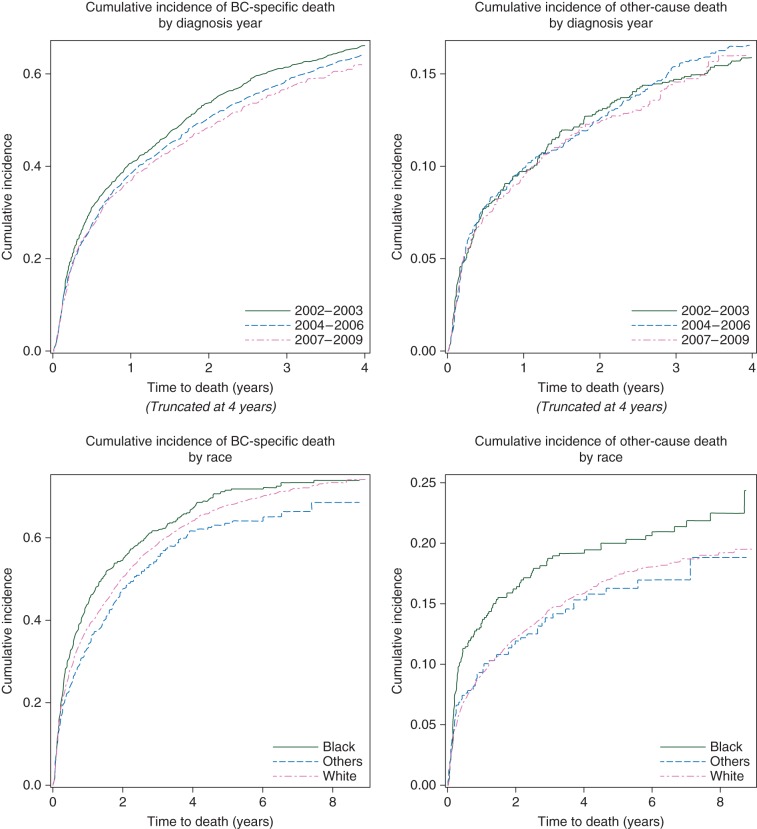
We plotted the cumulative incidence functions of cause-specific mortalities by diagnosis period and race, respectively (Figure 2). Gray's tests suggested that breast cancer-specific mortality had marginally improved over the three diagnosis periods (P = 0.04), whereas other-cause mortality showed little improvement (P = 0.84). The 3-year, breast cancer-specific mortality for each chronologic diagnosis period was 68.6%, 65.4%, and 63.1%, respectively, and the corresponding 3-year, other-cause mortality was 23.6%, 24.8%, and 22.5%, respectively (supplementary Table S5, available at Annals of Oncology online).
Figure 2.
Cumulative incidence and Gray's test by diagnosis year in three categories and race: Gray's test for breast cancer-specific death by the diagnosis period, P = 0.04; Gray's test for other-cause death by the diagnosis period, P = 0.84; Gray's test for breast cancer-specific death, P = 0.02 by race; Gray's test for other-cause death by race, P = 0.05.
Non-Hispanic black women had significantly higher mortality rates due to both breast cancer (P= 0.02) and other causes (P = 0.05; Figure 2). The 3-year breast cancer-specific mortality for non-Hispanic black women, non-Hispanic white women, and patients of other races was 71.4%, 65.0%, and 61.4%, respectively, and the corresponding 3-year other-cause mortality for each race group was 31.3%, 23.1%, and 20.2%, respectively (supplementary Table S5, available at Annals of Oncology online). We plotted these cumulative incidence functions by diagnosis period and race (supplementary Figure S1, available at Annals of Oncology online).
Table 2 provides the results of the multivariable models for cause-specific mortalities. After controlling for the other risk factors in Table 2, the risk of breast cancer-specific death was significantly decreased (HR = 0.90, 95% CI 0.81–0.98, P = 0.02) for patients diagnosed between 2007 and 2009, whereas the risk of other-cause mortality changed little (HR = 0.99, 95% CI 0.81–1.19, P = 0.88) compared with patients diagnosed between 2002 and 2003. Non-Hispanic black women had the highest risk of death compared with non-Hispanic white women: HR = 1.14 (95% CI 1.03–1.27, P = 0.01) for breast cancer-specific death and HR = 1.27 (95% CI 1.04–1.54, P = 0.02) for other-cause death. After adding an interaction term between race and the period of diagnosis to the two Cox models, we found neither to be statistically significant (supplementary Table S6, available at Annals of Oncology online), which indicates that the racial disparity in cause-specific mortality has neither improved nor widened over the three diagnosis periods. The comorbidity score was a significant predictor for other-cause mortality, but not for breast cancer-specific mortality. The risk of breast cancer-specific mortality was statistically significantly different among the four geographic regions, with patients in the Northeast and West having a lower risk than those in the South and Midwest. No significant geographic variation was found for the risk of other-cause mortality.
Table 2.
Multivariable Cox models for cause-specific mortality
| BC-specific survival |
P-value for the overall effect | Other-cause survival |
P-value for the overall effect | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |||
| Year of diagnosis | ||||||||
| 2002–2003 | 1 | 0.08 | 1 | 0.96 | ||||
| 2004–2006 | 0.95 | 0.87–1.03 | 0.21 | 1.01 | 0.85–1.20 | 0.93 | ||
| 2007–2009 | 0.90 | 0.81–0.98 | 0.02 | 0.99 | 0.81–1.19 | 0.88 | ||
| Race | ||||||||
| White | 1 | 0.02 | 1 | 0.05 | ||||
| Black | 1.14 | 1.03–1.27 | 0.01 | 1.27 | 1.04–1.54 | 0.02 | ||
| Others | 0.91 | 0.80–1.05 | 0.20 | 0.93 | 0.71–1.22 | 0.60 | ||
| Age at diagnosis | ||||||||
| 66 | 1 | 1 | ||||||
| One-year increase | 1.02 | 1.01–1.02 | <0.0001 | 1.03 | 1.02–1.04 | <0.0001 | ||
| Comorbidity score | ||||||||
| 0 | 1 | 0.77 | 1 | <0.0001 | ||||
| 1 | 1.03 | 0.94–1.13 | 0.49 | 1.61 | 1.36–1.90 | <0.0001 | ||
| 2+ | 1.02 | 0.91–1.14 | 0.76 | 2.10 | 1.72–2.51 | <0.0001 | ||
| Region of patient's residence | ||||||||
| Midwest | 1 | 0.008 | 1 | 0.09 | ||||
| Northeast | 0.88 | 0.78–0.98 | 0.02 | 1.10 | 0.88–1.38 | 0.41 | ||
| South | 0.99 | 0.88–1.11 | 0.88 | 1.19 | 0.95–1.50 | 0.14 | ||
| West | 0.87 | 0.78–0.98 | 0.02 | 0.95 | 0.75–1.19 | 0.64 | ||
| Hormone receptor | ||||||||
| Negative | 1 | <0.0001 | 1 | <0.0001 | ||||
| Positive | 0.46 | 0.41–0.50 | <0.0001 | 0.49 | 0.40–0.60 | <0.0001 | ||
| Unknown | 0.80 | 0.72–0.89 | <0.0001 | 1.05 | 0.85–1.31 | 0.64 | ||
| Chemotherapy | ||||||||
| No | 1 | 1 | ||||||
| Yes | 0.64 | 0.59–0.70 | <0.0001 | 0.53 | 0.45–0.63 | <0.0001 | ||
| Radiation therapy | ||||||||
| No | 1 | 1 | ||||||
| Yes | 0.88 | 0.82–0.95 | 0.001 | 0.64 | 0.54–0.75 | <0.0001 | ||
| Surgery | ||||||||
| No | 1 | 1 | ||||||
| Yes | 0.62 | 0.57–0.67 | <0.0001 | 0.69 | 0.59–0.80 | <0.0001 | ||
BC, breast cancer; HR, hazard ratio; CI, confidence interval.
discussion
Utilizing the contemporary SEER–Medicare linked data over the past decade for patients older than 65 years with newly diagnosed stage IV breast cancer, we found that the overall mortality rates had moderately improved since 2002, and the improvement in breast cancer-specific mortality was a main contributor to this overall decrease in the mortality rate as the other-cause mortality did not change much over the last decade. The launch of targeted therapies in the market and their dissemination into clinical practice at the population level during the last decade may have contributed to the improved breast cancer-specific mortality for stage IV disease [17, 18].
Among the study cohort, non-Hispanic black women had the worst other-cause mortality, which continued over the last decade. The other-cause mortality changed little for all racial groups. Focusing on breast cancer-specific mortality, non-Hispanic black women had the highest mortality rate in 2002–2003, which gradually decreased during the period of 2004–2009, until it reached the breast cancer-specific mortality rate of white/other races in 2002–2003.
Previous studies reporting temporal changes in the mortality of patients with stage IV breast cancer have been limited to small institutional retrospective reviews or to patients diagnosed before 2003 using SEER data [10]. None of the published work investigated cause-specific mortality using competing risk models. Unlike the earlier findings of Dawood et al. [10] based on SEER data from 1998 to 2002, which suggested a widening disparity between the mortality rates among white and black women with stage IV breast cancer, the interaction term between the time period of diagnosis and race in our model was not statistically significant. This indicates that the racial gap has not widened over the past 10 years. There was a moderate improvement for non-black women from 2002 to 2003 until the most recent time period and a ‘marginal’ improvement for non-Hispanic black women during recent years to the level of non-black women in 2002–2003. One plausible explanation is that the increasing attention on the racial disparity in breast cancer care may have led to improved clinical practice, such as reducing the barriers to care or using navigation services for better coordination and management of cancer treatment.
Although the cumulative incidence of breast cancer-specific death has steadily decreased over time, the other-cause mortality rate for all races of these patients has not changed much over the last decade. One significant risk factor for other-cause mortality is the comorbidity score, which does not significantly predict breast cancer-specific mortality (Table 2). Of interest, we expected comorbid conditions to limit a patient's access to treatments, which could subsequently impact breast cancer-specific mortality. After adjusting for other factors, however, we did not find this to be the case. For older patients with stage IV breast cancer, patients who do and do not have surgery and/or radiation can be very different. It is likely that patients who received surgery were healthier than their counterparts, regardless of whether there is any benefit of surgery. Hence, the estimated hazard ratio per treatment cannot be interpreted as the treatment benefit for this population.
Given that 20% of the deaths among this cohort were not due to breast cancer, more efforts should be devoted to reducing the risk of death from chronic diseases by better controlling hypertension and diabetes and encouraging healthy life styles. Another interesting observation is the difference in breast cancer-specific mortality across geographic regions that did not exist for other cause-specific mortality. It appears there is still room for improvement in breast cancer care, especially in the South and Midwest.
There are several limitations of this study that should be considered. This cohort of older patients with breast cancer represented 26% of the US population in a similar age category; however, the overall health status of individuals not included in the SEER–Medicare cohort may be different, which may limit the generalizability of our results. Among patients with late-stage breast cancer, the absolute number of non-Hispanic black women is moderate; but the racial/ethnic distribution of the data is representative of older patients in the US breast cancer population (12.5%). The temporal mortality trend documented in our study for older patients with stage IV breast cancer might be different from the trend for a younger counterpart cohort. A future study should explore the temporal change of mortality by race using other national databases. We truncated follow-up times at 31 December 2010 in the survival analysis because the causes of death were unavailable for deaths observed between 1 January 2011 and 31 December 2011. The patients diagnosed in 2008–2009 have relatively limited follow-up compared with others. In the analysis cohort, we did not include detailed treatment-related information such as timing of treatments, types of chemotherapy, and the use of hormonal therapy. In addition, unmeasured risk factors may attribute to and explain some of the racial disparity in the overall mortality and cause-specific mortality rates.
Continual improvement in the management of late-stage breast cancer will require sustained and increased efforts to provide high-quality breast cancer care and effective management of co-existing chronic diseases across all segments of the population. National guidelines recommend that physicians take into account life expectancy, functional status, organ function, and individual patient preference when considering treatment options for older women with breast cancer [19]. Although the difference in mortality rates between blacks and other races for older women with late-stage breast cancer did not increase over the last decade, it has not decreased, either. Efforts to reduce and eliminate the existing racial disparities in the overall mortality rate for patients with breast cancer should continue to receive high priority until we accomplish the goal of eradicating cancer disparities.
funding
This work was supported in part by the National Institutes of Health (grant numbers CA079466, CA016672, and AHRQ R01 HS02026) and Duncan Family Institute fund.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.American Cancer Society. Cancer Facts and Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin 2014; 64(1): 52–62. [DOI] [PubMed] [Google Scholar]
- 3.Ademuyiwa FO, Groman A, Hong CC et al. . Time-trends in survival in young women with breast cancer in a SEER population-based study. Breast Cancer Res Treat 2013; 138(1): 241–248. [DOI] [PubMed] [Google Scholar]
- 4.Ward E, Jemal A, Cokkinides V et al. . Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004; 54(2): 78–93. [DOI] [PubMed] [Google Scholar]
- 5.Parsons HM, Lathrop KI, Schmidt S et al. . Breast cancer treatment delays in a majority minority community: is there a difference? J Oncol Pract 2015; 11(2): e144–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hershman DL, Tsui J, Wright JD et al. . Household net worth, racial disparities, and hormonal therapy adherence among women with early-stage breast cancer. J Clin Oncol 2015; 33: 1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silber JH, Rosenbaum PR, Clark AS et al. . Characteristics associated with differences in survival among black and white women with breast cancer. JAMA 2013; 310(4): 389–397. [DOI] [PubMed] [Google Scholar]
- 8.Smith BD, Jiang J, McLaughlin SS et al. . Improvement in breast cancer outcomes over time: are older women missing out? J Clin Oncol 2011; 29: 4647–4653. [DOI] [PubMed] [Google Scholar]
- 9.Parise CA, Caggiano V. Disparities in race/ethnicity and socioeconomic status: risk of mortality of breast cancer patients in the California Cancer Registry, 2000–2010. BMC Cancer 2013; 13: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawood S, Broglio K, Gonzalez-Angulo AM et al. . Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol 2008; 26(30): 4891–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16(3): 1141–1154. [Google Scholar]
- 12.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94(446): 496–509. [Google Scholar]
- 13.Pintilie M. Analysing and interpreting competing risk data. Stat Med 2007; 26(6): 1360–1367. [DOI] [PubMed] [Google Scholar]
- 14.Patnaik JL, Byers T, Diguiseppi C et al. . The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst 2011; 103: 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klabunde CN, Legler JM, Warren JL et al. . A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol 2007; 17(8): 584–590. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000; 53(12): 1258–1267. [DOI] [PubMed] [Google Scholar]
- 17.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med 2007; 357(1): 39–51. [DOI] [PubMed] [Google Scholar]
- 18.Perez EA, Romond EH, Suman VJ. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014; 32(33): 3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson RW, Moench S, Hurria A et al. . NCCN Task Force Report: breast cancer in the older woman. J Natl Compr Canc Netw 2008; 6(Suppl 4): S1–25. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.