Abstract
Purpose/Objective
Although there has been little research on the adaptive behavior of people with congenital compared to acquired disability, there is reason to predict that people with congenital conditions may be better adapted because they have lived with their conditions for their entire lives (Smart, 2008). We examined whether people with congenital facial paralysis (FP), compared to people with acquired FP, compensate more for impoverished facial expression by using alternative channels of expression (i.e. voice and body).
Research Method/Design
Participants with congenital (n = 13) and acquired (n = 14) FP were videotaped while recalling emotional events.
Main Outcome Measures
Expressive verbal behavior was measured using the Linguistic Inquiry Word Count (Pennebaker, Booth & Francis, 2007). Nonverbal behavior and FP severity were rated by trained coders.
Results
People with congenital FP, compared to acquired FP, used more compensatory expressive verbal and nonverbal behavior in their language, voices, and bodies. The extent of FP severity had little effect on compensatory expressivity.
Conclusions/Implications
This study provides the first behavioral evidence that people with congenital FP use more adaptations to express themselves than people with acquired FP. These behaviors could inform social functioning interventions for people with FP.
Keywords: Facial Paralysis, Moebius syndrome, congenital and acquired adaptation to disability, expressive channels
Introduction
People with FP are at risk for considerable social disability (Bogart & Matsumoto, 2010; Coulson, O’Dwyer, Adams, & Croxon, 2004). The face is the primary communication channel for social interaction, serving to communicate emotion, initiate and regulate the dynamics of conversation, develop rapport, and build social connectedness (Ekman, 1986; Tickle-Degnen, 2006). People with impoverished facial expression are perceived by others as unfriendly, depressed, disinterested, or unintelligent (Lyons, Tickle-Degnen, Henry, & Cohn, 2004; Tickle-Degnen & Lyons, 2004), and others are less interested in pursuing friendships with them (Hemmesch, Tickle-Degnen, & Zebrowitz, 2009). In a sample of individuals with unilateral Bell’s palsy, Coulson et al. (2004) found that impairment in forming just one of six basic expressions of emotion resulted in significantly poorer social functioning. However, in this article, we propose that people with FP may compensate for impoverished facial expression to some extent by becoming more expressive with their language, voice, and body. We suggest that people with congenital FP, who have lived with their conditions all of their lives, compared to people who acquire FP later in life, develop more adaptations to compensate for their impoverished facial expression by becoming more expressive with their bodies, voices, and language.
Adaptation to Disability
Adaptation to disability can be broadly defined as affective, cognitive, and behavioral changes that gradually approach an optimal state of person-environment congruence (Livneh & Antonak, 1997). Vash (1981) described the following 12 attributes of a disability that may influence an individual’s response to his or her disability: time of onset, type of onset, functions impaired, severity, visibility, degree of disfigurement, degree of stigma, course, prognosis, and treatment. One of the least studied factors in the above list is time of onset, particularly whether the disability is congenital or acquired. Smart (2008) suggested that adaptation to disability is best when onset is congenital or early in childhood for the following reasons: 1. children are cognitively and affectively resilient and flexible; 2. there is no premorbid identity or functional loss; 3. children have not internalized society’s prejudices about disability; 4. children have not fully developed their body image.
There have only been a handful of studies that examined whether an earlier age of onset is associated with a better response to disability, and all of these studies used survey methodology (Alfano, Nielson, & Fink, 1993; Krause, 1992; Li & Moore, 1998; Woodrich & Patterson, 1983). For example, in a survey of 1,266 people with disabilities, Li and Moore (1998) found that people with congenital disabilities had higher levels of acceptance of disability compared to people with acquired disabilities. To our knowledge, no one has gone beyond survey research to examine adaptation behaviorally in people with congenital compared to acquired disability. In this study, we examine the behavioral adaptations of people with congenital compared to acquired FP in response to constraints on their facial expressiveness.
Types of Facial Paralysis
Although there are a variety of conditions that result in FP, the social consequences for these conditions are similar: an impaired ability to communicate with the face (Ekman, 1986). Congenital FP can result from prenatal maldevelopments (e.g. Moebius syndrome or Hemifacial Microsomia) or birth trauma (e.g. from forceps delivery). Estimates for the occurrence of congenital FP vary widely from 3,410 to 8,960 American births per year (Hughes, Harley, Milmoe, Bala, & Martorella, 1999). A notable example is Moebius syndrome, a condition resulting in FP, which is usually severe and bilateral, and impaired lateral movement of the eyes (Briegel, 2006; Möbius, 1888).
Acquired FP is estimated to occur in 118,000 Americans each year and can result from a variety of causes, including Bell’s palsy, infections, damage to the facial nerve from neoplasms (e.g. acoustic neuroma, parotid tumors), and trauma (Bleicher, Hamiel, Gengler, & Antimarino, 1996). Bell’s palsy is the most common cause of FP, resulting in FP that is usually unilateral (Bleicher et al., 1996).
Psychosocial Effects of Facial Paralysis
No one to our knowledge has compared the psychosocial effects of congenital and acquired FP. Unfortunately, even in the broader literature on facial disfigurement, researchers have not distinguished between congenital and acquired conditions in their studies, although Newell (2000) suggested that people with congenital disfigurement may respond better to their condition and called for research in this area. Due to the difficulty of recruiting people with congenital FP, samples for FP studies are mostly comprised of people with acquired conditions. Several studies of people with various types of FP have found an increased incidence of anxiety and depression in people with these conditions (e.g. VanSwearingen, Cohn, Bajaj-Luthra, 1999; VanSwearingen, Cohn, Turnbull, Mrzai, & Johnson, 1998), and until recently, it was assumed that there was an equal risk for psychological distress across different types of conditions. For example, a study of 48 people with FP (only two participants had congenital FP), found that participants had high rates of anxiety and depression, with 65% of participants scoring in the clinical depression range of the Beck Depression Inventory (VanSwearingen et al. 1999).
However, in a condition-specific study of Moebius syndrome, Bogart and Matsumoto (2010) examined self-report measures of anxiety, depression, social functioning, and satisfaction with life in adults with Moebius syndrome, compared to age and gender matched control participants without FP and normative data. Of the factors examined, the only significant difference found between the Moebius participants compared to the control group or normative data was that the Moebius group reported lower social functioning. These findings suggest that people Moebius syndrome may be less likely to experience psychological distress compared to previous studies consisting mostly of acquired FP samples.
Compensatory Expressive Behavior
Although people with FP have difficulty communicating with facial expression, there are other expressive channels, the voice and body, which also communicate social information, including emotion and personality (Ambady, Bernieri, & Richeson, 2000). Although there has been considerable research on these channels among normal populations (for a review, see Noller, 1985), their use has not been studied in people with FP. In a qualitative study of adults with Moebius syndrome, participants reported compensating for their lack of facial expression by using expressive behaviors including body language, prosody, and verbal disclosure to express emotion (Bogart, Tickle-Degnen, & Joffe, in press).
Purpose of Study
We aim to follow up the qualitative and survey-based research described above by quantitatively examining the actual adaptive behavior of people with FP, and by directly comparing people with congenital and acquired FP. Adaptation is operationally defined in this study as the use of compensatory expressive behaviors of the body and voice. We suggest that compensatory expressive behaviors may be among the most useful adaptations for people with FP to improve their social functioning because these behaviors aid in communication of social information such as emotion, interest, and friendliness (Ambady et al., 2000). We hypothesized that people with congenital FP would be more expressive in compensatory verbal and nonverbal channels than people with acquired FP.
Method
Overview
Participants with congenital and acquired FP were videotaped while they recalled sad and happy events in their lives in order to capture their expressive behavior. Trained coders viewed portions of the videos and rated the compensatory nonverbal behaviors and FP severity of each participant. Participants’ compensatory verbal behavior was analyzed using the Linguistic Inquiry Word Count (LIWC; Pennebaker, Booth, & Francis, 2007), a software program that counts the usage of emotion words. This study was approved by the Tufts University Institutional Review Board, and all participants provided informed consent.
Participants
Recruiting flyers were posted at the Facial Nerve Center at Massachusetts Eye and Ear Hospital, and flyers and web postings were posted in the United States Moebius Syndrome Foundation newsletter and on their website. Although we attempted to recruit both acquired and congenital participants from the Facial Nerve Center, we were only able to recruit individuals with acquired FP from that site because few congenital patients attended the Center. Thus, the acquired participants were obtained through the Center, and the congenital participants were obtained through the Moebius Syndrome Foundation. Inclusion criteria were: 18 years or older, paralysis/paresis of at least part of the face, and ability to hold a comprehensible conversation in English. Of 30 participants who agreed to participate, three were excluded. One was excluded because the person had fully recovered from FP. One was excluded because the video was not properly recorded due to equipment failure. One was excluded because the participant did not complete the questionnaires. Thirteen participants had congenital FP and 14 had acquired FP. All congenital participants reported diagnoses of Moebius syndrome. For people with acquired FP, the reported diagnoses were as follows: benign facial tumors such as acoustic neuroma (n = 6), unremitted Bell’s palsy (n = 4), infection (n = 2), facial nerve trauma (n = 1), brainstem tumor (n = 1). All participants were community-dwelling. Due to the rarity of the FP population, it was not feasible to balance FP severity or laterality between congenital and acquired paralysis; they varied naturally across groups. See Table 1 for additional participant information.
Table 1.
Participant Characteristics
| Congenital FP | Acquired FP | |
|---|---|---|
| Total n | 13 | 14 |
| Male n | 5 | 4 |
| Unilateral n | 2 | 14 |
| Age in years M (SD) | 43.54 (14.32) | 45.57 (11.24) |
| Age range in years | 23 – 62 | 32 – 60 |
| Duration in years M (SD) | 43.54 (14.32) | 11.79 (9.76) |
| Duration range in years | 23 – 62 | 0 – 31 |
| FP severity M (SD) | 1.68 (.59) | 2.67 (.48) |
| HADS Depression M (SD) | 3.69 (2.90) | 4.43 (4.20) |
| Ethnicity | 12 Caucasian, 1 African American | 12 Caucasian, 2 Hispanic |
| Percent with college degree | 77% | 79% |
| Percent employed | 77% | 64% |
Note. FP severity is the average of 5 raters’ ratings of facial expressivity on a scale from 1 to 5, with higher numbers mean more expressivity.
Procedure and Materials
Procedures
Prior to coming in for a videotaped interview, participants completed a questionnaire packet containing demographic questions and the Hospital Anxiety and Depression Scale (HADS; Zigmond & Snaith, 1983), which is described below. All interviews were conducted by the same interviewer (the first author). The interviews lasted approximately 45 minutes, and included the following tasks, which are described in detail below: participants attempted standardized facial movements to be later assessed using the Sunnybrook Facial Grading Scale (FGS; Ross, Fradet, & Nedzelski, 1996), answered semi-structured questions about their experience living with FP (not reported in this study) and performed the autobiographical recall tasks. At the end of the study, participants were debriefed, and none were distressed.
Measurement of depression
As described in the introduction, some studies have found high incidences of depression in FP (VanSwearingen et al., 1998; 1999). Since depression could have a dampening effect on verbal and nonverbal expressivity (Schwartz, Fair, Salt, Mandel, & Klerman, 1976), we administered HADS, a brief 14-item questionnaire measuring depression and anxiety (Zigmond & Snaith, 1983). In a literature review of 747 studies using HADS, Bjelland, Dahl, Haug, and Neckelmann (2002) concluded that it is a reliable and valid measure for assessing depression and anxiety disorders in medical patients and the general population. HADS is unlikely to confound with physical symptoms, and it has been used to measure depression and anxiety among people with FP (Bogart & Matsumoto, 2010).
Autobiographical recall
Towards the end of the interview, participants were asked to perform autobiographical recall of a sad event, followed by a happy event (Ekman, Levenson, & Friesen, 1983). They were instructed to remember an event when they were feeling sad/happy, and to try to relive that event. Then they were instructed to describe the event while feeling that emotion. As a manipulation check, participants rated how happy and sad they were feeling on a scale from 1 to 5, with 5 being the most intense, at the beginning of the interview and after each recall.
Measurement of compensatory expressive verbal behavior
Participants’ verbal responses to the sad and happy autobiographical recall were transcribed. The transcriptions were then analyzed using LIWC, a software program that quantitatively analyzes the extent to which certain words are used (Pennebaker et al., 2007). We were interested in the degree to which participants used positive (e.g. happy, joy, peaceful) and negative emotion words (e.g. sad, hate, hurt). LIWC utilizes a dictionary of almost 4,500 words that were categorized by expert judges. The positive and negative word subscales each have a Cronbach’s α of .97 (Linguistic Inquiry and Word Count, n.d).
Measurement of compensatory expressive nonverbal behavior
A 20 s excerpt was taken from each of the 27 participants’ videotapes at a standardized time point during the participant’s autobiographical recall of a sad event and a happy event, resulting in a total of 54 clips. The standardized clip selection procedure was as follows: the clip included the last 30 seconds before the endpoint of the person’s response minus the last ten seconds of the response. If this segment contained interviewer speech, videotapes were fast forwarded until a 20 s segment with the least amount of interviewer interruptions was found.
Eight items modified from the Interpersonal Communication Rating Protocol for Individual Expressive Behavior (ICRP-IEB; Tickle-Degnen, 2010) were used to rate the compensatory expressive behaviors of participants with FP. The items were: inflection, laugh, talkativeness, loudness, gesture, head movement, trunk movement, and leg movement. The items were chosen because they were conceptually related to compensatory expressive behaviors; they assessed behaviors that people could adapt, and they did not involve facial expression. Five trained raters viewed the clip excerpts and rated the quality, intensity, and frequency of each behavior on a scale of 1 to 5, with 5 being the most expressive. This method of sampling and rating short clips of social behavior is called thin slicing, and has been shown to be a highly reliable and valid representation of a person’s interpersonal behavior (Ambady et al., 2000). Several studies of people with impoverished facial expression resulting from Parkinson’s disease have used rating protocols similar to the ICRP to rate expressive behavior from thin slices (Lyons et al., 2004; Tickle-Degnen & Lyons, 2004).
Raters were blind to the hypotheses as well as FP type and severity. While compensatory expressive nonverbal behavior was being rated, participants’ faces were not visible. Raters observed and rated one channel at a time to avoid being influenced by other channels. When rating talkativeness and laughter in the voice channel, the video was not visible. When rating inflection and loudness in the voice channel, the video was not visible, and in order to prevent raters from being influenced by speech content, the audio track was content-filtered to remove speech content but retain the sound qualities of the voice (van Bezzoijen & Boves, 1986). When rating the body channel, the audio was turned off and the face was cropped out. The effective reliability, or the reliability of the average of the five raters as a group (Rosenthal & Rosnow, 2008), of each of the eight items ranged from .75 to 0.95.
We averaged ratings for each item across all five raters. A varimax rotated principal component analysis (PCA) constrained to two factors supported two internally consistent subscales of our modified ICRP: a voice scale consisting of the following items: inflection, laugh, talkativeness, loudness (Cronbach’s α = 0.75), and a body scale consisting of the following items: gesture, head movement, trunk movement, and leg movement, (Cronbach’s α = 0.78). We formed two compensatory expressivity composites based on the averages of the items in each of these scales.
Measurement of FP severity
Commonly used measures of FP severity such as FGS were not designed for use with bilateral FP (Ross et al., 1996). For instance, FGS instructs raters to compare the paralyzed side of the face to the normal side, but people with bilateral FP do not have a normal side. We preferred a measure that would be sensitive to spontaneous expressions that occur during social interaction, rather than a clinical measure like the FGS that assesses the ability to voluntarily form certain standard expressions. Thus, we measured FP severity using a method similar to our measurement of compensatory nonverbal expressivity with an ICRP item that instructed raters to rate the frequency, duration, and intensity of the overall expressivity of each side of the participant’s face using a rating scale of 1 to 5, with 5 being the most expressive. The same five raters viewed video clips taken from the autobiographical recall of a happy event. We chose to rate the clips from the happy event because this is the context in which the face would be maximally expressive (Schwartz et al., 1976; Takahashi, Tickle-Degnen, Coster, & Latham, 2010). To prevent raters from being influenced by other expressive channels, the body was cropped from the video and the audio was turned off. Raters viewed and rated each side of participants’ faces separately. The effective reliability for the five raters as a group was .87. Ratings were averaged across raters, and then each participant’s left and right facial expressivity ratings were averaged to create a FP severity score for each participant.
In order to validate our measure of FP severity, we also assessed participants using FGS, a widely used measure in clinical practice to assess severity of FP (Ross et al., 1996). During the videotaped interview, participants attempted five standardized facial movements: eyebrow raise, gentle eye closure, open-lipped smile, snarl (nose wrinkling and upper lip raise), and lip pucker. An occupational therapy graduate student research assistant and the first author viewed these expressions and graded them using the FGS. As we discussed above, this measure was designed to assess people with unilateral FP, but many of our participants had bilateral FP. To account for this, raters scored each side of the face separately, and considered the degree of muscle excursion independent of the participant’s other side. The FGS scores of each participants’ left and right sides were averaged to form a FGS total score. The interrater reliability of the FGS total score was ICC = 0.98. The FGS total score showed good convergent validity with the FP severity scores, r = 0.79.
Data Analysis Overview
In order to determine whether our autobiographical recall tasks were successful in eliciting emotion and whether the mood manipulation differentially affected participants with congenital or acquired FP or severe or mild FP, we conducted a 2 (onset: congenital or acquired) by 2 (FP severity dichotomized by median split: severe or mild) by 2 (emotion: happy or sad) by 3 (time: baseline, post-sad recall, post-happy recall) ANOVA with repeated measures on the last two factors on self-reported emotion ratings.
In order to test our hypothesis that participants with congenital FP use more compensatory expressive verbal behavior than those with acquired FP, we conducted a mixed 2 (onset) × 2 (dichotomized FP severity) × 2 (LIWC emotion word type: positive or negative) × 2 (emotion recall: happy or sad) ANOVA on LIWC emotion word percentages, with repeated measures on the last two factors. In order to test our hypotheses that participants with congenital FP use more compensatory expressive nonverbal behavior than participants with acquired FP, we conducted mixed 2 (onset) × 2 (dichotomized FP severity) × 2 (emotion recall: happy or sad) ANOVAs separately for the voice and body expressivity composites, with repeated measures on the last factor. We expected to find main effects of onset indicating that participants with congenital FP used more emotion words, vocal expressivity, and body expressivity. We included FP severity in our ANOVAs as a blocking variable to control for the possibility that the confounding of onset with severity would account for differences in expressivity.
Preliminary analyses found two participants in the study had HADS scores of 11 or higher, the recommended cutoff for “definite depression” (Zigmond & Snaith, 1983). Indeed, these two participants self-reported having been diagnosed with depression. In order to ensure that the participants’ depression was not affecting our results, we conducted our analyses with and without the data from these two individuals. With or without their data, the pattern of results and significance remained the same. We present the higher-powered findings from the full sample here.
Results
Autobiographical Recall Manipulation Check
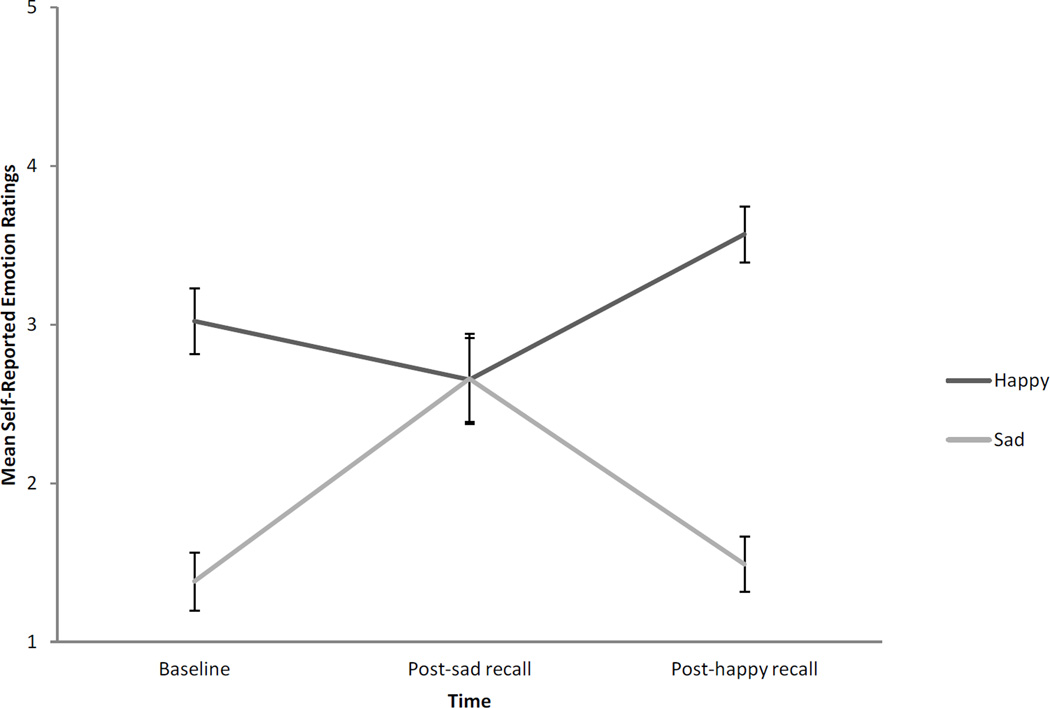
Results showed that participants’ moods changed during the autobiographical recall task in the expected direction. Figure 1 displays the means and standard errors representing participants’ change in emotion during autobiographical recall. There was a significant interaction of emotion and time, F(2,42) = 15.25, p < .001, ηp2= .42, a main effect of emotion, F(1,21) = 20.03, p < .001, r = .70, and a main effect of time, F(2,42) = 4.57, p = .02, ηp2 =.17. Planned pairwise comparisons indicated that happiness marginally decreased from baseline to post-sad recall (p = .14, r = −.23), increased from post-sad recall to post-happy recall (p < .001, r = .49), and from baseline to post-happy recall (p < .0001, r = .31). Similarly, planned pairwise comparisons indicated that sadness increased from baseline to post-sad recall (p < .001, r = .58), and from post-sad recall to post-happy recall (p < .001 r = .59), but not from baseline to post-happy recall (p = .60, r = .01). Thus, participants’ emotions changed after the mood manipulations in the expected direction. There was a main effect of onset, indicating that people with congenital FP reported less extreme emotion overall (M = 2.17, SE = 0.14), compared to people with acquired FP (M = 2.75, SE = 0.11), F(1,21) = 10.97, p < .001, r = .59). There was a significant main effect of FP severity, indicating that people with severe FP reported more emotion (M = 2.69, SE = 0.12) than people with mild FP (M = 2.24, SE = 0.13), F(1,21) = 6.48, p = .02, r = .49. There were no significant interactions of onset or severity with emotion and time, indicating that the mood manipulation did not differentially affect congenital and acquired participants. There were no other significant effects.
Figure 1.
Manipulation check showing change in self-reported emotion during autobiographical recall. Ratings are self-reports of emotion intensity on a scale from 1 to 5, with higher numbers indicating a greater intensity of emotion. Error bars represent standard errors.
Verbal Expressivity Analysis
In support of our hypothesis, there was a main effect of FP onset on LIWC emotion word percentages, indicating that people with congenital (M = 3.62, SE = .28), compared to acquired FP (M = 2.64, SE = .21), used more emotion words, F(1,21) = 7.02, p = .02, r = .50. There were no other significant effects. An interaction of emotion recall and emotion word type replicated the manipulation check, indicating that the emotional word usage of participants was congruent with the topic of the autobiographical recall, F(1,21) = 33.70, p < .001, r = .78. Planned simple effects tests revealed that participants used more positive words when describing a happy event compared to a sad event, F(1,21) = 14.52, p < .001, r = .64, and more negative words when describing a sad event compared to a happy event, F(1,21) = 36.04, p < .001, r = .80. When recalling a happy event, participants used more positive than negative words F(1,21) = 43.00, p < .001, r = .82, but when recalling a sad event, participants used an equal amount of positive and negative words, F(1,21) = 1.80, p = .2, r = .28.
Nonverbal Expressivity Analysis
For the voice expressivity composite, supporting our hypothesis, there was a significant main effect of onset, indicating that people with congenital FP (M = 2.94, SE = 0.13) use more vocal expression than people with acquired FP (M = 2.52, SE = 0.11), F(1,23) = 6.08, p = .02, r = .46. There was also a significant main effect of facial expressivity, indicating that people with more facial expressivity (M = 2.92, SE = 0.13) were more expressive with their voices (M = 2.55, SE = 0.11), F(1,23) = 4.91, p = .04, r = .42. There were no other significant effects.
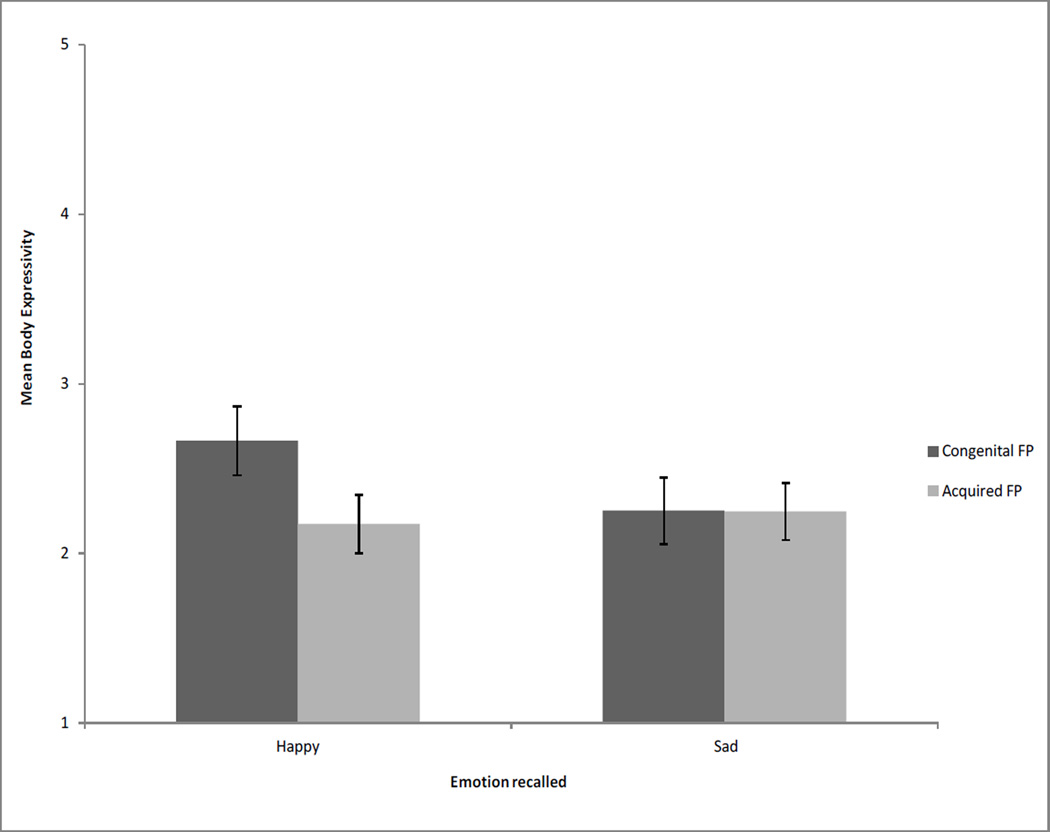
For the body expressivity composite, there were no significant main effects, but there was a significant interaction of onset by emotion, F(1,23) = 5.36, p = .03, r = .43. Figure 2 shows the M and SE of onset and emotion. Simple effects tests revealed that people with congenital FP used more body expression in the happy condition relative to the sad condition, F(1,23) = 6.63, p = .02, r = .47, but people with acquired FP did not differ in the amount of expression used when discussing a happy or sad topic. In partial support of our hypothesis, there was a trend towards a simple effect revealing that when recalling a happy event, people with congenital FP tended to be more expressive than people with acquired FP, F(1,23) = 3.40, p = .08, r = .36. There was no significant difference between people with congenital and acquired FP in their expressivity when recalling a sad event, F(1,23) = 0.00, p = .98, r = .01.
Figure 2.
Interaction of FP onset and emotion recall on body expressivity. Participants with congenital FP, compared to acquired FP, tended to display more body expressivity, but only when recalling a happy event. Body expressivity is a composite containing ratings of gesture, head movement, trunk movement, and leg movement. Possible body expressivity composite rating scores ranged from 1 to 5, with higher scores indicating more expressivity. Error bars represent standard errors.
Discussion
In this study, we recorded the actual interpersonal behavior of people with congenital and acquired FP on videotape and compared their use of compensatory expressive behavior. In support of our hypothesis, people with congenital FP used more emotion words, vocal expression (inflection, laughter, talkativeness, and vocal loudness), and tended to show increased body expression (gestures, head movements, trunk movements, and leg movements) but only when recalling a happy event. The expression of sadness may not involve bodily activity; the body may remain relatively still (Van den Stock, Righart, & DeGelder, 2007).
The increased compensatory expressive behavior in people with congenital FP could be attributed to several factors. People with congenital FP have had longer to adapt to FP. They went through early childhood development with their condition, a time when individuals are cognitively and affectively resilient (Smart, 2008). They did not experience functional losses, since they never relied on their faces to express themselves. It is possible that they have learned to adapt their behavior to communicate emotion, avoid misunderstandings and achieve positive outcomes like friendly interactions.
Some people with FP, particularly those with congenital FP, were aware of their compensatory behavior and considered it a compensatory strategy. During his interview, one man with Moebius syndrome said, “The tone, the volume, the rate, the timbre of the voice, and body language, I use to supplement in ways that my face can’t provide…I have a whole repertoire of laughs that I use to respond to different situations.” This is consistent with previous qualitative research on people with Moebius syndrome in which participants described using compensatory expressive behaviors (Bogart, Tickle-Degnen, & Joffe, in press).
Methodological Considerations
Like all studies of FP, this study was limited by its recruiting method and small sample size (Briegel, 2006; Bleicher et al., 1996). The incidence of FP is too low to recruit from the general population. Thus, researchers must recruit from FP medical clinics and support groups. Our sampling was nonrandom, and participants who were willing to discuss their conditions on videotape self-selected into the study. Thus, both samples may have been skewed towards people who were better adjusted to their conditions. Additionally, the sample was not large enough to rule out that individual idiosyncratic behavior was captured and drove the results. Our acquired FP group consisted of a heterogeneous mix of conditions; in contrast, our congenital group was more homogenous, consisting only of people with Moebius syndrome. This reflects the fact that there are fewer known conditions resulting in congenital FP (Bleicher et al., 1996). The fact that our congenital and acquired samples were recruited from different sources is a limitation. However, despite potential differences discussed above, the two groups had similar backgrounds: all participants were community-dwelling, and most were college-educated and employed.
One concern is that the congenital group had more severe FP than the acquired group. We accounted for FP severity in our ANOVA models, and severity did not affect expressive verbal or nonverbal behavior in all but one case: people with less severe FP were more vocally expressive. This finding is likely due to a confounding of FP severity with speech and vocal problems. We excluded participants who were not able to communicate clearly, and all of the participants’ responses were readily comprehensible and transcribable. However, people with more severe FP may have had mouth and vocal cord weakness that subtly limited their ability to inflect their voices (Meyerson & Foushee, 1978). In all cases, the effect sizes for FP onset were larger than the effect sizes for FP severity on compensatory expressivity, indicating that FP onset is a more important factor in the development of compensatory behavior than severity of disability.
A related concern is that most participants in the congenital sample had bilateral FP, while all participants in the acquired sample had unilateral FP. It may be argued that these differences in laterality, rather than onset, are the reason for the increased compensatory expressive behavior in people with congenital FP. Although the social functioning of people with unilateral compared to bilateral FP has not been compared, both groups have been found to experience significant social disability (Bogart & Matsumoto, 2010; Coulson et al., 2004), and could benefit from compensatory expressive behavior. Even though people with unilateral FP are able to move one side of their face, it may be difficult for others to interpret their expressions correctly. For example, a unilateral smile may not be recognizable as a smile, but rather, it may look like a contempt expression (Ekman & Friesen, 1986). Additionally, asymmetrical expressions may be particularly disfiguring, since symmetry is an important component of attractiveness (Rhodes, Yoshikawa, & Clark, 2003). We calculated FP severity by averaging expressivity across both sides of the face, so FP severity scores were generally less severe for those with acquired FP compared to those with congenital FP. Thus, by including the severity scores in the ANOVA model, we were able to control for some of the potential effects of laterality as well.
Some previous research has found high rates of depression in people with FP (VanSwearingen et al. 1998; 1999), and it could be argued that depression has the potential to dampen expressive behavior. Whether or not we included people categorized by HADS as definitely depressed in our analyses, our results remained the same. When Schwartz et al. (1976) used an emotional recall task similar to ours, they found that the expressive behavior of depressed patients was restored to a normal level. Thus, our autobiographical recall task may have prevented expressive dampening that might have otherwise occurred due to depression.
Another potential limitation is that the first author served as the interviewer for all participants. The part of the interview analyzed for this study, the autobiographical recall task, was a structured task, and the interviewer took care to read the script in a professional and neutral manner. Although there is the possibility that unintended experimenter bias was introduced, having a single interviewer was the most feasible, and it prevented interviewer effects that might have occurred if multiple interviewers were used.
This study has several novel strengths. This was the first study to go beyond qualitative or survey methodology to measure the actual adaptive behavior of people with FP, and the first to sample relatively equal numbers of acquired and congenital FP. Indeed, it is one of the first to our knowledge to compare the adaptive behavior of people with any sort of congenital or acquired disability. Our study was strengthened by our use of multiple methods to measure expressive behavior. As such, this study is an important place to start this line of research. Due to the limitations described above, our results may not be generalizable; it is best to view them as descriptive of this sample, and to use them to inform future research.
Implications for Future Research and Clinical Application
Although a control group is not necessary to test the hypothesis of this study, that people with congenital FP display more compensatory expressivity relative to people with acquired FP, future research could include a control group of people without FP to examine whether one or both of the FP groups displays more expressivity relative to the typical population.
There is a surprising lack of behavioral studies examining the role of onset in adaptation to disability; we hope that this study inspires researchers to examine this among other populations of people with disabilities and health conditions. Future research could reveal useful compensations that could be taught to people who are having difficulty adapting to their conditions.
Rehabilitation professionals should be aware of the compensatory expressive behaviors that may be used by people with FP. Attending to these behaviors could improve therapeutic relationships and rapport. A social functioning intervention that encourages the use of compensatory expressive behaviors may be useful for people with FP, particularly for those with acquired conditions.
Impact.
-
◦
This is the first study to examine behaviorally the way people with facial paralysis (FP)—an understudied population—compensate for their lack of facial expression using other expressive behaviors.
-
◦
This study is among the first to advance theories of adaptation to disability by examining behavioral adaptation to congenital compared to acquired disability.
-
◦
The adaptive behaviors of people with congenital FP, who may be better adapted than people with acquired FP, can inform a novel social functioning intervention for people with FP. Social functioning interventions may be particularly useful to help people with acquired FP adapt to their condition.
-
◦
To improve therapeutic relationships, rehabilitation professionals should be aware of the compensatory expressive behaviors that may be used by people with FP.
Acknowledgments
This research was supported by a National Institute of Dental and Craniofacial Research NRSA Predoctoral Fellowship award number F31DE021951 and partially supported by a Moebius Syndrome Foundation travel grant. We thank the Facial Nerve Center at Massachusetts Eye and Ear Infirmary and the Moebius Syndrome Foundation for aiding recruitment. We thank research assistants Rebecca Fitzhugh, Christine George, Jennifer Perlmutter, Tara Saloman, Tiffany Tu, and Caroline Wilkes for their assistance in coding nonverbal behaviors.
Contributor Information
Kathleen R. Bogart, Tufts University
Linda Tickle-Degnen, Tufts University.
Nalini Ambady, Stanford University.
References
- Alfano DP, Neilson PM, Fink MP. Long-term psychosocial adjustment following head or spinal cord injury. Cognitive and Behavioral Neurology. 1993;6(2):117. [Google Scholar]
- Ambady N, Bernieri FJ, Richeson JA. Toward a histology of social behavior: Judgmental accuracy from thin slices of the behavioral stream. Advances in Experimental Social Psychology. 2000;32:201–271. [Google Scholar]
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. Journal of Psychosomatic Research. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Bleicher JN, Hamiel S, Gengler JS, Antimarino J. A survey of facial paralysis: Etiology and incidence. Ear, Nose, & Throat Journal. 1996;75(6):355–358. [PubMed] [Google Scholar]
- Bogart KR, Matsumoto D. Living with Moebius syndrome: Adjustment, social competence, and satisfaction with life. Cleft Palate-Craniofacial Journal. 2010;47(2):134–142. doi: 10.1597/08-257_1. [DOI] [PubMed] [Google Scholar]
- Bogart KR, Tickle-Degnen L, Joffe M. Social interaction experiences of adults with Moebius syndrome: A focus group. Journal of Health Psychology. doi: 10.1177/1359105311432491. (in press). [DOI] [PubMed] [Google Scholar]
- Briegel W. Neuropsychiatric findings of Möbius sequence: a review. Clinical Genetics. 2006;70:91–97. doi: 10.1111/j.1399-0004.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- Coulson SE, O’Dwyer N, Adams R, Croxson GR. Expression of emotion and quality of life following facial nerve paralysis. Otology and Neurotology. 2004;25:1014–1019. doi: 10.1097/00129492-200411000-00026. [DOI] [PubMed] [Google Scholar]
- Ekman P. Psychosocial aspects of facial paralysis. In: May M, editor. The Facial Nerve. New York: Thieme; 1986. [Google Scholar]
- Ekman P, Friesen WV. A new pan-cultural facial expression of emotion. Motivation and Emotion. 1986;10:159–168. [Google Scholar]
- Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221(4616):1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- Hemmesch AR, Tickle-Degnen L, Zebrowitz LA. The influence of facial masking and sex on older adults’ impressions of individuals with Parkinson’s disease. Psychology and aging. 2009;24(3):542. doi: 10.1037/a0016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CA, Harley EH, Milmoe G, Bala R, Martorella A. Birth trauma in the head and neck. Archives of Otolaryngology-Head and Neck Surgery. 1999;125(2):193. doi: 10.1001/archotol.125.2.193. [DOI] [PubMed] [Google Scholar]
- Krause JS. Adjustment to life after spinal cord injury: A comparison among three participant groups based on employment status. Rehabilitation Counseling Bulletin. 1992;35(4):218–229. [Google Scholar]
- Li L, Moore D. Acceptance of disability and its correlates. The Journal of Social Psychology. 1998;138(1):13–25. doi: 10.1080/00224549809600349. [DOI] [PubMed] [Google Scholar]
- Linguistic Inquiry and Word Count. (n.d) Retrieved from: http://www.liwc.net/descriptiontable1.php. [Google Scholar]
- Livneh H, Antonak RF. Psychosocial adaptation to chronic illness and disability. Gaithersburg, MD: Aspen; 1997. [Google Scholar]
- Lyons KD, Tickle-Degnen L, Henry A, Cohn E. Impressions of Personality in Parkinson’s Disease: Can Rehabilitation Practitioners See Beyond the Symptoms? Rehabilitation Psychology. 2004;49(4):328–333. [Google Scholar]
- Meyerson M. Resiliency and success in adults with Moebius syndrome. Cleft Palate—Craniofacial Journal. 2001;38(3):231–235. doi: 10.1597/1545-1569_2001_038_0231_rasiaw_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Meyerson M, Foushee D. Speech, Language and Hearing in Moebius Syndrome: a Study of 22 Patients. Developmental Medicine & Child Neurology. 1978;20(3):357–365. doi: 10.1111/j.1469-8749.1978.tb15225.x. [DOI] [PubMed] [Google Scholar]
- Möbius PJ. Ueber angeborene doppelseitige Abducens-Facialis-Lahmung. Munchener med Wochenschr. 1888;35:91–94. [Google Scholar]
- Newell R. Body image and disfigurement care. London, England: Routledge: Psychology Press; 2000. [Google Scholar]
- Noller P. Video primacy—A further look. Journal of Nonverbal Behavior. 1985;9(1):28–47. [Google Scholar]
- Pennebaker J, Booth R, Francis M. Linguistic Inquiry Word Count 2007 Operator’s Manual. Austin, Texas: LIWC.net; 2007. [Google Scholar]
- Rhodes G, Yoshikawa S, Clark A. Attractiveness of Facial Averageness and Symmetry in Non-Western Cultures. Search of Biologically Based Standards of Beauty. 2001;35(4):218–229. doi: 10.1068/p3123. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow R. Essentials of behavioral research: Methods and data analysis. New York, NY: McGraw-Hill; 2008. [Google Scholar]
- Ross BG, Fradet G, Nedzelski JM. Development of a sensitive clinical facial grading system. Otolaryngology-Head and Neck Surgery. 1996;114(3):380–386. doi: 10.1016/S0194-59989670206-1. [DOI] [PubMed] [Google Scholar]
- Schwartz G, Fair P, Salt P, Mandel M, Klerman G. Facial expression and imagery in depression: an electromyographic study. Psychosomatic Medicine. 1976;38(5):337–347. doi: 10.1097/00006842-197609000-00006. [DOI] [PubMed] [Google Scholar]
- Smart J. Disability, Society, and the Individual. 2nd ed. Austin, TX: Pro-Ed; 2008. [Google Scholar]
- Takahashi K, Tickle-Degnen L, Coster WJ, Latham NK. Expressive behavior in Parkinson’s disease as a function of interview context. The American Journal of Occupational Therapy. 2010;64(3):484–495. doi: 10.5014/ajot.2010.09078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickle-Degnen L. Nonverbal behavior and its functions in the ecosystem of rapport. In: Manusov V, Patterson M, editors. The SAGE Handbook of Nonverbal Communication. Thousand Oaks, CA: Sage; 2006. [Google Scholar]
- Tickle-Degnen L. The Interpersonal communication rating protocol: A manual for measuring individual expressive behavior. 2010 http://ase.tufts.edu/hql/documents/researchICRP-IEB.pdf. [Google Scholar]
- Tickle-Degnen L, Lyons KD. Practitioners' impressions of patients with Parkinson's disease: The social ecology of the expressive mask. Social Science & Medicine. 2004;58(3):603–614. doi: 10.1016/s0277-9536(03)00213-2. [DOI] [PubMed] [Google Scholar]
- van Bezzoijen R, Boves L. The effects of low-pass filtering and random splicing on the perception of speech. Journal of Psycholinguistic Research. 1986;15(5):403–417. doi: 10.1007/BF01067722. [DOI] [PubMed] [Google Scholar]
- Van den Stock J, Righart R, de Gelder B. Body expressions influence recognition of emotions in the face and voice. Emotion. 2007;7(3):487–494. doi: 10.1037/1528-3542.7.3.487. [DOI] [PubMed] [Google Scholar]
- VanSwearingen JM, Cohn JF, Bajaj-Luthra A. Specific impairment of smiling increases the severity of depressive symptoms in patients with facial neuromuscular disorders. Aesthetic Plastic Surgery. 1999;23(6):416–423. doi: 10.1007/s002669900312. [DOI] [PubMed] [Google Scholar]
- VanSwearingen JM, Cohn JF, Turnbull J, Mrzai T, Johnson P. Psychological distress: linking impairment with disability in facial neuromotor disorders. Otolaryngology-Head and Neck Surgery. 1998;118(6):790–796. doi: 10.1016/S0194-5998(98)70270-0. [DOI] [PubMed] [Google Scholar]
- Vash C. Psychology of disability: Springer series on rehabilitation. Vol. 14. The New York: Springer Publishing Company; 1981. [Google Scholar]
- Woodrich F, Patterson JB. Variables related to acceptance of disability in persons with spinal cord injuries. Journal of Rehabilitation. 1983;49(3):26. [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]