Abstract
Background
The circle of Willis is a major collateral pathway important in ischemic conditions. The aim of our study was to assess the structural characteristics of the circle of Willis within the Turkish adult population, along with variations and arteries involved in the measurement of diameters and lengths on cranial computed tomography angiography (CTA).
Material/Methods
One hundred adult patients who underwent CTA images were evaluated retrospectively.
Results
Results of the study revealed 82% adult, 17% fetal, and 1% transitional configurations. A complete polygonal structure was observed in 28% of cases. Variations of the circle of Willis were more common in the posterior portion. Hypoplasia was found to be the most common variation and was observed as a maximum in the posterior communicating artery (AComP).
Conclusions
The patency and size of arteries in the circle of Willis are important in occlusive cerebrovascular diseases and cerebrovascular surgery. Although CTA is an easily accessible non-invasive clinical method for demonstrating the vascular structure, CTA should be evaluated taking into account image resolution quality and difficulties in the identification of small vessels.
MeSH Keywords: Cerebral Angiography; Cerebral Arteries; Circle of Willis; Tomography Scanners, X-Ray Computed
Background
The circle of Willis, whose function is to protect the brain from ischemia, is the main structure that provides a constant and regular blood flow to the brain. The circle of Willis is an arterial polygon and is a vascular ring composed of branches of arteria (a) carotis interna and a. basilaris. This circle is composed of bilateral A1 segments of anterior cerebral arteries (ACAs), anterior communicating artery (AComA) that links these 2 arteries, bilateral P1 segments of posterior cerebral arteries (ACPs), and posterior communicating arteries (AComP) which links a. carotis interna (ACI) to ACP. This communicating pathway allows equalization of the blood flow between the 2 sides of the brain and permits anastomotic circulation, if a part of the circulation becomes occluded. In recent years, detailed knowledge about the circle of Willis has generally been based on cadaver studies [1–5]. The Willis polygon and its principal arteries can be imaged angiographically using a non-invasive, easily accessible method such as computed tomography angiography (CTA). Obtaining a 3-dimensional data set became possible with the introduction of multi-detector CT devices, and, when used in conjunction with recently developed image processing techniques, it has become possible to obtain angiographic images of the Willis polygon and its principal arteries with better resolution at each layout [6–8]. The aim of this study was assessment of the structural characteristics and variations, and to measure the diameter and length of the arteries of the circle of Willis on CTA in the Turkish population.
Material and Methods
Patient population
In the present study, the Willis polygon was retrospectively evaluated among adult patients who had reported CTA normally at Ege University School of Medicine Department of Radiology. This study was approved by the Ege University ethical committee. One hundred patients (57 male, 43 female, age range 16–83 years, median age 60.06) were selected. Patients who were excluded from the study were those with cerebrovascular malformations such as aneurysm, arteriovenous malformation, arteriovenous fistula, and acute ischemic, hemorrhagic cerebrovascular strokes.
Image technique
In Multi Detector CTA examination, a CT scan with 64 detectors was used (Somatom Sensation; Siemens, Erlangen, Germany). The examination was performed by using scans with contrast administration, so as to cover the supra-aortic and cranial area. Application of contrast was performed by giving intravenous (4–5 ml/s) 80 ml non-ionic contrast material iopromide (Ultravist 370; Bayer group, Berlin, Germany) and, immediately after that, 25 ml serum physiologic.
Data analysis
The data were transferred to a workstation for post-processing. The diameter and length of the arteries which constituted the circle of Willis were measured after proper magnification. Variations were evaluated (an invisible artery was evaluated as aplasia, while the diameter of an artery smaller than 1 mm was evaluated as hypoplasia). The structures of the circle of Willis were evaluated by being complete or incomplete images (complete image means all of the arteries forming the circle without hypoplasia, and incomplete image means with aplasia) and according to the diameter of AComP-P1 relationship. There are 3 configurations according to diameters of AComP and ACP: Adult configuration, with diameter of AComP smaller than P1; fetal configuration, with diameter of AComP larger than P1; and the main vascularization of the occipital lobe are provided by the ACI. The transitional configuration has a diameter of AComP the same as with P1 [9]. Data were transferred to SPSS 20 (Statistical Packages for Social Sciences) program for statistical analysis. Wilcoxon test was used for comparing right and left sides. Statistical significance was calculated with to corrected nominal alpha values according to the Bonferroni method.
Results
The study group consisted of 43 females (43%) and 57 males (57%) and the mean age was 60.06 years (range 16–83). All of the arteries forming the circle of Willis were found in 71 samples (71%). Complete polygonal structure included all arteries forming the circle, however, hypoplastic arteries were not evaluated – this was obtained in just 28 samples (28%). Incomplete circles, which included invisible arteries, were found in 29 samples (6 in the anterior, 22 in the posterior, and 1 in both) (29%). Adult configuration was detected in 82 samples (82%). Fetal configuration was detected in 17 (17%) and transitional configuration was detected in 1 (1%) (Tables 1, 2). Variations were found more in the posterior part. Hypoplasia was the most common variation and was found to be most common in AComP (38%). Aplasia was the second leading variation and was also detected in AComP (23%). AComA hypoplasia is the most common variation seen in the anterior part (23%). Hypoplasia was found to be most common in AComA following AComP. The most common multiple variation was the coexistence of AComA and AComP hypoplasia, which was found in 12 samples (12%) (Figures 1–4).
Table 1.
The number and rate of forming the circle of Willis arteries.
| Complete polygon (with hypoplasia) | 71 (71%) |
| Complete polygon (without hypoplasia) | 28 (28%) |
| Incomplete polygon (with aplasia) | |
| Anterior segment | 7 |
| Posterior segment | 21 |
| Anterior-Posterior | 1 |
| Total | 29 (29%) |
Table 2.
The number and rate of forming the circle of Willis arteries.
| Adult configuration | 82 (82%) |
| Fetal configuration | |
| Right | 8 |
| Left | 5 |
| Bilateral | 4 |
| Total | 17 (17%) |
| Transitional configuration | 1 (1%) |
Figure 1.
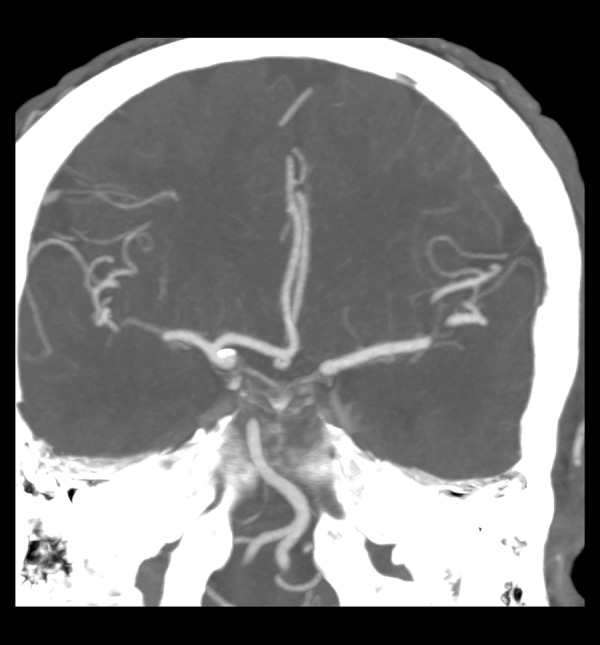
A1 aplasia in the left.
Figure 2.
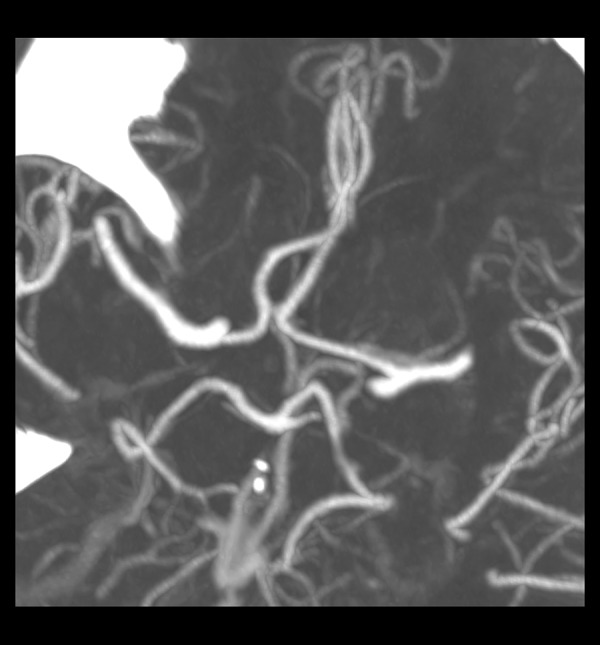
Bilateral AComP aplasia.
Figure 3.
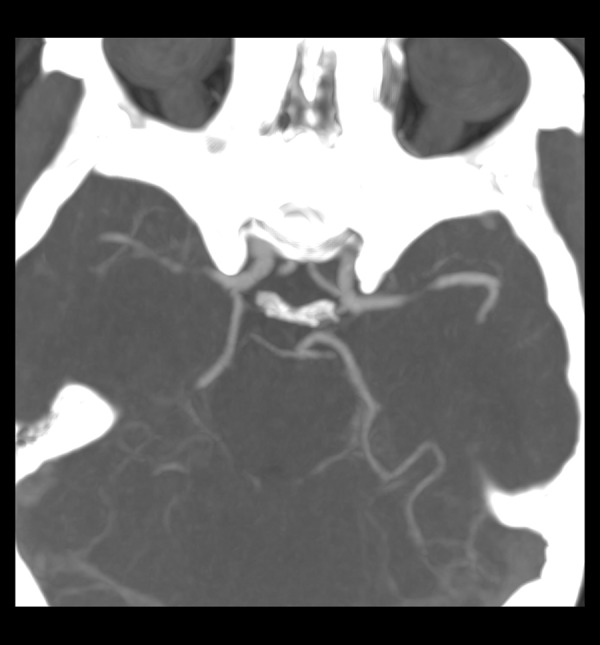
The fetal originated AComP and hypoplastic P1 in the right.
Figure 4.
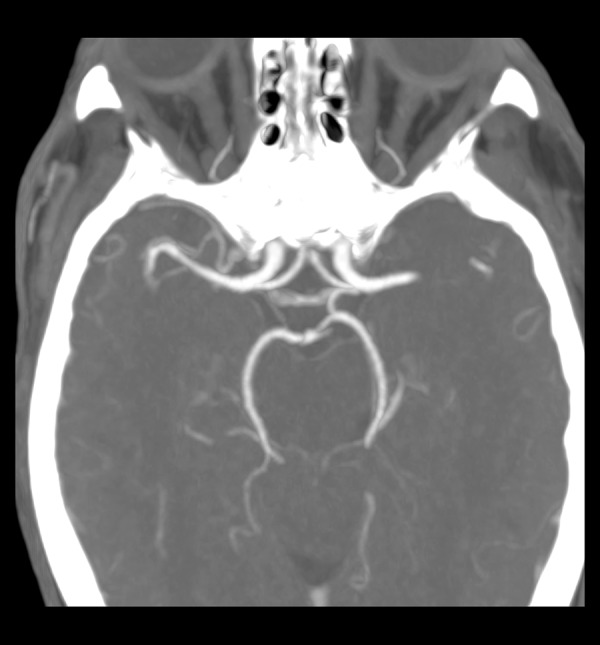
AComP hypoplasia in the right.
No statistically significant difference was noted between the groups in terms of age, sex, or bilaterally measured values.
Arteria cerebri anterior
A1 length was measured as 15.61±2.79 mm in the right and 15.13±2.54 mm in the left. A1 diameter was measured as 2.15±0.63 mm in the right and 2.26±0.61 mm in the left. A1 hypoplasia was detected 3 (2 single, 1 combined; together with other variations) in the right, and 2 (combined) in the left. Aplasia was seen 5 (3 single and 2 combined) in the right, and 2 (1 single, 1 combined) in the left.
Arteria communicans anterior
The mean length of the AComA was 1.48±1.45 mm and the diameter was 1.39±0.83 mm. Twenty-three (7 single, 16 combined variations) hypoplasia and 1 combined aplasia were detected.
Arteria communicans posterior
The length of the AComP was 10.99±3.07 mm in the right and 9.87 ±3.32 mm in the left. AComP diameter was 1.30±0.50 mm in the right and 1.27±0.55 mm in the left. A total of 38 hypoplasia (3 right, 12 left, 5 bilateral, and 18 combined) and 23 aplasia (8 right, 7 left, 1 bilateral, and 7 combined) were detected in AComP.
Arteria cerebri posterior
ACP (P1) length was mean 9.01±3.24 mm in the right and 8.14±3.29 mm in the left. P1 diameter was measured as 2.22±0.67 mm in the right and 2.12±0.52 mm in the left. P1 hypoplasia was detected in 1 single (right) and 2 combined (1 right, 1 bilateral). P1 aplasia was not observed.
Discussion
CTA may reveal various pathologies and their variations, such as carotid system stenosis, aneurysms, vasospasm, arteriovenous malformations, dissections, and venous thrombus [10]. Although CTA is a fast and non-invasive imaging method, it also has some disadvantages, most significantly its requirement for a contrast agent, exposure to radiation, lengthy data processing times, and difficulties encountered in assessing arteries at the base of the skull due to bone structure or contrasting uptake of cavernous sinuses. For intracranial aneurysms in particular, the accuracy provided by a CTA is close to that of DSA, and is sufficient for the planning of endovascular and surgical treatments, particularly for aneurysms that are larger than 5 mm [11]. The sensitivity decreased to 50% for aneurysms less than 2 mm [12]. Nevertheless, CTA can still be considered superior to MRA, since it is less sensitive to motion, can be easily used on intubated patients, and circumvents the MR-compatibility problem noticed among patients with a clipped aneurysm. Contrary to autopsy studies, a CTA allows real images to be obtained from patients and enables an accurate assessment of arterial calibers, as it does not depend on flow rate.
The patency and size of arteries in the circle of Willis is important in occlusive cerebrovascular diseases and cerebrovascular surgery. Circulus arteriosus cerebri is in fact not a real circle, but rather a nonagon with 9 borders (3 anterior, 2 anterolateral, and 4 posterior) [13]. A complete circle of Willis is composed of 10 arterial parts. A complete circle that does not have hypoplastic arteries is seen in less than half of the cases and reported in a range as wide as 4.6–72.2% in cadaver studies [1–5]. The cause of this wide range is considered to be differences in definition, hypoplasia criteria, and not being able to make real measurements in the studies. We compared our findings with previous CTA studies. However, there are few studies concerning the configuration of the circle of Willis as a whole with CTA. Generally, some part of the circle has been examined among patients with cerebrovascular diseases.
Li et al. carried out a CTA study to examine the Willis polygon among 160 Chinese healthy adults, and observed that 27% of the cases had a complete polygon, which is in accordance with our study; 56% were partial, 17% were concomitant anterior, and posterior incomplete status was reported. Arterial segments that were less than 1 mm were classified as hypoplastic, and if invisible, as aplasia. The most frequent variation was a bilateral AComP hypoplasia or aplasia. They observed a significantly higher percentage of incomplete posterior part compared with the anterior part of the circle. The fetal-type posterior circulation was noted in 11%. They found a higher prevalence of incomplete posterior part in this Chinese population compared to the Western and Japanese populations, which could be explained by different ethnicity. However, they compared their results with previous MRA study findings [6].
Dodevski et al. carried out a CTA study to examine the type of ACP in 53 Macedonian adult patients. They found 70% adult, 23% fetal, and 7% transitional configurations. P1 diameter was measured as 1.74±0.31 mm in the right and 1.98±0.04 mm in the left [14].
Waaijer et al. assessed anatomic variations in the circle of Willis among 91 patients with symptomatic carotid artery stenosis, and compared this to 91 control adult subjects with multislice CTA. The study was performed as follows for each arterial segment: invisible, hypoplastic (<1 mm), or normal (≥1 mm). In the control group, 1% aplasia was detected in AComA; 1% aplasia and 4% hypoplasia was detected in A1; and 27% aplasia and 40% hypoplasia was detected in AComP or P1. The study results revealed 85% adult, 14% fetal, and 1% transitional configurations in the control group. These results were similar to our study. They concluded there was a relationship between symptomatic carotid stenosis and incomplete circle of Willis [8].
Urbanski et al. performed a study using CTA for the pre-operative neurovascular assessment of 99 adult patients who were to undergo aortic surgery, and found that 59 patients had a complete polygon, 18 patients had a single abnormality (hypoplasia or aplasia within anterior or posterior part), 13 patients had a bilateral AComP abnormality (hypoplasia or aplasia), and 9 patients had coexisting AComA and AComP abnormalities. However, despite these abnormalities, the patients developed no neurological deficits after the surgical procedure, which involved carotid clamping [7].
Han et al. analyzed the arterial segments of Willis among 117 subarachnoid hemorrhage patients CTA and DSA images. In the study, no flow was defined as “aplasia”, arteries luminal narrowing more than 50% were accepted as “hypoplasia”. In the AComA, 30% aplasia and 3% hypoplasia was detected. In the A1, 8% aplasia and 7% hypoplasia was detected. In the AComP, 65% aplasia and 5% hypoplasia was detected. In the P1, 9% aplasia and 8% hypoplasia was detected. The CTA results were compared with findings on the corresponding DSA images. They concluded that CTA is highly accurate in the assessment of anatomical variations of the circle of Willis. However, CTA has limited sensitivity in depicting hypoplastic segments, although it is quite specific [10].
In the present study, variations in the Willis polygon were more common in the posterior part of the polygon, consistent with the literature, the most common of which was hypoplasia, being most frequently observed in AComP (38%) [6,8]. Hypoplasia was found to be concomitant at 15% with unilateral, 5% with bilateral, and 18% with other abnormalities. The second most common variation was aplasia, which was also most frequently observed in AComP (23%). In the AComA, hypoplasia was the second most common variation (23%), although AComA hypoplasia was the most common variation observed in the anterior region. The most common multiple variation was the coexistence of the AComA and AComP hypoplasia, which was encountered in 12% of the cases. A1 aplasia was observed in 7% of the cases, while AComA aplasia was observed in 1%. Aplasia was not detected in P1. A1 hypoplasia was present in 5% of cases, while P1 hypoplasia was present in 3%.
In the present study, 71% of the patients had all arteries constituting the Willis polygon. A complete polygonal structure without the evaluation of hypoplasic arteries was observed in just 28% of cases. An incomplete polygon with invisible arteries was encountered in 29 cases (29%). In our previous cadaver study among the adult Turkish population, 91% the patients had all arteries constituting the Willis polygon. The complete and incomplete polygonal ratios were 8% and 9%, respectively, in that study [15]. The increased frequency of aplasia and decreased frequency of hypoplasia encountered in the CTA group has been associated with the technical limitations of the CTA in the measurement of arteries with smaller diameters, which prohibits clear detection of hypoplasia and aplasia, as in an autopsy.
Our study results revealed 82% adult, 17% fetal, and 1% transitional configurations. When we compared these results with our cadaveric study, fetal configurations were detected more frequently with CTA [15]. The higher percentage of fetal configurations can be explained by the infrequent occurrence of hypoplasic AComP and the significantly higher AComP diameters observed in the group. This is a result of the technical difficulties of CTA in the assessment of hypoplasic arteries with diameters smaller than 1 mm.
Some arteries of the polygon may be so thin that intervention by radiological means is impossible, or they may not be present at all, and this is important in the treatment of occlusive cerebrovascular diseases and cerebrovascular surgery. Knowing the variations and understanding any abnormalities prior to a cerebrovascular (aneurysm, endartectomy, bypass) operation is important in ensuring safe surgery. The presence of hypoplastic or aplastic AComP is an independent risk factor for ischemic cerebral infarction when the ACI is occluded [13]. However, Chuang et al. demonstrated that AComP hypoplasia appears to be a contributor to the risk of ischemic stroke, even in the absence of ACI occlusion [16]. Miyazawa et al. also found a higher frequency of lacunes in the basal ganglia in patients with hypoplasia or aplasia in the anterior part of the circle of Willis [17]. CTA can be used practically in the evaluation of cerebral vascular structures, particularly for screening and pre-operative examination purposes in asymptomatic or normal individuals. CTAs should be evaluated considering the image quality, venous contamination, insufficient contrast dosage, and limitations in the determination of arteries with small diameters. The structure of the Willis polygon, as well as the status and sufficiency of AComA and AComP, should be assessed. Aside from studies of the healthy population, the clinical implications of variations of the Willis polygon should be examined through studies of patients with cerebral arterial diseases.
The present study shows that although CTA is a valuable imaging method for clinical examination of the vascular structure, studies performed on cadavers are still important in this area, since CTA faces technical limitations in the imaging of some arteries. On the other hand, the abundance of variations observed in the Willis polygon among individuals implies that the utility of CTA as a guide will be significant.
Conclusions
Among patients due to undergo surgery, because of common variations of the Willis polygon, a pre-operative examination of the structure and variations of the Willis polygon through easily accessible and non-invasive techniques with CTA will decrease the potentially significant neurological complications and associated secondary risks of morbidity and mortality. However, it should be noted that diagnosis of hypoplasia and aplasia during the CTA will not be sufficiently reliable, since the CTA has technical limitations in the assessment of small arteries.
Footnotes
Declaration of interest
The authors declare that they have no conflicts of interest.
Source of support: Departmental sources
References
- 1.Alpers BJ, Berry RG, Paddison RM. Anatomical studies of the circle of Willis in normal brain. Arch Neurol Psychiat. 1959;81:409–18. doi: 10.1001/archneurpsyc.1959.02340160007002. [DOI] [PubMed] [Google Scholar]
- 2.Baptista AG. Studies on the arteries of the brain. Acta Neurol Scand. 1964;40:398–414. doi: 10.1111/j.1600-0404.1964.tb02765.x. [DOI] [PubMed] [Google Scholar]
- 3.Battacharji SK, Hutchinson EC, Mc Call AJ. The circle of Willis the incidance of developmental abnormalities in normal and infarcted brains. Brain. 1967;90:747–58. doi: 10.1093/brain/90.4.747. [DOI] [PubMed] [Google Scholar]
- 4.De Silva KRD, Silva R, Gunasekera WSL, Jayesekera RW. Prevelence of typical circle of Willis and the variation in the anterior communicating artery: A study of a Sri Lankan population. Ann Indian Acad Neurol. 2009;12:157–61. doi: 10.4103/0972-2327.56314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher CM. The circle of Willis: anatomical variations. Vasc Dis. 1965;2:99–105. [Google Scholar]
- 6.Li Q, Li J, Lv F, et al. A multidetector CT angiography study of variations in the circle of Willis in a Chinese population. J Clin Neurosci. 2011;18:379–83. doi: 10.1016/j.jocn.2010.07.137. [DOI] [PubMed] [Google Scholar]
- 7.Urbanski PP, Lenos A, Blume JC, et al. Does anatomical completeness of the circle of Willis correlate with sufficient cross-perfusion during unilateral cerebral perfusion? Eur J Cardiothorac Surg. 2008;33:402–8. doi: 10.1016/j.ejcts.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Waaijer A, van Leeuwen MS, van der Worp HB, et al. Anatomic variations in the circle of Willis in patients with symptomatic carotid artery stenosis assessed with multidedector row CT Angiography. Cerebrovasc Dis. 2007;23:267–74. doi: 10.1159/000098326. [DOI] [PubMed] [Google Scholar]
- 9.Padget DH. The circle of Willis. Its embriyology and anatomy. In: Dandy WE, editor. Intracranial arterial aneurysms. Comstock publishing Co; NY: 1947. pp. 67–90. [Google Scholar]
- 10.Han A, Yoon DY, Chang SK, et al. Accuracy of CT angiography in the assessment of the circle of Willis: comparison of volume-rendered images and digital subtraction angiography. Acta Radiol. 2011;52:889–93. doi: 10.1258/ar.2011.110223. [DOI] [PubMed] [Google Scholar]
- 11.White P, Teasdale E, Wardlaw J, Easton V. Intracranial aneurysms: CT angiography and MR angiography for detection-prospective blinded comparison in a large patient cohort. Radiology. 2001;219:739–49. doi: 10.1148/radiology.219.3.r01ma16739. [DOI] [PubMed] [Google Scholar]
- 12.Wintermark M, Uske A, Chalaron M, et al. Multislice computerized tomography angiography in the evaluation of intracranial aneurysms; a comparison with intraarterial digital subtraction angiography. J Neurosurg. 2003;98:828–36. doi: 10.3171/jns.2003.98.4.0828. [DOI] [PubMed] [Google Scholar]
- 13.Osborn AG. Diagnostic cerebral angiography. 2nd ed. Lippincott Williams Wilkins; Philadelphia: 1999. pp. 105–6. [Google Scholar]
- 14.Dodevski A, Tosovska Lazarova D, Mitreska N, et al. Posterior cerebral artery – variation in the origin and clinical significance. Prilozi. 2014;35:163–68. [PubMed] [Google Scholar]
- 15.Karatas A, Yilmaz H, Coban G, et al. The anatomy of circulus arteriosus cerebri (circle of Willis). Paper presented at: 2014 European Congress of Neurosurgery; 2014; Prague, Czech Republic. [Google Scholar]
- 16.Chuang YM, Liu CY, Pan PJ, Lin CP. Posterior communicating artery hypoplasia as a risk factor for acute ischemic stroke in the absence of carotid artery occlusion. J Clin Neurosci. 2008;15:1376–81. doi: 10.1016/j.jocn.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Miyazawa N, Shinohara T, Yamagata Z. Association of incompleteness of the anterior part of the circle of Willis with the occurrence of lacunes in the basal ganglia. Eur J Neurol. 2011;18:1358–60. doi: 10.1111/j.1468-1331.2011.03400.x. [DOI] [PubMed] [Google Scholar]


