Belvisi et al. show in human bronchi that the bitter taste receptor (TAS2R) agonist chloroquine evokes marked relaxation which is in agreement with our findings in mouse airways1. They note, however, an equivalent efficacy (degree of maximal relaxation) with the β-agonist isoproterenol in human bronchi. In our paper, we performed the vast majority of intact airway physiology in mice, where we found that bitter tastants had a greater efficacy compared to isoproterenol. We never stated that TAS2R agonists were more potent than β-agonists, and we note the differences in the potency (the EC50) between isoproterenol and chloroquine are comparable between our study and Belvisi et al. The three-fold greater efficacy of TAS2R agonists compared to the β-agonist isoproterenol in our paper was specifically noted in the text in regard to mouse airways. With the limited number of human airways that we studied, we were not in position to provide quantitative efficacy data in human airways. We have now examined additional human rings after contraction with methacholine (Fig. 1a-c) and can adequately compare the efficacy of bitter tastants and isoproterenol. We find a 77 ± 4.3 % relaxation by isoproterenol (n=15 experiments), while chloroquine and quinine achieved essentially 100% relaxation (106 ± 4.7 and 99 ± 2.9 %, respectively (Fig. 1d). So, we still note a somewhat greater efficacy of bitter tastants compared to isoproterenol, but recognize that it is certainly less than the three-fold difference we observed in mice1, and attribute this difference to a species-effect. Thus we agree that in the ex vivo human airway ring model, the efficacy of these TAS2R agonists is similar to that of isoproterenol. We would like to point out, however, that the full β-agonist isoproterenol is not used clinically. The most commonly utilized inhaled β-agonist is albuterol, a partial agonist with, at best, a ~60% efficacy for relaxation in human airways compared to isoproterenol2,3. So from a clinical standpoint, it remains to be seen whether inhaled bitter tastants are more efficacious than this benchmark inhaled β-agonist.
Figure 1.
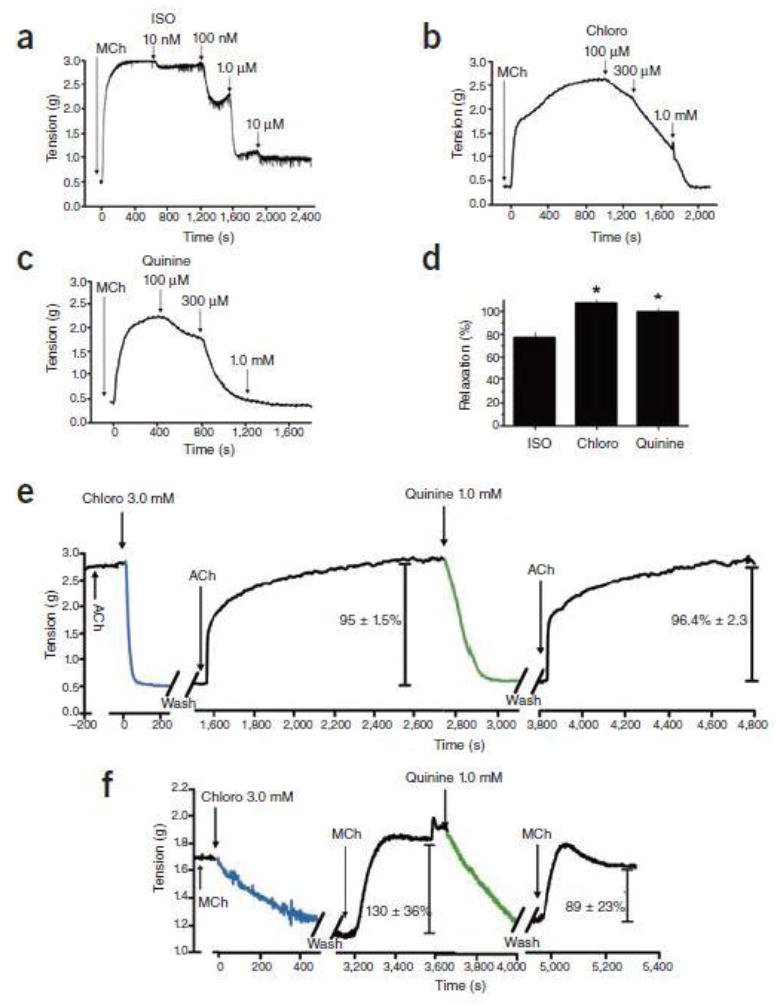
TAS2R agonist-mediated relaxation of human and mouse airways. a-c) Dose-dependent relaxation of human airway rings by isoproterenol (ISO), chloroquine (Chloro) and quinine. Shown are representative experiments in rings contracted with 0.1 mM methacholine (Mch). d) Maximal relaxation effects derived from dose-response studies with the indicated agonists against 0.1 mM methacholine-contracted human airway rings (n = 15 isoproterenol, n = 11 chloroquine, n = 7 quinine; *P < 0.005 vs. ISO). e,f) The onset of action of chloroquine and quinine is rapid and readily reversible. Shown are representative tracings of airway tension from 4 experiments performed with mouse trachea contracted with acetylcholine (ACh) (e) and 4 experiments performed with human airway rings contracted with MCh (f). The values provided within the figures are mean ± SEM from the 4 experiments.
We note differences in the methods of Belvisi et al compared to ours that might explain the modest decreased efficacy of isoproterenol that we find in human airways compared to quinine or chloroquine: inclusion of indomethacin in the myograph bath, contraction of the bronchi with different agents, the setting of a greater resting tension, and in the two experiments performed, they apparently combined data from both open strips of main bronchi and closed rings from lower order bronchi. Ironically, the authors normalized the data in their Fig. 1a,b, to the papaverine response, which further complicates interpretation because papaverine is recognized4 as an agonist for the two highest expressing TAS2Rs in airway smooth muscle (TAS2R10 and 14, ref.1). Finally, we caution about interpretations of data derived from two experiments (with a representative experiment shown), as there can be variability in contractile and relaxation responses in human airway rings from intrinsic inter-individual factors, chronic drug effects, and disease states.
In contrast to our paper1, additional human airway data shown in Fig. 1a-d, and the correspondence from Belvisi et al., Morice et al. found little relaxation of human bronchi to chloroquine or quinine within the expected time-frame for agonist activation of G-protein coupled receptors such as a TAS2R, and, a lack of reversibility of the effect. They also find a minimal response to saccharin. In regards to saccharin, it should be noted that there is not an orthologous TAS2R for saccharin in mice, so we do not utilize this bitter tastant for physiologic studies in mice. We have limited experience with saccharin in intact human airways and would not be surprised to find that this agent acts as a partial agonist at these receptors for this response. The issue is not particularly relevant, given the wide variety of TAS2R agonists that are available, and the fact that saccharin does not activate a bitter taste receptor in mice or guinea pigs, which are commonly used for models of human airways disease.
The kinetic data with chloroquine and quinine in human airways reported by Morice et al. is in contrast to what we have found1. In fact, these investigators suggest these agents promote “irreversible” relaxation or cell injury. However, we have shown that blockade of [Ca2+]i re-uptake, or antagonism of the BKCa channel, inhibits mouse airway relaxation to chloroquine, and in isolated human airway smooth muscle cells the relaxation to bitter tastants is blocked by a PLC inhibitor1. The results of these interventions, which are pathway specific, would be highly unlikely if TAS2R agonists cause nonspecific effects or cell injury. The assertions of Morice et al. are based on their finding of a slow onset of action and an inability to reverse the relaxation effect by washing and then re-contracting with methacholine in human airways obtained from surgical specimens. Since the primary set of observations from our paper were with inbred mice, we first turned to our ex vivo method in this species (where there is no confounding by genetic differences, chronic drug treatment, or disease) to specifically address the rate and reversibility issues. In new studies using a single concentration of chloroquine (3 mM) or quinine (1 mM), the time to maximal relaxation was found to be 4.9 ± 0.24 min and 4.4 ± 0.38 min for the two drugs, respectively (n=4, Fig. 1e). After contraction with acetylcholine and relaxation by chloroquine or quinine, we utilized four exchanges of Krebs buffer in the bath, and then re-challenged with the same dose of acetylcholine. The mean time from the last wash to 90% of the maximal contraction by the subsequent acetylcholine dose was 7.2 ± 0.85 min for chloroquine and 7.8 ± 0.48 min for quinine (n=4). The maximal extent of that subsequent contraction to acetylcholine compared to the first challenge was 95 ± 1.5%, and 96 ± 2.3%, respectively (P > 0.05, Fig. 1e). Having derived these kinetic values in mice, which are compatible with what we had previously observed1, we then applied this same approach to human airway rings, using 0.1 mM methacholine as the contractile agent (Fig. 1f). The times to maximal response to chloroquine and quinine were 8.6 ± 0.05 and 5.3 ±0.14 min, respectively (n=4), which is comparable to what was observed in mouse airways. These values clearly differ from Morice et al., who give mean times of 34 and 23 minutes for the two bitter tastants. Furthermore, like in the mouse airways, in human airways we observed ~90% reversal of the relaxant effect after washout and re-stimulation with methacholine (Fig. 1f). Again, these results differ markedly from those reported by Morice et al. who failed to observe any return of the contractile effect to methacholine once rings were exposed to chloroquine or quinine. Thus we demonstrate rapid on-rates, with virtually complete reversibility, in these human airways. Taken together, both mouse and human models indicate a rapid response to TAS2R agonists in a manner consistent with a typical agonist-GPCR interaction in airways, marked efficacy, and complete reversibility. Indeed, once re-contracted, airways can be relaxed again in a dose-dependent manner by another TAS2R agonist (Fig. 1e,f) or isoproterenol1. The suggestion by Morice et al. that these bitter tastants evoke cell injury is not compatible with these functional studies.
There are differences between our technique and Morice et al. that might explain the slow onset of action and apparent irreversibility that they report for chloroquine and quinine. For our original studies1 and those reported here, we set the resting (passive) tension at ~5-10 mN (equivalent to ~0.50 to 1.0 g), while they utilized tensions of 2-3 g. This lower baseline stretch placed on rings in our studies might favor TAS2R mediated relaxation and functional recovery, while the greater tensions used by Morice et al. may not be conducive to relaxation by this specific mechanism or may promote cell injury in the context of the higher concentrations of TAS2R agonists that are required.
We have thus now shown the relative efficacies of isoproterenol and two TAS2R agonists in relaxing human airway smooth muscle. The efficacy of the bitter tastants, in human airways studied under our conditions, is modestly greater than the full β-agonist, but the relevance of this difference at the physiologic or clinical level has not been defined. We show rapid on- and off-kinetics of the relaxation to these agonists, and functional recovery of contraction and a repeat relaxation, in mouse and human airways. Together with our original studies1 using inhibitors of the TAS2R pathway, we demonstrate airway relaxation via a receptor-mediated mechanism. These results continue to show that this previously unknown pathway may provide a novel mechanism to relax smooth muscle in the treatment of obstructive airway disease. Ultimately, human trials will be required to ascertain the clinical effectiveness of TAS2R agonists in the context of diseases such as asthma and chronic obstructive pulmonary disease.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
For Methods, see Supplementary Material.
References
- 1.Deshpande DA, et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nature Medicine. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naline E, et al. Relaxant effects and durations of action of formoterol and salmeterol on the isolated human bronchus. Eur Resp J. 1994;7:914–920. [PubMed] [Google Scholar]
- 3.Naline E, Trifilieff A, Fairhurst RA, Advenier C, Molimard M. Effect of indacaterol, a novel long-acting beta2-agonist, on isolated human bronchi. Eur Respir J. 2007;29:575–581. doi: 10.1183/09031936.00032806. [DOI] [PubMed] [Google Scholar]
- 4.Meyerhof W, et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]


