Abstract
The adenosine A2b-receptor (Adora2b) has been implicated in cardio-protection from myocardial ischemia. As such the Adora2b was found to be critical in ischemic preconditioning (IP) or ischemia reperfusion (IR) injury of the heart. While the Adora2b is present on various cells types, the tissue specific role of the Adora2b in cardio-protection is still unknown.
To study the tissue specific role of Adora2b signaling on inflammatory cells, endothelia or myocytes during myocardial ischemia in vivo, we intercrossed floxed Adora2b mice with Lyz2-Cre+, VE-Cadherin-Cre+ or Myosin-Cre+ transgenic mice, respectively. Mice were exposed to 60 minutes of myocardial ischemia with or without IP (4×5min) followed by 120 minutes of reperfusion.
Cardio-protection by IP was abolished in Adora2bf/f-VE-Cadherin-Cre+ or Adora2bf/f-Myosin-Cre+, indicating that Adora2bs signaling on endothelia or myocytes mediates IP. In contrast, primarily Adora2b signaling on inflammatory cells was necessary to provide cardio-protection in IR injury, indicated by significantly larger infarcts and higher troponin levels in Adora2bf/f-Lyz2-Cre+ mice only. Cytokine profiling of IR injury in Adora2bf/f-Lyz2-Cre+ mice pointed towards PMNs. Analysis of PMNs from Adora2bf/f-Lyz2-Cre+ confirmed PMNs as one source of identified tissue cytokines. Finally, adoptive transfer of Ador2b−/− PMNs revealed a critical role of the Adorab2 on PMNs in cardio-protection from IR-injury.
Adora2b signaling mediates different types of cardio-protection in a tissue specific manner. These findings have implications for the use of Adora2b agonists in the treatment or prevention of myocardial injury by ischemia.
INTRODUCTION
Myocardial infarction (MI) is the leading cause of death worldwide and according to the World Health Organization responsible for 7.25 million deaths each year. In the USA, about every 44 seconds somebody will have a new heart attack. About 34 percent of the people who experience a coronary attack in a given year will die from it (1). Despite standard therapies such as early coronary artery reperfusion for the treatment of acute ST-elevation myocardial infarction, morbidity and mortality from MI remain significant. Based on this, the incidence of congestive heart failure continues to increase, and there is a need to provide better therapy that reduces the amount of necrosis that may be coupled with better clinical outcome in the setting of MI (2, 3).
Substantial research efforts have been dedicated to identify agents modulating the inflammatory response after MI which represent one mechanism in myocardial injury by reperfusion (IR-injury). Multiple studies have suggested that adenosine is critical for protection against IR-injury (4). The mechanism of adenosine dependent cardio-protection involves most likely a shift in parenchymal cells metabolism, vasodilatation of coronary arteries or inhibition of leukocyte-mediated inflammatory responses (4, 5).
Adenosine elicits protective effects through four adenosine receptors [ARs; Adora1, Adora2a, Adora2b and Adora3 (6–10)]. All ARs have been associated with cardiac tissue protection in different settings. In particular, the Adora2b has been implicated in ischemic preconditioning (IP) (11, 12) and post-conditioning (13) effects of the heart. Both represent powerful cardio-protective mechanisms where the heart tissue at risk is exposed to short repeated ischemic periods either prior to the onset of ischemia or at the onset of reperfusion (14–16).
Although in vivo experiments have shown the cardio-protective effect of the Adora2b (11, 13, 17–20), these experiments have not dissected the major cellular targets (myocytes, endothelium, or bone-marrow derived cells) responsible for the salutary effect of Adora2b activation in different settings such as cardiac IP or IR-injury of the heart.
In the present study, we used state-of-the-art Cre-lox mouse models to generate tissue specific Adora2b deletion on bone marrow derived inflammatory cells (Adora2bf/f-Lyz2-Cre+), endothelia (Adora2bf/f-VE-Cadherin-Cre+) or myocytes (Adora2bf/f-Myosin-Cre+). Exposing those mice to a murine in-situ model for IP or IR-injury indicated that the Adora2b has a differential tissue specific function in different settings. These findings implicate that tissue specific targeting of the Adora2b seems to be desirable when using Adora2b agonists to prevent or treat myocardial ischemia.
MATERIALS & METHODS
Mice
All animal procedures were performed in an AAALAC-accredited facility in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the University of Colorado Denver Institutional Animal Care and Use Committee. For all studies we used male mice 8–16 weeks old. Studies were in accordance with the NIH guidelines for use of live animals. C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, USA). Adora2b-floxed (Adora2bf/f) mice were generated by Ozgene (Pert, Australia). Lyz2-Cre+ (B6.129P2-Lyz2tm1(cre)Ifo/J) (21)], VE-Cadherin-Cre+ (B6.Cg-Tg(Cdh5-cre)7Mlia/J) (22) and tamoxifen inducible Myosin-Cre+ (Tg(Myh6-cre/Esr1*)1Jmk/J) (23) mice were purchased from Jackson laboratories. To obtain tissue specific Adora2b−/− mice, we crossbred Adora2bf/f (Ozgene) mice with the appropriate tissue specific Cre recombinase mouse. For studies using Myosin-Cre+ mice, all mice were induced by treatment with tamoxifen 1 mg/day i.p, dissolved in peanut oil for five days. Experiments were performed after additional 14 days following tamoxifen administration. Genotyping PCRs for tissue specific animals were performed by GeneTyper, NY, USA.
Isolation of adult cardiomyocytes
8 – 12 weeks old Myosin Cre+ or Adora2bf/f -Myosin-Cre+ mice were anesthetized and the heart was quickly removed from the chest cavity and immediately placed in ice-cold KHB buffer. After weighing, the aorta was cannulated and the heart were perfused with Ca2+-free KHB for 3 min followed by 8–12 min perfusion with Ca2+-free KHB containing collagenase and elastase. After perfusion, ventricles were removed, minced and incubated with the collagenase/elastase solution for an additional 3–7 min. The cells were filtered through a nylon mesh (300 μm) and collected in a 15 ml sterile tube. Myocytes were washed and calcium was slowly re-introduced in a stepwise fashion. Finally, cells were resuspended in MEM and plated on laminin. Cell were harvested the next day and immediately resuspenden in Trizol for mRNA analysis (19).
Isolation of myeloid cells
Heart tissue from Lyz2Cre+ or Adora2bf/f -Lyz2-Cre+ mice was minced and digested for 45 minutes using collagenase solution. Myeloid cells were isolated using EasySep CD11b-PE positive selection (StemCell Technologies), according to the manufacturer’s instructions. After Trypan Blue staining to confirm cell viability, cells were immediately resuspenden in Trizol for mRNA analysis.
Isolation of endothelia cells
Heart tissue from VE-Cadherin-Cre+ or Adora2bf/f -VE-Cadherin-Cre+ mice was minced and digested for 45 minutes using collagenase solution. Endothelial cells were isolated using EasySep CD31-Biotin positive selection (StemCell Technologies), according to the manufacturer’s instructions. After Trypan Blue staining to confirm cell viability, cells were immediately resuspenden in Trizol for mRNA analysis.
Adora2b mRNA analysis from heart tissue cells
Total RNA was isolated from cells using the Trizol Reagent according to the manufacturer’s instructions (Invitrogen, 15596–018). For this purpose, cells were homogenized in the presence of Trizol Reagent and chloroform was added. After spinning at 12,000xg for 15 minutes, the aqueous phase was removed and the RNA was precipitated with isopropanol. RNA was pelleted, washed with ethanol, treated with DNAse, and the concentration was quantified. The PCR reactions contained a mix of forward and reverse oligonucleotides with SYBR Green (Bio-Rad, 170–8880). Each target sequence was amplified using increasing numbers of cycles of 94°C for 1 min, 58°C for 0.5 min, 72°C for 1 min. Quantification of transcript levels was determined by real-time RT-PCR (iCycler; Bio-Rad Laboratories Inc.). The primers were Quantitect from Qiagen (Mm_Adora2b, QT01543444).
Murine Model for cardiac IP in IR-injury
Anesthesia was induced (70 mg/kg i.p.) and maintained (10 mg/kg/h i.p.) with sodium pentobarbital. Mice were placed on a temperature-controlled heated table (RT, Effenberg, Munich, Germany) with a rectal thermometer probe to maintain body temperature at 37°C. The tracheal tube was connected to a ventilator (Servo 900C, DRE, USA). Animals were ventilated with a pressure controlled ventilation mode (pressure of 10 mbar, frequency 110 breaths/min, positive end-expiratory pressure of 5 mbar, FiO2 = 0.4). After induction of anesthesia, animals were monitored with a surface electrocardiogram (ECG, Hewlett Packard, DRE, USA). Fluid replacement was performed with normal saline, 0.2ml/h i.a. until the onset of reperfusion, and with 1 ml/h i.a. during reperfusion. Operations were performed under an upright dissecting microscope (Olympus SZX12). Following left anterior thoracotomy, exposure of the heart and dissection of the pericardium, the left coronary artery (LCA) was visually identified and an 8.0 nylon suture (Prolene, Ethicon, USA) was placed around the vessel. Atraumatic LCA occlusion for ischemia and IP studies was performed using a hanging weight system (24, 25). Successful LCA occlusion was confirmed by an immediate color change of the vessel from light red to dark violet, and of the myocardium supplied by the vessel from bright red to white (pale), as well as the immediate occurrence of ST-elevations in the ECG. During reperfusion, the changes of color immediately disappeared when the hanging weights were lifted and the LCA was perfused again. Infarct sizes were determined by calculating the percentage of myocardium that underwent infarction compared to the area at risk (AAR) using a previously described double staining technique with Evan’s blue and triphenyltetrazolium chloride (TTC). AAR and the infarct size were determined via planimetry using the NIH software Image 1.0 and the degree of myocardial damage was calculated as percent of infarcted myocardium from the AAR (12, 18).
Heart Enzyme Measurement
Blood was collected by central venous puncture for troponin I (cTnI) measurements using a quantitative rapid cTnI assay (Life Diagnostics, Inc., West Chester, PA) (18–20, 25, 26).
Analysis of β-gal and F4/80 expression in hearts of Adora2b reporter mice
To localize the Adora2b in heart tissue, we analyzed β-galactosidase (β-gal) expression in Adora2b-KO/β-gal–knock-in mice (27). Hearts were harvested following perfusion fixation in 4% PFA/0.1 M PB (phosphate buffer without saline) and post fixed in the same fixative for 3 hours, followed by cryoprotection in 20% sucrose in 0.1 M PB pH 7.2 overnight at 4°C. Using a cryostat, 16-μm sections were collected onto Superfrost Plus slides (Fisher Scientific). The slides underwent three 10-minute washes in 0.1 M PBS before incubation in blocking solution (2% normal goat serum, 1% BSA, 0.3% Triton in PBS) for 1 hour at room temperature. The samples were then incubated overnight at 4°C in guinea pig anti–β-gal (1:1000, AB was a generous gift from Professor Thomas E. Finger at UC Denver) diluted in blocking solution. To stain for macrophages and determine whether they co-localize with β-gal staining the goat anti-rat F4/80 (1:100) (Serotec, Oxford, UK) was co-incubated with the anti-β-gal antibody diluted in blocking solution. Following three washes in PBS, samples were incubated for 2 hours at room temperature with Alexa Fluor 594 goat anti-guinea pig (1:400)(Invitrogen) and Alexa Flour 488 goat anti-rat (1:400) (Invitrogen) diluted in blocking buffer. Slides were then washed twice in PBS, followed by a 10-minute wash in 0.1 M PB and cover slipped using Vectashield containing DAPI (Vector Laboratories). Immunofluorescent images were taken using a Zeiss 780 LSM.
Cytokine multiplex ELISA
To measure cytokine tissue levels after 60 minutes of ischemia, Lyz2Cre+ or Adora2f/f-Lyz2Cre+ mice were euthanized following 120 minutes of reperfusion. Remaining blood was removed, the myocardial tissue (area at risk) was excised after delineation with Evans’ blue and immediately frozen with liquid nitrogen and stored at −80°C. Tissues were homogenized on ice using a Tissue Master 125 (OMNI International) in T-PER (Thermo Scientific, 78510) containing Pierce Protease Inhibitors according to the manufacturer’s recommendations (Thermo Scientific, 88665). After spinning at 10,000 RPM for 5 minutes the supernatant was removed and diluted to a final concentration of 10ug/100uL per well. The cytokine ELISA array (Signosis, EA-4005) was performed according to the manufacturer’s instructions.
IL6 and TNFa mRNA analysis from in PMNs
PMNs were harvested from bone marrow according to the product manual for preparing a single cell suspension (STEMCELL Technology, 19762A). The PMNs were subsequently isolated from the bone marrow according to the EasySep protocol (STEMCELL Technology, 19762A) using the purple EasySep magnet (STEMCELL Technology, 18000). The isolated PMNs were transferred immediately to ice to avoid activation. Total RNA was isolated from PMNs using the Trizol Reagent according to the manufacturer’s instructions (Invitrogen, 15596–018). For this purpose, liquid nitrogen frozen cells were homogenized in the presence of Trizol Reagent and chloroform was added. After spinning at 12,000xg for 15 minutes, the aqueous phase was removed and the RNA was precipitated with isopropanol. RNA was pelleted, washed with ethanol, treated with DNAse, and the concentration was quantified. The PCR reactions contained a mix of forward and reverse oligonucleotides with SYBR Green (Bio-Rad, 170–8880). Each target sequence was amplified using increasing numbers of cycles of 94°C for 1 min, 58°C for 0.5 min, 72°C for 1 min. Quantification of transcript levels was determined by real-time RT-PCR (iCycler; Bio-Rad Laboratories Inc.). The primers were Quantitect from Qiagen (TNFα, QT00104006; IL-6, QT00098875).
ROS assay
Cardiac protein carbonyl was measured using OxiSelect protein carbonyl ELISA kit (Cell Biolabs, San Diego, CA).
Neutrophil depletion and adoptive transfer
Mice were treated with a Ly6G-specific mAb 1A8 [Bio X Cell (28)]. This depletes circulating neutrophils but does not affect circulating GR-1 positive monocytes, as described previously (28). In a subset of experiments mice were treated with an anti-GCSF antibody (PeproTech) that prevents recruitment of endogenous PMNs (29). To perform an adoptive transfer of PMNs into PMN depleted animals, we first euthanized donor wildtype mice (BL6/C57, 6–8 weeks) and harvested the bone marrow by flushing the femoral bones. PMNs we separated by negative selection using the EasySep™ Mouse Neutrophil Enrichment Kit (StemCell Technologies Inc) and counted by a hemocytometer. Then 1 × 106 cells were injected into the neutrophil-depleted mice via an arterial catheter over 10 min. After a waiting period for 60 min, the mice underwent myocardial ischemia and reperfusion injury as described above.
Statistics
Data were compared by Student’s t test where appropriate. Values are expressed as mean ± SD from 3–6 animals per condition. The chosen numbers of animals per group was based on findings in previous studies and a subsequent samples size analysis. The studies are designed to be able to reject the null hypothesis that the population means of the experimental and control groups are equal with probability (power) 0.8. The Type I error probability associated with this test of this null hypothesis is 0.05. Data are expressed as mean ± SD. P < 0.05 was considered statistically significant. For all statistical analysis GraphPad Prism 5.0 software for Windows was used.
RESULTS
Experimental setup and animal models to investigate the tissue specific role of Adora2b-dependent cardio-protection
The experimental protocols are displayed in Figure 1a. For myocardial ischemia and reperfusion injury we used 60 minutes of ischemia followed by 120 minutes of reperfusion (Figure 1a, Model 1). For ischemic precondition (IP) we performed four cycles of 5 minutes ischemia and 5 minutes of reperfusion prior to 60 min of ischemia and 120 min reperfusion (Figure 1a, Model 2). After reperfusion we visualized the infarcted area using Evan’s Blue and triphenyltetrazolium chloride (TCC). In addition, we determined troponin I from serum samples.
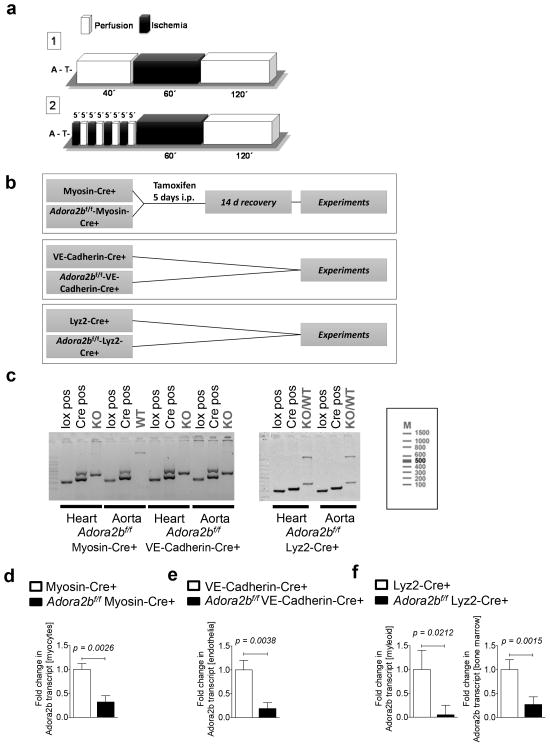
Figure 1. Overview of the experimental protocol and tissue-specific Adora2b-deficient mice.
(a) Experimental protocols used. Model 1. Myocardial ischemia consisted of 60 minutes of ischemia followed by 2 h of reperfusion. Model 2. For ischemic preconditioning studies, 4 cycles of 5 min ischemia and 5 min reperfusion prior to the onset of myocardial ischemia was performed. (b) Overview of tissue-specific Adora2b deletion using a Cre-lox-system. (c) Genotyping data. Tissue was collected from the organs as labeled and genotyped using conventional PCR (GeneTyper, NY, USA). Genotyping analyzed for Adora2b floxed (lox), Cre-recombinase (Cre), Adora2b wildtype (WT) and for the Adora2b knockout (KO) signal. Left, representative PCR results from heart or aortic tissue from Adora2bf/f -Myosin-Cre+ mice (cardio-myocyte specific); note: the positive KO band in cardiac tissue, in contrast to only WT band present in the aortic tissue. Middle, representative PCR result from Adora2bf/f-VE-Cadherin-Cre+ mice (endothelial specific); note: the positive KO signal in heart and aorta. Right, representative PCR results from Adora2bf/f-Lyz2-Cre+ mice (bone-marrow derived cell specific); note: positive KO and WT band in heart and aorta. The Cre signal for Myosin-Cre+ or VE-Cadherin-Cre+ was different than the signal from Lyz2-Cre+ as a different PCR strategy was used (according to Jackson Lab protocols); Adora2b flox signal: 200 base pairs (bp); Adora2b KO signal: 366 bp, Adora2b WT signal: 1600 bp, Myosin-Cre+ and VE-Cadherin-Cre+ signal: double band at 270 and 497 bp, Lyz2-Cre+ signal: 260 bp; ladder used is depicted to the right. (d–f) RT-PCR data showing Adora2b mRNA transcript levels from the respective tissues of the tissue specific- Adora2b deleted mice. (d) Cardiomyocytes were isolated from Myosin Cre+ and Adora2bf/f -Myosin-Cre+ mice and cultured overnight, (e) endothelia and (f) myeloid cells were isolated via positive selection (CD31+ for endothelia from VE-Cadherin-Cre+/ Adora2bf/f -VE-Cadherin-Cre+ hearts and CD11b+ for myeloid cells from Lyz2-Cre+/ Adora2bf/f -Lyz2-Cre+ hearts) using magnetic beads (EasySep) and bone marrow cells were isolated from the Lyz2-Cre and Adora2bf/f -Lyz2-Cre femurs.
To understand the tissue specific contribution of the Adora2b in cardio-protection we used state-of-the-art Cre-lox mouse models. Figure 1b displays the different Adora2b tissue specific mouse models that were used in the current studies. For Adora2b deletion on cardio-myocytes we generated Adora2bf/f-Myosin-Cre+ mice, for deletion on endothelial cells we used Adora2bf/f-VE-Cadherin-Cre+ mice, and for a bone-marrow derived cell deletion we used Adora2bf/f-Lys2Cre+ mice (Figure 1b). The Myosin-Cre+ mice have an inducible Cre-recombinase (Figure 1b, upper panel) and therefore the Adora2bf/f-Myosin-Cre+ and Myosin-Cre+ mice received 1 mg tamoxifen intraperitoneal for 5 days in order to induce Cre-recombinase activity. The other Cre-strains (VE-Cadherin-Cre+, Lyz2-Cre+) express constitutively Cre-recombinase and therefore no induction was necessary.
As shown in Figure 1c, the tissue-specific deletion of the Adora2b in the different tissue specific mouse models was confirmed using a genotyping PCR-analysis from hearts and aortas to understand how the cardiovascular system was affected. Adora2b floxed (lox), Cre recombinase (Cre), Adora2b knockout (KO) or Adora2b wildtype (WT) signal is depicted in each case. PCR-analysis confirmed that all tissue specific strains were positive for Cre recombinase and the floxed Adora2b gene. In Adora2bf/f-Myosin-Cre+ mice we found – as expected - a KO signal in the heart but a WT signal in the aorta. In Adora2bf/f-VE-Cadherin-Cre+ mice we found KO signal in the heart and the aorta, as both tissues are abundant in endothelial cells. Adora2bf/f-Lys2Cre+ mice had a heterozygous signal in the heart and the aorta (KO/WT signal in heart and aorta), probably as a result of resident inflammatory cells in these tissues. To analyze the efficiency of Adora2b gene deletion in the respective tissues of the tissue specific mice we isolated mRNA from the cells in question and determined Adorab2 transcript levels using real time RT-PCR. Cardiomyocytes were isolated and cultured over night from Adora2bf/f-Myosin-Cre+ or Myosin-Cre+ mice. Endothelial cells were isolated from Adora2bf/f-VE-Cadherin-Cre+ or VE-Cadherin-Cre+ hearts. Myeloid cells or bone marrow cells were isolated from Adora2bf/f-Lyz2Cre+ or Lyz2-Cre+ hearts or femurs, respectively. As shown in Figure 1d–f, Adora2b mRNA was significantly depleted in the different cell compartments from our tissue specific- Adora2b deleted mice. In summary, we generated three state-of-the-art Cre-lox mouse models in order to assess the tissue-specific contribution of Adora2b to IP or myocardial IR injury.
Adora2b signaling on bone marrow derived cells during ischemic preconditioning of the heart
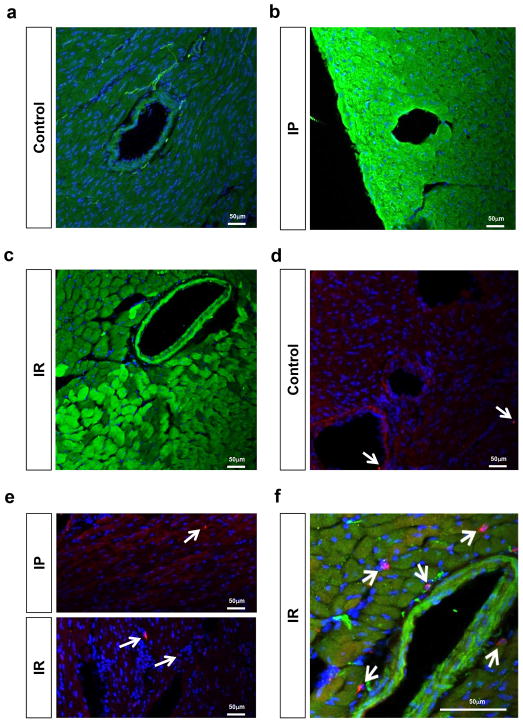
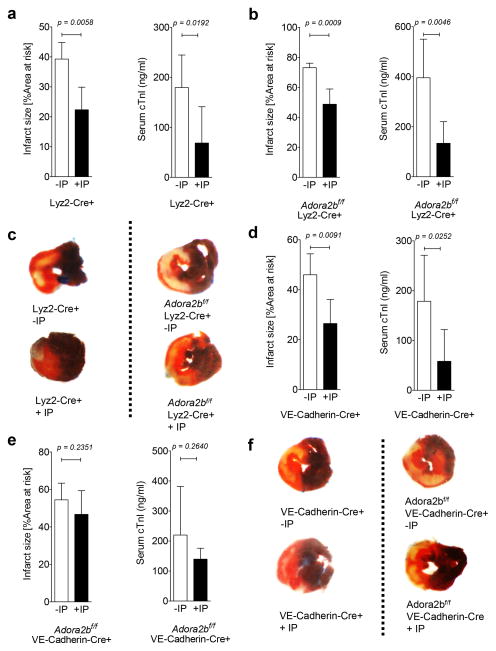
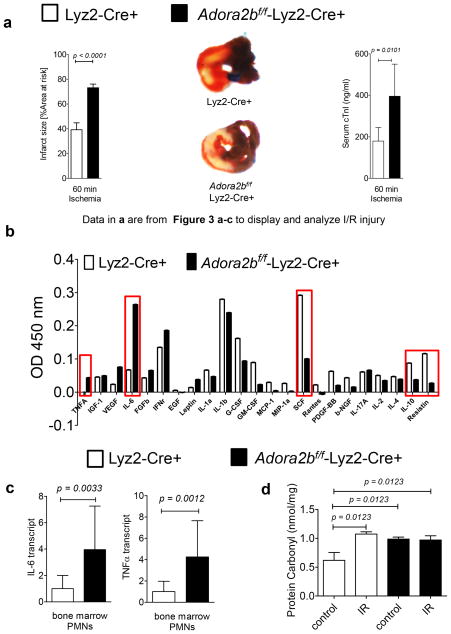
Based on earlier studies using germline Adora2b−/− mice, the Adora2b is crucial for the cardio-protective effects of IP (12). To get further insight into a tissue specific function of the Adora2b, we recapitulated what cell types express Adora2b in the heart. To obtain a very specific expression profile of the Adora2b receptor in the mouse heart tissue, we used an Adora2b-knockout-β-gal knock-in reporter mouse (27). As shown in Figure 2a–c, β-gal staining (green) of representative heart sections from an Adora2b-β-gal-reporter mouse revealed dominantly Adora2b positive cells in the vessel walls at baseline and a strong and significant up-regulation of the Adora2b on endothelia and cardiomyocytes upon IP or ischemia-reperfusion (IR) injury. While some resident macrophages were detected in the heart (red staining, Figure 2d, e), none co-expressed the Adora2b (Figure 2f). Indeed, expression could be different in circulating vs. tissue resident macrophages (27). In addition, only IR treatment slightly increased the number of resident macrophages (Figure 2f). Based on the fact that IP consists of short non-lethal repeated ischemic periods prior to ischemia and based on our expression profile of cardiac Adora2b receptors, we hypothesized that bone marrow derived inflammatory cells do not play an important role during IP of the heart. To test this hypothesis we used Adora2bf/f-Lyz2-Cre+ mice and their respective controls (Lyz2Cre+) and exposed them to 60 min of ischemia with ischemic preconditioning (+IP; 4 cycles of 5 min of ischemia followed my 5 minutes of reperfusion) or without IP (−IP) followed by 120 minutes of reperfusion. Infarct sizes were measured by double staining using Evan’s blue and triphenyl-tetrazolium chloride, while serum troponin I was determined using ELISA. As shown in Figure 3a, c, IP significantly decreased infarct sizes from 39.3±5.5% (n=5) to 22.4±7.5% (n=4) and troponin I serum levels from 179.6±65.1 ng/ml to 68.7±72.7 ng/ml (n=6 per group) in controls (Lyz2-Cre+) mice. However, studies Adora2bf/f-Lyz2-Cre+ mice (Figure 3b, c) also showed significantly decreased infarct sizes from 73.1±3.0% to 48.8±10.2% (n=5 per group) and troponin I serum levels from 396.0 ± 154.4 ng/ml to 133.9 ± 86.5 ng/ml (n=6 per group) when hearts had been pretreated with IP prior to ischemia.
Figure 2. Effect of ischemic preconditioning or ischemia reperfusion injury on Adora2b and F4/80 positive cell expression.
(a–c) Beta-gal staining of representative sections of an Adora2b-beta-gal-reporter mouse at baseline (a), after ischemic preconditioning (b) or ischemia reperfusion injury (c). Green stain: beta-gal-positivity indicating Adora2b promoter activation; Note: Adora2b positive cells are dominantly located in the vessel wall at baseline, while ischemic preconditioning or ischemia reperfusion injury significantly upregulates Adora2b gene promoter activity on endothelia but also on myocardial cells. (d–f) F4/80 staining (red) at baseline (d), after ischemic preconditioning (e) or ischemia reperfusion injury (e,f). Note: while F4/80 positive cells are rarely found at baseline or after ischemic preconditioning, a slight increase of F4/80 cells was found after ischemia reperfusion injury (f). Note: No beta gal staining was observed on residential macrophages (f); Green stain: Adora2b; Red stain: Macrophages; Blue stain: DAPI; blue stain representing nuclei.
Figure 3. Effect of ischemic preconditioning on myocardial injury in bone-marrow derived cell and endothelial-specific Adora2b-deficient mice.
(a–f) Mice underwent 60 min of ischemia with ischemic preconditioning (+IP; 4 cycles of 5 min of ischemia followed my 5 minutes of reperfusion) or without IP (−IP) followed by 120 minutes of reperfusion. Infarct sizes were measured by double staining with Evan’s blue and triphenyl-tetrazolium chloride. Infarct sizes are expressed as the percent of the area at risk (AAR) that underwent infarction. Serum troponin I concentrations were measured by enzyme-linked immunosorbent assay (ELISA). (a, b) Infarct sizes and serum troponin I levels in Lyz2-Cre+ (controls) or Adora2bf/f-Lyz2-Cre+ with and without IP. (c) Representative infarct staining from Lyz2Cre (controls) or Adora2bf/f-Lyz2-Cre+ mice; (n=5–6; ±SD).
(d, e) Infarct sizes and serum troponin I levels in VE-Cadherin-Cre+ (controls) and Adora2bf/f-VE-Cadherin-Cre+ with and without IP. (f) Representative infarct staining from VE-Cadherin-Cre+ (controls) and Adora2bf/f-VE-Cadherin-Cre+ (n=5–6, ±SD).
Taken together, these data are in support of our initial hypothesis that Adora2b receptors on bone marrow derived inflammatory cells do not play an important role in mediating cardio-protection by IP. Since within the heart, the Adora2b is mainly expressed in the vessel wall and to some extent also in cardio-myocytes, those tissues might be important for cardio-protection by IP.
Adora2b signaling on endothelia and myocytes during ischemic preconditioning of the heart
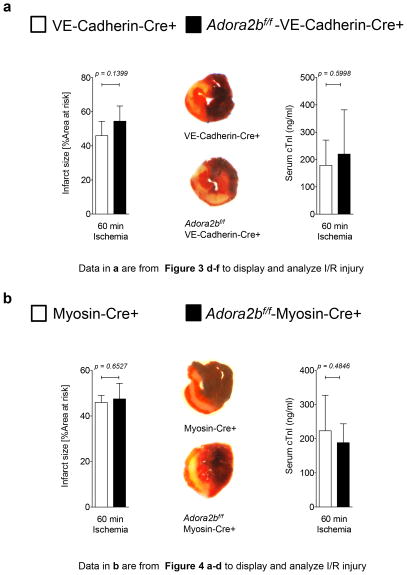
Given that there is no apparent role for Adora2b on bone marrow derived inflammatory cells during cardiac IP, we next investigated the role of the endothelia or myocytes. First, we used our endothelial specific Adora2b deficient mice (Adora2bf/f-VE-CadherinCre+) and appropriate controls (VE-Cadherin-Cre+). As shown in Figure 3d, f, IP significantly decreased infarct sizes from 45.9±8.4% to 26.5±9.6% (n=5 per group) and troponin I serum levels from 178.6±92.4 ng/ml to 58.2±63.6 ng/ml (n=6 per group) in VE-Cadherin-Cre+ mice. In contrast, IP had no significant effect on infarct sizes (−IP: 54.5±8.9%; n=6) vs. +IP: 46.7±12.7%; n=6) or troponin I serum levels (−IP: 219.9±162.1 ng/ml vs. +IP: 139.6±36.3 ng/ml; n=6 per group) in Adora2bf/f-VE-Cadherin-Cre+, as shown in Figure 3e, f.
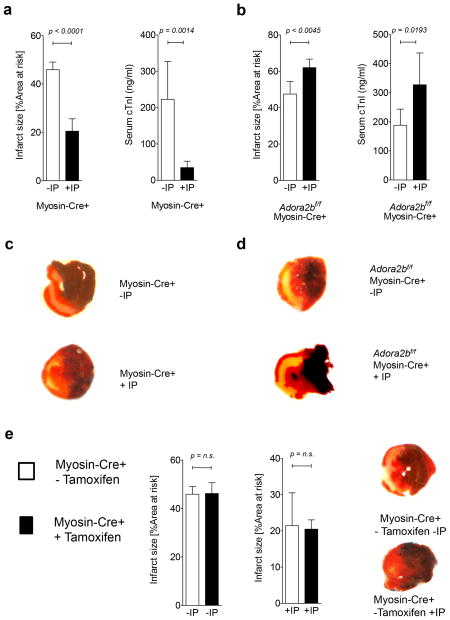
Next we analyzed the contribution of the Adora2b on cardio-myocytes as shown in Figure 4a–d. Here we found, that while IP significantly decreased infarct sizes from 45.9±3.1% (n=5) to 20.4±5.1% (n=4) and troponin I serum levels from 223.1±103.9 ng/ml to 34.9±17.7 ng/ml (n=6 per group) in control mice (Myosin-Cre+), IP in cardio-myocyte-specific Adora2b-deficient mice (Adora2bf/f-Myosin-Cre+) significantly increased infarct sizes from 47.5±6.8% to 62.1±4.7% (n=5 per group) or troponin I serum levels from 188.2±55.3 ng/ml to 327.5±109.3 ng/ml (n=6 per group). As Myosin-Cre+ controls had been pretreated with tamoxifen and this could have anti-inflammatory effects (30), we performed infarct size studies in Myosin-Cre+ mice without tamoxifen treatment. As shown in Figure 4e, no significant differences in infarct sizes were observed between tamoxifen treated and untreated Myosin-Cre+ mice.
Figure 4. Effect of ischemic preconditioning on myocardial injury in cardiomyocyte-specific Adora2b-deficient mice.
(a–e) Mice underwent 60 min of ischemia with ischemic preconditioning (+IP; 4 cycles of 5 min of ischemia followed my 5 minutes of reperfusion) or without IP (−IP) followed by 120 minutes of reperfusion. Infarct sizes were measured by double staining with Evan’s blue and triphenyl-tetrazolium chloride. Infarct sizes are expressed as the percent of the area at risk (AAR) that underwent infarction. Serum troponin I concentrations were measured by enzyme-linked immunosorbent assay (ELISA). (a, b) Infarct sizes and serum troponin I levels in Myosin-Cre+ (controls) and Adora2bf/f-MyosinCre+ with and without IP. (c, d) Representative infarct staining from Myosin-Cre+ (controls) and Adora2bf/f-MyosinCre+. (e) Infarct sizes in Myosin-Cre+ (controls) with and without tamoxifen pretreatment. (n=3–6, ±SD).
Taken together these data show an important role of the Adora2b on endothelial cells and myocytes in mediating cardio-protection by IP.
IR injury in cardio-myocyte or endothelial specific Adora2b deletion
After we found an important role of the Adora2b on myocytes and endothelia in mediating cardio-protection from ischemia by IP we next analyzed their role in IR injury. Surprisingly, as shown in Figure 5a and Figure 5b, infarct sizes and troponin I serum values were unchanged between control mice and mice with a tissue specific Adora2b deletion on endothelia or myocytes (VE-Cadherin-Cre+: Infarct sizes 54.4±8.8%, cTnI 219.9±162.1 ng/m vs. Adora2bf/f-VE-Cadherin-Cre+: infarct sizes 45.9±8.4%, cTnI 178.60±92.4 ng/ml; n=6 per group or Myosin-Cre+: Infarct sizes 45.9±3.1%, cTnI 223.1±103.9 ng/ml vs. Adora2bf/f-Myosin-Cre+: Infarct sizes 47.5±6.8%, cTnI 188.2±55.3 ng/ml; n=6 per group). Taken together, these data indicate that Adora2b expressing cardiac cells like endothelia or myocytes do not play a major role in cardiac IR injury.
Figure 5. Effect of myocardial ischemia-reperfusion injury on myocardial damage in endothelial or cardio-myocytes specific Adora2b deficient mice.
(a,b) Mice underwent 60 min ischemia and 120 min reperfusion. Infarct sizes were measured by double staining with Evan’s blue and triphenyl-tetrazolium chloride. Infarct sizes are expressed as the percent of the AAR that underwent infarction. Serum troponin I concentrations were measured by enzyme-linked immunosorbent assay (ELISA) (a) Infarct sizes and serum troponin I levels in VE-Cadherin-Cre+ (controls) and Adora2bf/f-VE-Cadherin-Cre+ with representative infarct staining [Note: Data in a are from Figure 3 d–f to display and analyze I/R injury]. (b) Infarct sizes and serum troponin I levels in Myosin-Cre+ (controls) and Adora2bf/f-Myosin-Cre+ with representative infarct staining (middle); (n=6, ±SD); [Note: Data in b are from Figure 4 a–d to display and analyze I/R injury].
IR injury in bone marrow derived inflammatory cell specific Adora2b deletion
After we discovered that mice with a tissue specific deletion of the Adora2b on endothelia or myocytes do not have increased IR injury when compared to their respective controls, we next analyzed mice with an Adora2b deletion on bone marrow derived inflammatory cells which include macrophages, monocytes and PMNs. As shown in Figure 6a, Adora2bf/f-Lyz2-Cre+ mice have increased infarct sizes when compared to Lyz2-Cre+ mice (Lyz2-Cre+: 39.2±5.5% vs. Adora2bf/f-Lyz2-Cre+: 73.1 ± 3.0%) and troponin I serum levels (Lyz2-Cre+ mice: 179.6±65.1 ng/ml vs. Adora2bf/f-Lyz2-Cre+: 396.0±154.4 ng/ml; n=6 per group). To identify potential cell specific cytokines responsible for the increased damage within the heart tissue during IR injury we performed a cytokine screen by using a multiplex ELISA. We found that Adora2bf/f-Lyz2-Cre+ had higher levels of tumor necrosis factor alpha (TNFα) or Interleukin-6 (IL-6) in the area at risk after 60 minutes of ischemia and 120 minutes of reperfusion. In addition, lower levels of G-CSF, stem cell factor, IL-10 and resistin were found in Adora2bf/f-Lyz2-Cre+ when comparted to controls (n=2 per group; Figure 6b). Based on the cytokine profile, we hypothesized that PMNs could be the major source of these cytokine changes (17). While Lyz2-Cre+ mice affect other bone marrow derived cells such as macrophages, they have been found to be particularly effective in deleting gene targets on PMNs in a tissue specific manner (31). We therefore isolated PMNs from Lyz2-Cre+ or Adora2bf/f-Lyz2-Cre+ mice and determined transcript levels of IL6 or TNFα at baseline (Figure 6c). RT PCR studies revealed that IL6 baseline transcript values were increased by 3.8±0.3, and TNFα baseline transcript values were increased by 4.2±0.2-fold in Adora2bf/f-Lyz2-Cre+ mice when compared to controls (Lyz2-Cre+). Values were normalized to the housekeeping gene β-actin (n=6). Based on the pro-inflammatory phenotype in Adora2bf/f-Lyz2-Cre+ we next investigated if Adora2bf/f-Lyz2-Cre+ mice could also have more superoxide production (ROS) during IR. As shown in Figure 6d, IR significantly increased ROS levels in the ischemic area of control mice. Interestingly, Adora2bf/f-Lyz2-Cre+ had already increased ROS levels at baseline when compared to control animals. This is consistent with earlier studies showing an augmented pro-inflammatory phenotype in Adora2b−/− mice in conjunction with enhanced leucocyte rolling at baseline (27). Taken together, these data indicate an important anti-inflammatory role of the Adora2b in IR injury.
Figure 6. Myocardial IR injury in bone marrow-specific Adora2b-deficient mice.
(a) Mice underwent 60 min ischemia and 120 min reperfusion. Infarct sizes were measured by double staining with Evan’s blue and triphenyltetrazolium chloride. Infarct sizes are expressed as the percent of the AAR that underwent infarction. Infarct sizes in Lyz2-Cre+ (controls) or Adora2bf/f-Lyz2-Cre+ with representative infarct staining. Serum troponin I concentrations were measured by enzyme-linked immunosorbent assay (ELISA); [Note: Data in a are from Figure 3a–c re-arranged to display I/R injury]; (b) Multiplex Elisa from the are at risk (AAR) after 60 minutes of ischemia and 120 minutes of reperfusion comparing Lyz2-Cre+ (controls) and Adora2bf/f-Lyz2-Cre+. (c) Isolated PMNs from Lyz2-Cre+ (controls) or Adora2bf/f-Lyz2-Cre+ were analyzed for IL-6 or TNFα transcript levels; (n=5–6; ±SD). (d) Cardiac protein carbonyl, as indicator of ROS production, was measured using OxiSelect protein carbonyl ELISA kit in control or ischemic heart tissue from Lyz2-Cre+ (controls) or Adora2bf/f-Lyz2-Cre+ mice.
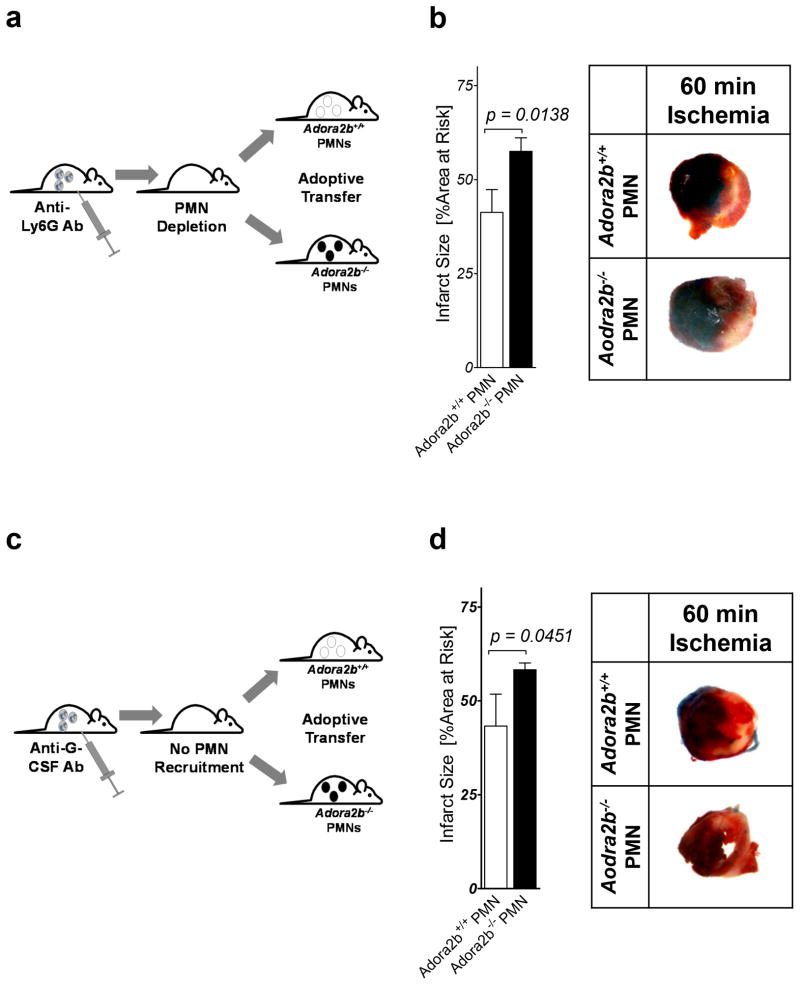
Adoptive transfer of Adora2b−/− or wildtype PMNs in IR injury
To further understand the role for Adora2bs on PMN cells in cardio-protection from IR injury, we next tested if adoptive transfer of Adora2b−/− PMNs into PMN depleted animals would increase infarct sizes when compared to a transfer of wildtype PMNs (see details of experimentally setup Figure 7a). First we treated wildtype animals with 1A8 Ly6G-specific antibodies 24h prior to the experiment. This treatment leads to a significant reduction of peripheral PMNs but not circulation monocytes in mice (28). On the day of experiment we isolated PMNs from wildtype or Adora2b−/− bone marrow and re-infused these cells via a carotid-catheter prior to the onset of myocardial ischemia. As seen in Figure 7b, Adora2b−/− PMN infusion significantly increased infarct sizes when compared to the wildtype PMN infusion (41.2± 6.1% (wildtype) vs. 57.5 ± 7.2% (Adora2b−/−); n=4 per group). To control for possible effects of the 1A8 Ly6G-specific antibody on transferred PMNs we repeated the experiment with an anti-GCSF antibody (Figure 7c). This antibody prevents the recruitment of endogenous PMNs but does not interfere with transferred PMNs (29). As shown in Figure 7d, a similar infarct size was observed with transferred Adora2b−/− PMNs. Therefore, in contrast to ischemic preconditioning, the Adora2b on bone marrow derived inflammatory cells, seemingly PMNs, represents an important therapeutic target during IR injury.
Figure 7. Effects of adoptive transfer of PMNs from Adora2b−/− into wildtype mice in IR injury.
(a,c) Model. Wildtype PMNs or Adora2b−/− PMNs were isolated and transferred into PMN depleted (1A8 Ly6G-specific antibody treatment 24 h prior to ischemia) or anti-GCSF (24 h prior to ischemia) treated animals. (b, d) Mice underwent 60 min ischemia and 120 min reperfusion. Infarct sizes were measured by double staining with Evan’s blue and triphenyltetrazolium chloride. Infarct sizes are expressed as the percent of the AAR that underwent infarction. Infarct sizes in PMN depleted wildtype mice that received either wildtpe PMNs (controls) or Adora2b−/− PMNs with representative infarct staining are shown; (n=3–4; ±SD)
DISCUSSION
Adora2b signaling has been shown to effectively protect the myocardium from ischemia in various settings such as IP or IR injury (11–13). In the present study, we investigated the cellular source of Adora2b-dependent cardio-protection. Studies using state-of-the-art Cre-lox mouse models for the Adora2b revealed an important role of myocytes or endothelial expressed Adora2b in IP of the heart as it was abolished in Adora2bf/f-VE-Cadherin-Cre+ or Adora2bf/f-Myosin-Cre+ mice. In contrast, protection from IR injury was primarily mediated by Adora2b signaling on bone marrow derived inflammatory cells. Characterization of the post-ischemic inflammatory response in Adora2bf/f-Lyz2-Cre+ mice revealed a PMN driven cytokine profile. In proof of principle studies, an adoptive cell transfer of Adora2b−/− PMNs confirmed the hypothesis that Adora2b signaling on PMNs had dominantly been responsible for the observed phenotype in Adora2bf/f-Lyz2-Cre+ mice. Taken together, these studies suggest that activation of Adora2bs on different tissues represent different therapeutic strategies.
The extent of myocardial cell death determines patient outcome after myocardial ischemia (32). Thus, it is not surprising that protective strategies to make the heart more resistant to ischemia or limit the damage during IR are an area of intense investigations (6, 16, 17, 19, 33–38). It is accepted that a number of G protein-coupled receptors can activate cardio-protective mechanisms (32). These receptors include the adenosine, opioid and bradykinin families (39). Over the past 20 years, substantial evidence indicates that adenosine, administered either prior to ischemia or during reperfusion, reduces myocardial injury (32). These effects are mediated via the activation of one or more of the four known adenosine receptor (AR) subtypes (Adora1, Adora2a, Adora2b and Adora3). All four ARs have been associated with cardiac tissue protection in different settings (12, 40, 41). Experimental studies in different species and models implicated that activation of the Adora1 or Adora3 prior to ischemia is cardio-protective (42, 43). Other studies revealed that the administration of Adora2a or Adora2b agonists during reperfusion can reduce MI (41, 44). However, while all ARs have been found to mediate cardio-protection from ischemia, the Adora2b might be the only one that was found to play a role in almost all known cardio-protective settings. As such, the Adora2b has been implicated in post-conditioning, which protects the re-perfused heart from infarction (44). Other studies have shown the importance of Adora2b signaling for cardio-protection mediated by IP (12) which seems to be associated with the initiation of a metabolic program to make heart more oxygenic efficient (19). Further mechanistic studies on Adora2b mediated cardio-protection revealed the involvement of hypoxia inducible factor 1 (HIF1), an important transcription factor implicated in tissue adaptation to hypoxia (18, 19). Other studies indicated reduction of superoxide generation from mitochondria through ERK, PI3K, and NOS as Adora2b mediated - all of which have been implicated in protection from ischemia (45). A very elegant study recently discovered that the Adora2b is present in or near mitochondria suggesting that Adora2b signaling results in inhibition of mitochondrial transition pores (46). Since it is believed that cardio-protective signaling pathways converge on the mitochondria, inhibition of mitochondrial transition pores is thought to be ‘the holy grail of cardio-protection’ (47). However, it needs to be pointed out that the Adora2b is the only one of the four-adenosine receptors whose cardiac expression was found to be induced by ischemia in both mice and humans and whose function is implicated in ischemic preconditioning and post conditioning of the heart (19).
Even though there is rising evidence for Adora2b signaling in mediating cardio-protection, no study has evaluated the tissue specific contribution yet. IP has been consistently demonstrated to be a potent protective mechanism in freshly isolated and cultured cardiomyocytes across multiple species, indicating that much of the innate protection of IP resides in cardiomyocytes (48). However, studies using state-of-the-art Cre-lox mouse models have not been performed yet. Based on earlier studies in germline Adora2b−/− mice (12, 17), we now generated tissue specific Adora2b knockout mice. Comparing an Adora2b tissue specific deletion in myocytes, endothelia or bone marrow derived inflammatory cells, indeed confirmed studies hypothesizing that cardio-myocytes are crucial for IP of the heart. In addition, Adora2b on the endothelium was also found to be important for the mechanism of IP. In fact, hypoxic preconditioning to model ischemic preconditioning in vitro has repeatedly been found to protect endothelial cells from subsequent long term hypoxia (19, 49, 50). In contrast, Adora2b signaling on inflammatory cells seems less important during IP. However, this is not surprising as IP consists of short nonlethal ischemic periods that are most likely not able to attract a significant amount of inflammatory cells (17). In addition, while we found some resident macrophages in the heart, Adora2bs were dominantly expressed on cardiomyocytes and endothelial cells, supporting our findings in IP of the heart.
Interestingly, the findings that Adora2b-elicited cardioprotection by IP involves vascular endothelial cells is also reflected in a recent study examining the role of HIF1A in cardioprotection. This study demonstrates convincingly, by using tissue specific HIF1A deficient mice, that vascular endothelial HIF1A is required for mediating the cardio-protective effects of IP. The authors conclude that HIF1A is functioning as a transcriptional activator, despite the acute nature of the response (51). As discussed above, HIF1A is a critical transcriptional enhancer of Adora2b signaling during IP (52). Therefore, it is conceivable that the transcriptional induction of the Adora2b via HIF1A is a critical component of cardioprotection elicited by IP.
While IP seems to be mainly linked to endothelial cells and cardio-myocytes, compelling evidence from both animal and clinical studies has indicated that leukocytes are the principal effector cells of IR injury (53). Reperfusion induces a vigorous inflammatory response and a dramatic increase in neutrophil adherence to the re-perfused endothelium (17, 54). As Adora2bs are widely distributed in hematopoietic cells (27, 37, 55, 56), studies using in vivo animal models have shown that Adora2b deficiency is associated with enhanced inflammation (20, 27, 37, 57). Other studies indicated that Adora2b mediated protection from vascular injury is based on anti-inflammatory processes (37, 58).
Based on its anti-inflammatory role, it is convincing that Adora2b signaling could dampen IR injury by interaction with bone marrow derived inflammatory cells. In fact, in the current study, we have established an important role for the Adora2b on bone marrow–derived cells in mediating cardio-protection against IR injury. Earlier studies using germline Adora2b−/− mice, showed that plasma levels of the pro-inflammatory cytokine TNFα was elevated at baseline (27) and in mice subjected to femoral artery or myocardial IR injury (17, 58). Thus, our findings on a TNFα guided pro-inflammatory phenotype in tissue specific deletion of the Adora2b on bone marrow derived cells (Adora2bf/f-Lyz2-Cre+) during cardiac IR are consistent with these previous findings.
In this study, as a proof of concept, we isolated Adora2b−/− PMNs from germline Adora2b−/− mice and transferred them into neutropenic mice. Following ischemia, we found significantly increased infarct sizes when compared to mice that were transferred with wildtype PMNs. These studies support our findings that only Adora2bf/f-Lyz2-Cre+ had larger infarcts when compared to controls. As bone marrow derived cells include neutrophils and macrophages, it seems compelling that PMNs play the dominant role as they are the dominant cell type during the first hours of reperfusion (17). However, it also indicates that our studies cannot completely rule out a role of the Adora2b on macrophages in IR injury. Future studies using tissue specific mice for different inflammatory cell types will be necessary to further elucidate the detailed mechanisms.
Interestingly, apart from the Adora2b dependent regulation of pro-inflammatory TNFα, we also found significantly lower levels of Stem cell factor (SCF) in Adora2bf/f-Lyz2-Cre+ mice. SCF is a cytokine that improves myocardial function (59) and enhances cardiac healing after myocardial injury (60). In addition, it has been found that adenosine can enhance SCF signaling in vitro (61). Thus, apart from a reduction of the post-ischemic inflammation, Adora2b signaling might act as a switch to start cardiac repair early in reperfusion. Indeed, pharmacological studies have indicated an important role of Adora2b in cardiac healing after IR injury (62).
Taken together, using a novel tissue specific approach for Adora2b signaling during IP or IR, we found different functions for the Adora2bs in different tissues. While Adora2b signaling in vascular endothelial cells and cardiac myocytes was critical for mediating IP-elicited cardioprotection, the extent of IR injury was determined by Adora2b signaling on inflammatory cells. These findings indicate that when using the Adora2b as therapeutic target it might be best when done in a tissue specific manner. One possible approach could be the administration of an Adora2b agonist into a coronary artery prior to a high risk intervention to “precondition” the heart, whereas systematic administration of an Adora2b agonist may be preferable during reperfusion. Future studies using specific Adora2b agonists in clinical trials will be necessary to get insight into tissue specific therapies for myocardial ischemia.
Acknowledgments
Sources of financial support for the work:
National Heart, Lung, and Blood Institute (NIH-NHLBI) Grant 1K08HL102267-01 and 1R01HL122472-01 to T.E and R01 DK097075, R01-HL0921, R01-DK083385, R01- HL098294, POIHL114457-01 and a grant by the Crohn’s and Colitis Foundation of America (CCFA) to HKE and a Deutsche Forschungsgemeinschaft (DFG) research fellowship to M. K
We would like to thank the Rocky Mountain Taste and Smell Center, Grant P30DC004657 for providing the guinea pig anti–β-gal antibody (AB_2316450).
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB C. American Heart Association Statistics, and S. Stroke Statistics. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB C. American Heart Association Statistics, and S. Stroke Statistics. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 4.Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol Ther. 2007;114:208–221. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 6.Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111:904–915. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koeppen M, Eckle T, Eltzschig HK. Interplay of hypoxia and A2B adenosine receptors in tissue protection. Advances in pharmacology. 2011;61:145–186. doi: 10.1016/B978-0-12-385526-8.00006-0. [DOI] [PubMed] [Google Scholar]
- 8.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eltzschig HK, Bonney SK, Eckle T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends in molecular medicine. 2013 doi: 10.1016/j.molmed.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eltzschig HK. Targeting purinergic signaling for perioperative organ protection. Anesthesiology. 2013;118:1001–1004. doi: 10.1097/ALN.0b013e3182874686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuno A, Critz SD, Cui L, Solodushko V, Yang XM, Krahn T, Albrecht B, Philipp S, Cohen MV, Downey JM. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol. 2007;43:262–271. doi: 10.1016/j.yjmcc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 13.Philipp S, Yang XM, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–314. doi: 10.1016/j.cardiores.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Granfeldt A, Lefer DJ, Vinten-Johansen J. Protective ischaemia in patients: preconditioning and postconditioning. Cardiovasc Res. 2009;83:234–246. doi: 10.1093/cvr/cvp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Seminars in cardiothoracic and vascular anesthesia. 2012;16:123–132. doi: 10.1177/1089253211436350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckle T. About Dogs, Mice, and Men: From Ischemic Preconditioning to Anesthetic Postconditioning of the Heart. Seminars in cardiothoracic and vascular anesthesia. 2014;18:247–248. doi: 10.1177/1089253214542253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koeppen M, Harter PN, Bonney S, Bonney M, Reithel S, Zachskorn C, Mittelbronn M, Eckle T. Adora2b signaling on bone marrow derived cells dampens myocardial ischemia-reperfusion injury. Anesthesiology. 2012;116:1245–1257. doi: 10.1097/ALN.0b013e318255793c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 19.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. The Journal of clinical investigation. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 22.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 23.Hall ME, Smith G, Hall JE, Stec DE. Systolic dysfunction in cardiac-specific ligand-inducible MerCreMer transgenic mice. Am J Physiol Heart Circ Physiol. 2011;301:H253–260. doi: 10.1152/ajpheart.00786.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dewald O, Frangogiannis NG, Zoerlein MP, Duerr GD, Taffet G, Michael LH, Welz A, Entman ML. A murine model of ischemic cardiomyopathy induced by repetitive ischemia and reperfusion. Thorac Cardiovasc Surg. 2004;52:305–311. doi: 10.1055/s-2004-821153. [DOI] [PubMed] [Google Scholar]
- 25.Eckle T, Grenz A, Kohler D, Redel A, Falk M, Rolauffs B, Osswald H, Kehl F, Eltzschig HK. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 2006;291:H2533–2540. doi: 10.1152/ajpheart.00472.2006. [DOI] [PubMed] [Google Scholar]
- 26.Eckle T, Koeppen M, Eltzschig H. Use of a hanging weight system for coronary artery occlusion in mice. J Vis Exp. 2011 doi: 10.3791/2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. The Journal of clinical investigation. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. Journal of leukocyte biology. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto S, Nava RG, Zhu J, Huang HJ, Ibrahim M, Mohanakumar T, Miller MJ, Krupnick AS, Kreisel D, Gelman AE. Cutting edge: Pseudomonas aeruginosa abolishes established lung transplant tolerance by stimulating B7 expression on neutrophils. Journal of immunology. 2012;189:4221–4225. doi: 10.4049/jimmunol.1201683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salman S, Kumbasar S, Kumtepe Y, Karaca M, Borekci B, Yildirim K, Alp HH, Cadirci E, Suleyman H. Role of adrenal gland hormones in the anti-inflammatory effect mechanism of tamoxifen, a partial antagonist for oestrogen receptors, and relation with COX levels. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2011;27:241–247. doi: 10.3109/09513590.2010.490610. [DOI] [PubMed] [Google Scholar]
- 31.Thompson AA, Elks PM, Marriott HM, Eamsamarng S, Higgins KR, Lewis A, Williams L, Parmar S, Shaw G, McGrath EE, Formenti F, Van Eeden FJ, Kinnula VL, Pugh CW, Sabroe I, Dockrell DH, Chilvers ER, Robbins PA, Percy MJ, Simon MC, Johnson RS, Renshaw SA, Whyte MK, Walmsley SR. Hypoxia-inducible factor 2alpha regulates key neutrophil functions in humans, mice, and zebrafish. Blood. 2014;123:366–376. doi: 10.1182/blood-2013-05-500207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Headrick JP, Lasley RD. Adenosine receptors and reperfusion injury of the heart. Handbook of experimental pharmacology. 2009:189–214. doi: 10.1007/978-3-540-89615-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonney S, Hughes K, Eckle T. Anesthetic Cardioprotection: The Role of Adenosine. Curr Pharm Des. 2014 doi: 10.2174/1381612820666140204102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonney S, Hughes K, Harter PN, Mittelbronn M, Walker L, Eckle T. Cardiac period 2 in myocardial ischemia: clinical implications of a light dependent protein. The international journal of biochemistry & cell biology. 2013;45:667–671. doi: 10.1016/j.biocel.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonney S, Kominsky D, Brodsky K, Eltzschig H, Walker L, Eckle T. Cardiac Per2 functions as novel link between fatty acid metabolism and myocardial inflammation during ischemia and reperfusion injury of the heart. PloS one. 2013;8:e71493. doi: 10.1371/journal.pone.0071493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckle T, Eltzschig HK. Toll-like Receptor Signaling during Myocardial Ischemia. Anesthesiology. 2011 doi: 10.1097/ALN.0b013e31820a4d78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warth A, Eckle T, Kohler D, Faigle M, Zug S, Klingel K, Eltzschig HK, Wolburg H. Upregulation of the water channel aquaporin-4 as a potential cause of postischemic cell swelling in a murine model of myocardial infarction. Cardiology. 2007;107:402–410. doi: 10.1159/000099060. [DOI] [PubMed] [Google Scholar]
- 39.Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol. 2008;103:203–215. doi: 10.1007/s00395-007-0687-7. [DOI] [PubMed] [Google Scholar]
- 40.Reichelt ME, Willems L, Molina JG, Sun CX, Noble JC, Ashton KJ, Schnermann J, Blackburn MR, Headrick JP. Genetic deletion of the A1 adenosine receptor limits myocardial ischemic tolerance. Circ Res. 2005;96:363–367. doi: 10.1161/01.RES.0000156075.00127.C3. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z, Day YJ, Toufektsian MC, Ramos SI, Marshall M, Wang XQ, French BA, Linden J. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 2005;111:2190–2197. doi: 10.1161/01.CIR.0000163586.62253.A5. [DOI] [PubMed] [Google Scholar]
- 42.Headrick JP, Gauthier NS, Berr SS, Morrison RR, Matherne GP. Transgenic A1 adenosine receptor overexpression markedly improves myocardial energy state during ischemia-reperfusion. J Mol Cell Cardiol. 1998;30:1059–1064. doi: 10.1006/jmcc.1998.0672. [DOI] [PubMed] [Google Scholar]
- 43.Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, Gross GJ, Kwok WM, Auchampach JA. The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3′-aminoadenosine-5′-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J Pharmacol Exp Ther. 2008;324:234–243. doi: 10.1124/jpet.107.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuno A, Solenkova NV, Solodushko V, Dost T, Liu Y, Yang XM, Cohen MV, Downey JM. Infarct limitation by a protein kinase G activator at reperfusion in rabbit hearts is dependent on sensitizing the heart to A2b agonists by protein kinase C. Am J Physiol Heart Circ Physiol. 2008;295:H1288–H1295. doi: 10.1152/ajpheart.00209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X, Xin W, Yang XM, Kuno A, Rich TC, Cohen MV, Downey JM. A2B adenosine receptors inhibit superoxide production from mitochondrial complex I in rabbit cardiomyocytes via a mechanism sensitive to Pertussis toxin. Br J Pharmacol. 2011;163:995–1006. doi: 10.1111/j.1476-5381.2011.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grube K, Rudebusch J, Xu Z, Bockenholt T, Methner C, Muller T, Cuello F, Zimmermann K, Yang X, Felix SB, Cohen MV, Downey JM, Krieg T. Evidence for an intracellular localization of the adenosine A2B receptor in rat cardiomyocytes. Basic Res Cardiol. 2011;106:385–396. doi: 10.1007/s00395-011-0151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol. 2010;105:151–154. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 48.Diaz RJ, Wilson GJ. Studying ischemic preconditioning in isolated cardiomyocyte models. Cardiovascular research. 2006;70:286–296. doi: 10.1016/j.cardiores.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Stubbs SL, Hsiao ST, Peshavariya HM, Lim SY, Dusting GJ, Dilley RJ. Hypoxic preconditioning enhances survival of human adipose-derived stem cells and conditions endothelial cells in vitro. Stem cells and development. 2012;21:1887–1896. doi: 10.1089/scd.2011.0289. [DOI] [PubMed] [Google Scholar]
- 50.Wu XD, Zhang ZY, Sun S, Li YZ, Wang XR, Zhu XQ, Li WH, Liu XH. Hypoxic preconditioning protects microvascular endothelial cells against hypoxia/reoxygenation injury by attenuating endoplasmic reticulum stress. Apoptosis : an international journal on programmed cell death. 2013;18:85–98. doi: 10.1007/s10495-012-0766-6. [DOI] [PubMed] [Google Scholar]
- 51.Sarkar K, Cai Z, Gupta R, Parajuli N, Fox-Talbot K, Darshan MS, Gonzalez FJ, Semenza GL. Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection induced by ischemic preconditioning. Proc Natl Acad Sci U S A. 2012;109:10504–10509. doi: 10.1073/pnas.1208314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eckle T, Kohler D, Lehmann R, El Kasmi KC, Eltzschig HK. Hypoxia-Inducible Factor-1 Is Central to Cardioprotection: A New Paradigm for Ischemic Preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 53.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 54.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. The New England journal of medicine. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 55.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Hoeven D, Wan TC, Gizewski ET, Kreckler LM, Maas JE, Van Orman J, Ravid K, Auchampach JA. A role for the low-affinity A2B adenosine receptor in regulating superoxide generation by murine neutrophils. J Pharmacol Exp Ther. 2011 doi: 10.1124/jpet.111.181792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Csoka B, Nemeth ZH, Rosenberger P, Eltzschig HK, Spolarics Z, Pacher P, Selmeczy Z, Koscso B, Himer L, Vizi ES, Blackburn MR, Deitch EA, Hasko G. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol. 2010;185:542–550. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen H, Yang D, Carroll SH, Eltzschig HK, Ravid K. Activation of the macrophage A2b adenosine receptor regulates tumor necrosis factor-alpha levels following vascular injury. Exp Hematol. 2009;37:533–538. doi: 10.1016/j.exphem.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiang FL, Lu X, Hammoud L, Zhu P, Chidiac P, Robbins J, Feng Q. Cardiomyocyte-specific overexpression of human stem cell factor improves cardiac function and survival after myocardial infarction in mice. Circulation. 2009;120:1065–1074. doi: 10.1161/CIRCULATIONAHA.108.839068. 1069 p following 1074. [DOI] [PubMed] [Google Scholar]
- 60.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 61.Hofer M, Vacek A, Pospisil M, Weiterova L, Hola J, Streitova D, Znojil V. Adenosine potentiates stimulatory effects on granulocyte-macrophage hematopoietic progenitor cells in vitro of IL-3 and SCF, but not those of G-CSF, GM-CSF and IL-11. Physiol Res. 2006;55:591–596. doi: 10.33549/physiolres.930854. [DOI] [PubMed] [Google Scholar]
- 62.Wakeno M, Minamino T, Seguchi O, Okazaki H, Tsukamoto O, Okada K, Hirata A, Fujita M, Asanuma H, Kim J, Komamura K, Takashima S, Mochizuki N, Kitakaze M. Long-term stimulation of adenosine A2b receptors begun after myocardial infarction prevents cardiac remodeling in rats. Circulation. 2006;114:1923–1932. doi: 10.1161/CIRCULATIONAHA.106.630087. [DOI] [PubMed] [Google Scholar]