Abstract
Objectives: Therapeutic benefits of omega-3 fatty acids (Ω3) for mood disorders, psychosis, and anxiety have been reported in the literature. The purpose of the present article is to provide a literature review of Ω3 supplementation for affective disorders and to illustrate the benefits of Ω3 with a case presentation of a young girl with a history of bipolar disorder-type 1 with psychotic features and generalized anxiety disorder.
Methods: Reviewed literature includes treatment studies of the impact of Ω3 on child mood disorders supplemented by review of meta-analyses within the adult mood disorders literature. The subject of this case report participated in 11 in-depth diagnostic and functional assessments over 5 years as part of an unrelated study. Three years were presupplementation and 2 years were with supplementation with no other medication changes, thus making a naturalistic multiple-baseline single-subject experiment.
Results: Augmentation over a 2 year period was notable for clinically significant and sustained improvement in depressive, manic, and psychotic symptoms.
Conclusion: Ω3 supplementation may be a safe, adjunct intervention for treating bipolar disorder in children and adolescents, even in the presence of psychotic and anxious features. The 2 year follow-up in this case offers hope of an accumulating and enduring benefit. Further research into mechanisms of Ω3 action and of combination treatment with other well-known interventions for mood disorders would be beneficial.
Introduction
Bipolar disorder (BD) is a debilitating illness for children and adolescents. First-line treatments, including atypical antipsychotics and mood stabilizers, are associated with adverse effects such as weight gain, gastrointestinal problems, thyroid function changes, and increased triglycerides, leading to noncompliance and treatment dropout (Biederman et al. 1998; Geller et al. 1998; Kowatch et al. 2000; Wagner et al. 2002). Although beneficial, medication does not fully ameliorate the symptoms of mania and depression inherent to bipolar disorder and can have many side effects. As an example, in a study of aripiprazole, Findling et al. (2009) reported that after 4 weeks on medication, only 25–50% of the sample (depending on dosage) reported symptom remission, whereas 75% of the overall sample reported one or more adverse events. Therefore, it is important to investigate alternative and complementary (adjunctive) treatments such as omega-3 (Ω3) supplementation, as a potentially safer means of decreasing psychotropic burden. High doses of Ω3 (usually >2 g) have the potential to cause side effects such as gastrointestinal upset (e.g., nausea, diarrhea/constipation, belching) or bleeding problems (albeit rare) if used in combination with an anticoagulant medication; however, the majority of clinical trials document no or minimal adverse symptoms related to Ω3 supplementation (Freeman et al. 2006).
The Ω3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are important to central nervous system functioning and are found in large amounts in the brain, particularly in neuronal membranes. Because Ω3 fatty acids are “essential,” meaning that they cannot be anabolized de novo, supplementation or other changes in dietary intake are the primary methods of increasing Ω3 levels in the body. Previous research on EPA and DHA suggests that polyunsaturated fatty acids (PUFAs) may improve functioning in a variety of disorders (Simopoulos 2002).
Evidence supporting the positive link between Ω3 levels and mood functioning exists. High resolution structural MRIs show that people with higher dietary intake levels of Ω3 have greater gray matter volume in the anterior cingulate cortex, the right hippocampus, and the right amygdala, areas involved in emotional arousal and emotion regulation that are reduced in people with mood disorders (Conklin 2007). Hirashima and colleagues (2004) demonstrated that patients who take Ω3 supplements evidence increased neuronal membrane fluidity, which is associated with improved brain functioning.
Additionally, decreased levels of Ω3 are associated with increased likelihood of inflammation by activation of pro-inflammatory cytokines such as interleukin-1, interleukin-2, interleukin-6, and tumor necrosis factor alpha (Maes et al. 1999). These inflammatory markers are associated with the immune pathophysiology of depression. Individuals who are low in Ω3 demonstrate higher stress-induced pro-inflammatory cytokines and report higher anxiety and perceived stress (Maes et al. 1998, 2000). These cytokines may interfere with serotonin synthesis, leading to dysregulation of serotonin neurotransmission, and may also lead to the production of anxiogenic and depressogenic tryptophan catabolites. Membrane fluidity and quaternary structure of membrane lipids and proteins may be changed as a result of Ω3 deficiency, which may contribute to further dysregulation of serotonin neurotransmission (Leonard and Maes 2012).
The neurological role of Ω3 has also been investigated in animal studies. Through direct measurement of Ω3 levels in various brain regions, investigators have shown that dopamine levels were reduced in the ventral striatum and in the prefrontal cortex in rats deficient in Ω3 relative to controls (Zimmer et al. 1999, 2002). Ω3 supplementation is hypothesized to raise dopamine transmission rates in these areas by increasing the numbers of dopamine storage vesicles and subsequent releases (Narendran et al. 2012). Improved dopamine neurotransmission in the ventral striatum and prefrontal cortex may thus improve attentional control and cognitive fluidity (Kehagia et al. 2010). With improvements in these areas of executive functioning, greater top-down emotion regulation is likely to confer greater protection from onset or exacerbation of depressive and manic symptoms (Drevets 1999).
Several biological pathways resulting from Ω3 depletion have been postulated to lead to mood disorder. Owen and colleagues (2008) posited four pathways that may link Ω3 depletion to depression. First, ratios of DHA to other fatty acids affect membrane fluidity and enzyme functioning, ion channels, and receptor binding. Second, Ω3 concentrations impact neuroplasticity and cell survival. Third, Ω3 concentrations affect gene expression for several fatty acid-mediated transcription factors essential to brain growth and synaptogenesis and responsible for production of cholesterol, bile acid, triacylglycerol, and glucocorticoids (Shahidi and Miraliakbari 2005). These first three pathways allow for healthy metabolic brain processes, such as neurotransmission, and development of functionally connected brain regions, which are often both disrupted when mood and other psychiatric disorders are present as mentioned earlier. Fourth, Ω3 fatty acids suppress production of pro-inflammatory cytokines (Tassoni et al. 2008), which are elevated in depressed patients and inhibited by some antidepressant medications. These pathways all offer possible points of intervention for mood disorders, as well as other psychiatric disorders such as anxiety and psychosis, by improving brain functioning and modifying inflammatory processes that co-occur with symptoms, via supplementation with Ω3.
Treatment of mood disorders
A meta-analysis of 10 studies that included 329 adults with mood disorders found a significant antidepressant effect of Ω3 within the overall sample (d=0.61, p=0.003), as well as in those with clearly defined depression (n=222, d=0.69, p=0.002) and with BD (n=105, d=0.69, p=0.0009) (Lin and Su 2007). Evidence for an EPA dose-response effect was found as well with increasing effect sizes for low dose (d=0.36), middle dose (d=0.79), and high dose (d=0.95) of EPA. Another meta-analysis of randomized controlled trials (RCTs) using Ω3 found small-to-moderate standardized mean effect sizes of −0.364 (p=0.025) for adults with BD and −0.551 (p=0.034) for depressive symptoms in adults with major depression. Adjunctive therapy with antidepressants (relative to Ω3 monotherapy) and higher proportions of EPA to DHA predicted greater therapeutic gain in independent meta-analyses (Martins 2009). A meta-analysis conducted by Appleton and colleagues (2010) found a small effect size of 0.10 (95% CI: 0.02, 0.17) favoring improvement in depressive symptoms with Ω3 supplementation. It is of note that participants with greater depressive severity and those meeting criteria for a depressive disorder demonstrated greater treatment gains. Other meta-analyses found significant small effect sizes for depressed mood states inclusive of bipolar depression but not for manic mood states (Kraguljac et al. 2009; Sarris et al. 2012). Many of these meta-analyses demonstrated great heterogeneity among reported effect sizes, suggesting potential moderators of effect or imprecision within the studies. These meta-analyses synthesized findings from reports of adult participants and not from youth. Efficacy of Ω3 supplementation in minimizing mood psychopathology in youth has not been investigated as extensively.
Only four studies of Ω3 treatment for juvenile-onset mood disorders have been published. Intervention length ranged from 6 to 16 weeks. The majority of participants in these studies demonstrated significant declines in depressive and manic symptoms as noted by clinicians on semistructured interviews or other clinical scales (Nemets et al. 2006; Wozniak et al. 2007; Clayton et al. 2009; Gracious et al. 2010). Clayton and colleagues (2009) conducted a 6 week open label (i.e., raters and patients were not blinded to study condition) trial of Ω3 in 18 youth with BD who were also concurrently taking a mood stabilizer. Erythrocyte levels of Ω3 increased significantly in participants postsupplementation. Clinician ratings of depression and mania decreased (p=0.002; p=0.004), whereas global functioning increased (p<0.001). Parent-rated internalizing and externalizing behaviors also decreased (p=0.009; p=0.014) (Clayton et al. 2009). Wozniak and colleagues (2007) completed an 8 week open label trial of Ω3 as monotherapy (with stimulants allowed) for 20 youth with BD. Participants received 1.3–4.3 g of Ω3 daily with 85% taking ≥2.0 g per day. After 8 weeks, manic symptoms decreased by 50% in 35% of participants. Depression ratings also decreased significantly (p=0.002). By study end, 40% of participants were rated much or very much improved for depression and mania. Nemets and colleagues (2006) examined Ω3 in 28 children with major depressive disorder. Participants were randomized to Ω3 or placebo for 16 weeks in a double-blind trial. Results in the 20 completers demonstrated highly significant effects of Ω3 on depressive symptoms: 70% of children who received Ω3 had >50% reduction in depressive symptoms compared with no children in the placebo group; 40% of children receiving Ω3 met remission criteria compared with no children in the placebo group. Most of the treatment response noted at 16 weeks had been achieved by week 12. Gracious and colleagues (2010) conducted a double-blind, RCT of alpha-linoleic acid (ALA) as flax seed oil in youth with bipolar I or II disorder. In this study, there was a high rate of noncompliance with taking study supplements because of the large number of capsules required (up to 12 per day). Those who took an adequate amount of ALA, and evidenced a significant change in ALA converted to serum EPA, demonstrated improved overall symptom severity relative to placebo group; however, depression and mania measures did not show significant differences between treatment and placebo groups. These findings, specifically from Gracious and colleagues (2010), suggest fish oil (containing EPA and DHA) as a more direct, potentially efficacious treatment than flax seed oil in treating BD, given that not having to convert ALA likely leads to faster and more pronounced symptom improvement (Gerster 1998).
Treatment/prevention of psychosis
Ω3 has been demonstrated to prevent onset of psychosis in a cohort of high-risk adolescents (Amminger et al. 2010). In this study, 4.9% of participants supplemented with Ω3 developed a full psychosis compared with 27.5% in the placebo group at 1 year follow-up (p=0.007). The time to noticeable effect of Ω3 relative to placebo was investigated in a post-hoc analysis. Significant improvement in positive symptoms was noted after 8 weeks of supplementation and significant improvement in negative symptoms and global functioning was noted after 12 weeks (Mossaheb et al. 2013).
Treatment of anxiety
Research examining the effect of Ω3 on anxiety disorders is limited. In one study of healthy volunteers, Ω3 reduced plasma noradrenaline (norepinephrine) levels following 8 weeks of supplementation with 400 mg EPA and 300 mg DHA per day, which is associated with decreased activation of the hypothalamic–pituitary–adrenal (HPA) axis (Hamazaki et al. 2005). These data suggested that Ω3 supplementation may lead to greater stability of the HPA axis that could result in lowered anxiety. Anxiety did decrease significantly with Ω3 supplementation relative to placebo in men with substance abuse (Buydens-Branchey et al. 2008) problems. Medical students randomized to Ω3 supplementation also demonstrated a 20% decrease in anxiety relative to a placebo group (Kiecolt-Glaser et al. 2011). For severe clinical anxiety disorders, Ω3 supplementation may not have much impact. Obsessive-compulsive disorder was not affected by Ω3 supplementation (Fux et al. 2004). Findings for posttraumatic stress disorder (PTSD) are mixed: one study reported that Ω3 supplementation worsened symptoms in 5 of 6 participants (Zeev et al. 2005) whereas another study of 11 adults reported that Ω3 supplementation may have had a protective effect in reducing risk of PTSD (Matsuoka et al. 2010). Most studies investigating Ω3 and anxiety have utilized small sample sizes and short treatment duration and, consequently, are underpowered. Further research is needed to more carefully examine the potential antianxiolytic potential of Ω3.
Summary
Ω3 appears to improve symptoms of mania, depression, psychosis, and, possibly, anxiety. Supplementation with EPA and DHA (relative to ALA) seems to be responsible for the greatest change in psychiatric symptoms. Additionally, EPA/DHA supplementation requires intake of fewer daily pills than ALA, which is likely more palatable to those taking the supplement and likely contributes to greater adherence to supplementation. As previously mentioned, Gracious and colleagues (2010) had high rates of nonadherence when using ALA supplementation, because of the high recommended daily pill intake. One shortcoming of the controlled trials reviewed was the short follow-up period, ranging from 6 to 16 weeks. The following case illustrates the impact over 2 years of adjunctive Ω3 in a child with BD type 1 with psychotic features and comorbid generalized anxiety disorder (GAD). It is unique in the detail provided regarding her clinical history and response to treatment, given her participation in an unrelated phenomenologic study.
Case Presentation
Course of illness
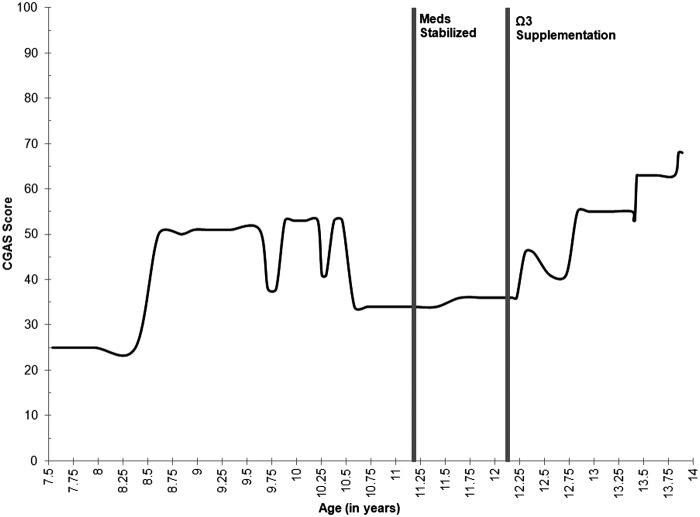
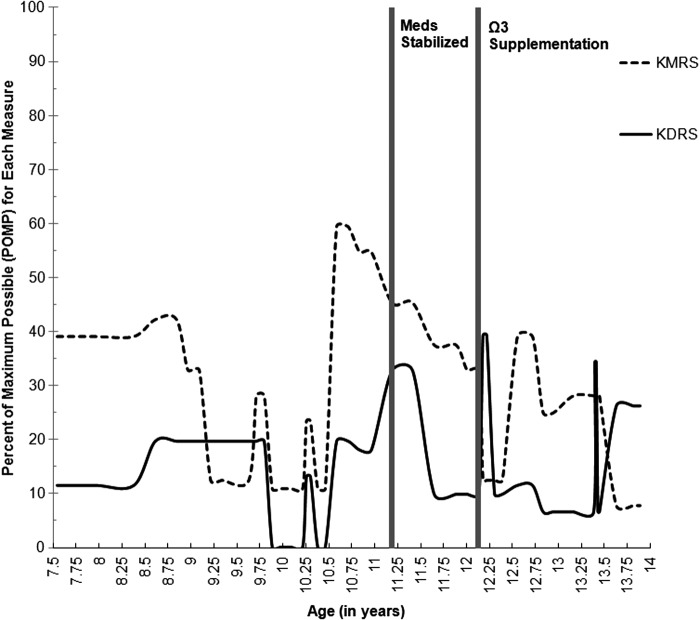
“Sarah” participated in 11 comprehensive assessments over 5 years, from age 8 to age 13, as part of the Longitudinal Assessment of Manic Symptoms (LAMS) research protocol (see Findling et al. 2010; Horwitz et al. 2010 for a full review of study procedures). Her overall functional capacity (as measured by the Children's Global Assessment Scale [CGAS]) throughout this interval is illustrated in Figure 1 (Lundh et al. 2010), and her depressive and manic severity (as measured by Kiddie Schedule for Affective Disorders and Schizophrenia Depression and Mania Rating Scales [KDRS and KMRS]) are illustrated in Figure 2. Jacobson and Truax (1991) outlined a statistical framework for determining a reliable change (RC) index. Using this methodology and the existing LAMS data set of children with elevated symptoms of mania (ESM) (see Findling et al. 2010), Sarah's RC index was determined to be 6.3, indicating a high degree of change when accounting for standard error of the CGAS (RC>1.96 indicates likelihood that posttest scores reflect true change). As was done for CGAS scores in Figure 1, RC indices for the KDRS and KMRS in Figure 2 were 3.0 and 1.0 respectively, indicating a high likelihood of true change for the KDRS but not for the KMRS. Statistically, Sarah demonstrated significant decline in depressive severity pre- to postsupplementation, with only marginal decrease in manic symptoms; however, as detailed in the subsequent clinical narrative, her symptoms of both depression and mania demonstrated notable clinically meaningful improvements.
FIG. 1.
Global functioning scores as measured by the Children's Global Assessment Scale (CGAS) across ages beginning with the onset of the index manic episode. CGAS is rated on a scale from 0 to 100, with higher scores indicating better overall functioning. Medications were last adjusted at age 11 years, 2 months and have been stable since. Ω-3 supplementation began at age 12 years, 2 months. These time points are noted in the figure. Lundh and colleagues (2010) found that interrater reliability was higher in trained experts (psychologists/psychiatrists) who arrive at these scores via consensus conference. For Sarah, these CGAS scores were derived from consensus conference rating between a licensed psychologist and a clinical psychology graduate student, both of whom have extensive experience with such rating scales.
FIG. 2.
Depressive and manic severity scores as measured by the Kiddie Schedule for Affective Disorders and Schizophrenia Depression and Mania Rating Scales (KDRS and KMRS respectively) by age, beginning with the onset of the index manic episode. Scores are presented as percent of maximum possible (POMP) to allow the measures to use similar metrics. Higher scores indicate worse symptom severity. Medications were last adjusted at age 11 years, 2 months and have been stable since. Ω3 supplementation began at age 12 years, 2 months. These intervals are noted in the figure.
At the baseline LAMS assessment, Sarah was diagnosed with BD type 1 with psychotic features, with onset at 7 years of age. At her age 10 assessment, Sarah additionally met diagnostic criteria for GAD. Over 5 years of follow-up, Sarah demonstrated three chronic manic episodes, with symptoms present most of every day for 6–11 months. Her index manic episode occurred at age 7 and lasted 10 months; her second mixed (simultaneous manic and major depressive) episode occurred at age 10 and lasted ∼11 months; her third manic episode occurred when she was 11 and lasted ∼6 months. During these episodes, Sarah's symptoms were characterized by expansive mood, pressured speech, increased goal-directed activities, and distractibility. Sarah would furiously complete artwork and take on other creative projects. If these projects were disrupted, she would become highly irritable and emotionally volatile. Sarah's mother reported that during one manic episode, Sarah planned an elaborate party with a meticulous schedule and began inviting classmates prior to discussing any details with her mother. Her mother noted that Sarah was “bouncing off the walls” during manic states and becoming focused on unproductive activities as if she were “in a hamster wheel.” Manic episodes often co-occurred with elevated psychotic symptoms that were transiently present at other times outside of mood episodes. Visual hallucinations included seeing brightly colored marbles that she “manipulated” with her fingers as well as seeing graphic images of herself and her family members being murdered. Auditory command hallucinations instructed her to harm others and “do bad things.” Persecutory delusions included thoughts that her family members, school personnel, and other students were plotting against her. During florid psychotic episodes, Sarah's irritability increased, she would become physically assaultive, and was “like a caged animal.” In one episode, Sarah described how she was morphing into Pegasus, and she could feel the hair growing from her skin. She also believed she was in a school theater production and tried to convince her mother this was true by demonstrating elaborate choreography.
Sarah had three significant major depressive episodes, often superimposed on manic symptoms. Sarah's first depressive episode occurred at age 9 and lasted for 2 months; her second depressive episode (during the course of a mixed episode) occurred at age 10 and continued for 11 months; her third depressive episode occurred at age 12 and lasted for 1 month. In addition, she consistently demonstrated subsyndromal depressive and manic symptoms, even when not in a mood episode, with protracted symptoms of negative self-esteem and depressive irritability. She experienced transient euthymic states (lasting for only a few weeks at a time) during which time she did not demonstrate active psychosis.
At age 10, with onset of a mixed mood episode, Sarah was hospitalized for 15 days because of increased suicidal and homicidal ideation, command auditory and visual hallucinations, hypersexuality (asking her sister to “hump” her and “do sexy stuff”), and somatic complaints (ruminating that her bones would break if others bumped into her). She believed she needed to be pushed in a wheelchair at the summer camp she attended prior to the admission. Hypersexual behavior persisted during hospitalization (asking other patients to engage in sexual behavior). She heard an “evil girl voice” commanding her to kill her immediate family members and to stab herself. At one point, while hospitalized, she attempted to stab herself with a pencil and attempted to break her own ankle by smashing it into a door.
In the year prior to her hospitalization, Sarah's mother noted that her anxiety symptoms began to “ramp up,” and believe that this was what contributed to her worsening mood. Her mother characterized this anxiety as excessive and “over the top.” Sarah would often complain of muscle tension and ask her mother for massages. She also seemed more “on edge” than usual, and her mother noted that her mind would “go blank” when having a conversation. The severity of Sarah's symptoms resulted in significant impairment in school, with peers, and at home. Her academic workload was modified commensurate with her ability to function (e.g., Sarah was excused from work if she was unable to concentrate, or was given easier or shorter assignments when her symptoms intensified). Notably, Sarah never met diagnostic criteria for attention-deficit/hyperactivity disorder (ADHD), although she demonstrated significant symptoms of inattention and impairment of executive functions because of her mood and anxiety symptoms. School functioning was particularly compromised when Sarah's paranoid delusions included believing that her school instructors were singling her out in class and that other students were plotting to make school life harder for her, including incessantly gossiping about her. Sarah's mother noted that the school was “working hard just to get her through.” Sarah was acutely sensitive to peer rejection, which caused her to become increasingly anxious about approaching others, and she would become easily irritated in social settings. Home life for Sarah and her family was often intolerable. Sarah's mother often considered placing Sarah in alternate care outside of their home because of her irritable and erratic moods and her associated functional impairment.
Sarah's manic symptoms were prototypical for most youth with BD. Her depressive symptoms were often present in a residual form, both between episodes of major depression and during manic episodes, leading to an overall mixed bipolar presentation. Sarah's mixed symptom presentation is frequent among other children with BD (Kraepelin 1921; Axelson et al. 2006; Duax et al. 2007; Algorta et al. 2011). Mixed presentations result in greater psychosocial impairment and can lead to an enhanced risk of suicide as a result of the coupling of dysphoric mood and hopelessness, with elevated energy and impulsivity (Algorta et al. 2011). Symptoms of psychosis occur in ∼20–35% of children with BD type 1 (Kowatch et al. 2005), placing Sarah in this higher severity category with respect to bipolar illness severity.
Treatment history
Given the severity of Sarah's symptoms and the persistence and resourcefulness of her mother, Sarah received consistent and extensive intervention. Her school provided numerous accommodations via an individualized education program (IEP). These included supplemental instruction, help with school projects, and in-school therapy sessions. Sarah also received community-based medication management and psychotherapy. This included weekly psychotherapy from a master's level social worker and multi-family psychoeducational psychotherapy (MF-PEP), a time-limited treatment program designed for children with mood disorders. Medication trials included aripiprazole, lithium, buspirone, quetiapine, lamotrigine, and oxcarbazepine in varying combinations and dosages. Sarah was sensitive to both the dosage and the timing of medications. Her daily medication regimen for the year prior to Ω3 augmentation included oxcarbazepine 1050 mg, buspirone 60 mg, quetiapine 200 mg, and lamotrigine 25 mg. No changes were made to these medications once Ω3 augmentation began, and no issues with compliance or barriers to obtaining medications were reported at any of Sarah's assessments.
Ω3 augmentation
Since the age of 12 years and 2 months, Sarah has taken Ω3 supplements, 1000 mg in the morning, containing 647 mg EPA, 253 mg DHA, and 1000 IU of cholecalciferol (vitamin D3); and 1500 mg in the evening containing 540 mg EPA and 360 mg DHA (with other fish oils) but without vitamin D3. It is of note that there was a higher concentration of EPA in the morning dose than in the evening dose; DHA was nearly equal in concentration in both the morning and evening doses. These supplements were obtained from the triple strength selections from a General Nutrition Center (GNC) store. Hogberg et al. (2012) recommended that depressed adolescents be supplemented with 2000–4000 IU of vitamin D3 in order to achieve an effect on depressive symptoms. Sarah's current supplements are below this recommended dosage; however, there is potential for an effect of vitamin D or an interactive effect between vitamin D3 and Ω3 on reported mood and/or anxiety symptom severity. These effects cannot be disentangled in the current analysis.
Effects of Ω3 augmentation
Sarah's mother noted significant improvement after ∼3 months of Sarah's taking Ω3. Emotional reactivity was dampened such that Sarah's relationships with family members were noted as being “average” with minor, transient conflicts. Sarah began to complete school assignments independently, although she still perceived schoolwork to be a struggle. After ∼8 months of Sarah's taking Ω3, her teachers gave her “glowing reports.” Sarah began taking greater initiative in completing school assignments and her social problem solving improved. Her manic and depressive symptoms did not change significantly at this time; however, her psychotic symptoms diminished greatly and no longer caused impairment. Additionally, at this assessment, her anxiety symptoms decreased such that she no longer met criteria for GAD. After 18 months, her manic symptoms decreased in severity and duration to twice weekly for 1–2 hours at a time. Positive interactions increased significantly, with Sarah and her mother laughing and talking with each other. Academically, Sarah is now functioning at a typical seventh grade level, and has required fewer academic accommodations. Although she still takes longer to understand concepts or complete projects than classmates, Sarah is demonstrating independent initiative in completing academic tasks. With respect to her scores on the Child and Adolescent Symptom Inventory, 4th ed. (CAASI-4), she demonstrated an ∼50% decrease on Category A (inattention/hyperactivity symptoms); these scores consistently ranged from 25 to 33 prior to supplementation and decreased postsupplementation, until most recently she scored a 14, providing quantitative evidence of her improved ability to focus attention. Peer relations also have improved markedly, with Sarah making prosocial efforts to help other students who are being bullied or who have special needs. Since beginning Ω3 supplementation, Sarah has not experienced any adverse effects, including gastrointestinal upset or fishy breath. Sarah's mother commented that for the first time in her life she understands “what other parents have described as being normal.”
Discussion
This case study illustrates the potential benefit that Ω3 might provide as an adjunct to psychotropic medication for treating bipolar disorder, even in the presence of anxiety and psychotic features. Notably, Sarah also received 1000 IU of vitamin D3, which may have contributed to symptom remission, although these effects cannot be parsed out for this case. Kaplan and colleagues (2007) have described the importance of considering the whole context in nutritional support of brain function, not just a single nutrient. It is possible that Ω3 may work best in the presence of adequate vitamin D and vice versa; therefore, interactive effects of these two nutrients cannot be ruled out. These assertions are currently speculative and require further research to understand such effects. Sarah's psychotropic medications have been taken at the same daily dosages beginning a year prior to Ω3 supplementation, remaining unchanged over the observation period. Although specific pathophysiological and executive functioning measures were not collected, attentional capacity and control improved by self-report and observation, followed by amelioration of Sarah's dysphoric, irritable mood (see Fig. 2). Findings from this case study are consistent with prior research findings in terms of the direction of symptom change with Ω3 supplementation. There are many possible mechanisms of action that can be considered for Sarah based on the extant literature on Ω3 and its supplementation.
The first possible explanation for Sarah's improvement may have been age related. In a study of youth with BD, it was demonstrated that small portions of variance (2–8%) for several manic symptoms (e.g., motor activity, aggression, thought content, racing thoughts) were significantly accounted for by age, with trends showing improvement of these symptoms with older age. However, age was a robust predictor of depressive symptom worsening in these youth (Demeter et al. 2013). In other studies, (hypo)mania is more common in younger age groups, whereas depression is a more common presentation in BD in middle adulthood and beyond (Kraepelin 1921; Judd et al. 2002). A decrease in manic symptoms may be the result of normative developmental improvements in neurocognitive functioning, such as inhibitory control, whereas a worsening in depressive symptoms may be the result of a greater likelihood of depressive episodes throughout adolescence as a result of psychosocial and biological/hormonal stressors. It is possible that Sarah's improvement in manic symptoms may have been age related, as she developed greater executive control capacity; however, this would not account for her improved depressive symptoms. It is also plausible that Sarah's improvements in mood symptoms were caused by the episodic nature of BD in general.
Second, Sarah's later improvements in mood and anxiety may have been in part the result of increased competence in tasks that were once difficult but had become easier as cognitive impairment lessened or increased with age, or both. Sarah demonstrated improved concentration and greater academic success, possibly because of improved working memory, which may be associated with increased dopamine neurotransmission. In human studies, performance on an n-back working memory task increased after 6 months of Ω3 supplementation in a sample of healthy, young adults; however, changes in vesicular monoamine transporter 2 (VMAT2) receptor densities in the striatum remained unchanged (Narendran et al. 2012). These results suggest that other mechanisms of dopamine transmission may be affected by Ω3 action, given the demonstrated positive effect of Ω3 on working memory and the nonsignificant effect on VMAT2 receptor density investigated in that study. Another study has shown that Ω3 supplementation improved working memory and reaction time in young adults whose diet was low in DHA (Stonehouse et al. 2013).
Third, reduction of depressive or anxious symptoms may have occurred as a result of improved physical health. Increased erythrocyte levels of Ω3 are associated with reduced bodily pain and with other physical health outcomes (Sinn et al. 2012). Prior to supplementation, Sarah experienced increased muscle tension such that she would ask her mother for nightly massages to alleviate perceived aches. With Ω3 supplementation, her somatic symptoms decreased significantly; her subjective complaints of anxiety and depression similarly diminished, consistent with the direct relationship reported between psychosomatic complaints and depressive/anxious symptoms (Chakraborty et al. 2010).
Fourth, many of Sarah's anxious ruminations and paranoid thoughts centered around social interactions and activities involving others. Decreased Ω3 fatty acid blood composition has been reported in individuals who suffer from clinically impairing social anxiety. Production of DHA is likely impeded by interactions with dysregulated neurotransmitters in these individuals (Green et al. 2006).
Fifth, Ω3 supplementation could have also been related to reducing stress-related immune dysfunction. Sarah experienced significant anxiety and depressive symptoms which in theory may have been associated with increased cytokine levels associated with oxidative metabolic stress. By introducing Ω3 supplementation, cytokine levels may have decreased and symptoms of depression and anxiety may have also decreased in response.
Finally, Sarah's improvement may have been the result of the vitamin D that she was taking during the course of Ω3 supplementation. Shaffer and colleagues (2014) conducted a meta-analytic review of RCTs investigating depression and vitamin D supplementation in adults. A medium effect size (d=0.60) demonstrated that vitamin D supplementation was beneficial for clinically depressed adults. Additionally, in a case series of depressed adolescents who were given vitamin D supplementation, there was a significant improvement in several symptoms including irritability, dysphoric mood, mood swings, and concentration (Hogberg et al. 2012). Oxcarbazepine, one of Sarah's medications, has the potential to contribute to vitamin D deficiency over time (Mintzer et al. 2006). This may have led to increased mood and psychotic symptoms, which may have been ameliorated with vitamin D supplementation (Fernandes et al. 2009; Gracious et al. 2012).
Conclusions
Sarah's improvement in mood, anxiety, and psychotic symptoms following Ω3 supplementation underscore the need for RCTs to evaluate the efficacy of Ω3 as monotherapy or adjunctive therapy in treating childhood bipolar and psychotic disorders. This case presentation is limited in that it does not include a control condition such as would be present in an ABA case design. However, the numerous assessments prior to supplementation provide the essentials of a multiple baseline design. Further intervention trials may provide greater support for Ω3 with or without vitamin D being included as a part of treatment for these disorders. Additionally, further work is needed to uncover the physiological mechanisms leading to symptom change, so that biomarkers may be considered as predictive of treatment response or as outcomes of treatment.
Clinical Significance
Ω3 supplementation is a safe and promising intervention for managing symptoms of mood disorder and comorbid anxiety. With supplementation, it may be possible that medication use can be maintained, without dose, type, or frequency changes, thus minimizing detrimental side effects that often occur with traditional pharmaceuticals. The current review and case report document the benefits of Ω3 supplementation for BD with co-occurring anxiety and psychotic features. The longitudinal tracking of this case over a 2 year period offers potential hope of accumulating and enduring benefit.
Disclosures
Mr. Anthony Vesco, Ms. Jennifer Lehmann, and Dr. Andrea S. Young have no financial disclosures. Dr. L. Eugene Arnold has received research funding from CureMark, Forest, Lilly, and Shire; advisory board honoraria from Biomarin, Novartis, Noven, Roche, Seaside Therapeutics, and Shire; consulting fees from Tris Pharma; and travel support from Noven. Dr. Barbara L. Gracious served as a consultant for Johnson and Johnson. Dr. Mary A. Fristad receives royalties from American Psychiatric Press, Inc., Child & Family Psychological Services, Inc., and Guilford Press, Inc.
References
- Algorta GP, Youngstrom EA, Frazier TW, Freeman AJ, Youngstrom JK, Findling RL: Suicidality in pediatric bipolar disorder: Predictor or outcome of family processes and mixed mood presentation? Bipolar Disord 13:76–86, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE: Long-chain {omega}-3 fatty acids for indicated prevention of psychotic disorders: A randomized, placebo-controlled trial. Arch Gen Psychiatry 67:146–154, 2010 [DOI] [PubMed] [Google Scholar]
- Appleton KM, Rogers PJ, Ness AR: Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr 91:757–770, 2010 [DOI] [PubMed] [Google Scholar]
- Axelson DA, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Bridge J, Keller M: Phenomenology of children and adolescents with bipolar spectrum disorders. Ach Gen Psychiatry 63:1139–1148, 2006 [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Bostic JQ, Prince J, Daly J, Wilens TE, Spencer T, Garcia-Jetton J, Russell R, Wozniak J, Faraone SV: The naturalistic course of pharmacologic treatment of children with manic like symptoms: A systematic chart review. J Clin Psychiatry 59:628–637, 1998 [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Hibbeln JR: Associations between increases in plasma n-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Prog Neuropsychopharmacol Biol Psychiatry 32:568–575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty K, Avasthi A, Kumar S, Grover S: Psychological and clinical correlates of functional somatic complaints in depression. Int J Soc Psychiatry 58:87–95, 2010 [DOI] [PubMed] [Google Scholar]
- Conklin SM: Dietary intake of the long-chain omega-3 fatty acids is associated with increased grey matter volume in the perigenual cingulate cortex. Poster session presented at the 65th Annual Scientific Meeting of the American Psychosomatic Society, Budapest, Hungary, March, 2007 [Google Scholar]
- Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL: Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutrit 63:1037–1040, 2009 [DOI] [PubMed] [Google Scholar]
- Demeter CA, Youngstrom EA, Carlson GA, Frazier TW, Rowles BM, Lingler J, McNamara NK, Difrancesco KE, Calabrese JR, Findling RL: Age differences in the phenomenology of pediatric bipolar disorder. J Affect Disord 147:295–303, 2013 [DOI] [PubMed] [Google Scholar]
- Drevets WC: Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci 877:614–637, 1999 [DOI] [PubMed] [Google Scholar]
- Duax JM, Youngstrom EA, Calabrese JR, Findling RL: Sex differences in pediatric bipolar disorder. J Clin Psychiatry 68:1565–1573, 2007 [DOI] [PubMed] [Google Scholar]
- Fernandes DA, Eyles D, Feron F: Vitamin D, a neuro-immunomodulator: Implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 34(Supp 1): S265–S277, 2009 [DOI] [PubMed] [Google Scholar]
- Findling RL, Nyilas M, Forbes RA, McQuade RD, Jin N, Iwamoto T, Ivanova S, Carson WH, Chang K. Acute treatment of pediatric bipolar I disorder, manic or mixed episode, with aripiprazole: A randomized, double-blind, placebo-controlled study. J Clin Psychiatry 70:1441–1451, 2009 [DOI] [PubMed] [Google Scholar]
- Findling RL, Youngstrom EA, Fristad MA, Birmaher B, Kowatch RA, Arnold LE, Frazier TW, Axelson D, Ryan N, Demeter CA, Gill MK, Fields B, Depew J, Kennedy SM, Marsh L, Rowles BM, McCue Horwitz S: Characteristics of children with elevated symptoms of mania: The Longitudinal Assessment of Manic Symptoms (LAMS) study. J Clin Psychiatry 71:1664–1672, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE, Marangell LB, Richardson AJ, Lake J, Stoll AL: Omega-3 fatty acids: Evidence basis for treatment and future research in psychiatry. J Clin Psychiatry 67:1954–1967, 2006 [DOI] [PubMed] [Google Scholar]
- Fux M, Benjamin J, Nemets B. A placebo-controlled cross-over trial of adjunctive EPA in OCD. J Psychiatr Res 38:323–325, 2004. [DOI] [PubMed] [Google Scholar]
- Geller B, Cooper TB, Sun K, Zimerman B, Frazier J, Williams M, Heath J: Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry 37:171–178, 1998 [DOI] [PubMed] [Google Scholar]
- Gerster H: Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int J Vitam Nutr Res 68:159–173, 1998 [PubMed] [Google Scholar]
- Gracious BL, Chirieac MC, Costescu S, Finucane TL, Youngstrom EA, Hibbeln JR: Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder. Bipolar Disord 12:142–154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracious BL, Finucane TL, Friedman-Campbell M, Messing S, Parkhurst MN: Vitamin D deficiency and psychotic features in mentally ill adolescents: A cross-sectional study. BMC Psychiatry 12:1–9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P, Hermesh H, Monselise A, Marom S, Presburger G, Weizman A: Red cell membrane omega-3 fatty acids are decreased in nondepressed patients with social anxiety disorder. Eur Neuropsychopharmacol 16:107–113, 2006 [DOI] [PubMed] [Google Scholar]
- Hamazaki K, Itomora M, Huan M, Nishizawa H, Sawazaki S, Masatoshi T, Watanabe S, Hamazaki T, Terasawa K, Yazawa K. Effect of omega-3 fatty acid-containing phospholipids on blood catecholamine concentration in healthy volunteers: A randomized, placebo-controlled, double-blind trial. Nutrition 21:705–710, 2005 [DOI] [PubMed] [Google Scholar]
- Hirashima F, Parrow AM, Stoll AL, Demopulos CM, Damico KE, Rohan ML, Eskesen JG, Zuo CS, Cohen BM, Renshaw PF: Omega-3 fatty acid treatment and T(2) whole brain relaxation times in bipolar disorder. Am J Psychiatry 161:1922–1924, 2004 [DOI] [PubMed] [Google Scholar]
- Hogberg G, Gustafsson SA, Hallstrom T, Klawitter B, Petersson M. Depressed adolescents in a case-series were low in vitamin D and depression was ameliorated by vitamin D supplementation. Acta Paediatr 101:779–783, 2012. [DOI] [PubMed] [Google Scholar]
- Horwitz SM, Demeter CA, Pagano ME, Younstrom EA, Fristad MA, Arnold LE, Birmaher B, Gill MK, Axelson D, Kowatch RA, Frazier TW, Findling RL: Longitudinal assessment of manic symptoms (LAMS) study: Background, design, and initial screening results. J Clin Psychiatry 71:1511–1517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson NS, Truax P: Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychology 59:12–19, 1991 [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, Leon AC, Rice JA, Keller MB: The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 59:530–537, 2002 [DOI] [PubMed] [Google Scholar]
- Kaplan BJ, Crawford SG, Field CJ, Simpson J, Steven A: Vitamins, minerals, and mood. Psychol Bull 133:747–760, 2007 [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW: Learning and cognitive flexibility: Frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol 20:199–204, 2010 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R: Omega-3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain Behav Immunity 25:1725–1734, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowatch RA, Suppes T, Carmody TJ, Bucci JP, Hume JH, Kromelis M, Emslie GJ, Weinberg WA, Rush AJ: Effect size of lithium, divalproex sodium, and carbamazepine in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 39:713–720, 2000 [DOI] [PubMed] [Google Scholar]
- Kowatch RA, Youngstrom EA, Danielyan A, Findling RL: Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disord 7:483–496, 2005 [DOI] [PubMed] [Google Scholar]
- Kraepelin E: Manic-Depressive Insanity and Paranoia. Edinburgh: Livingstone; 1921 [Google Scholar]
- Kraguljac NV, Montori VM, Pavuluri M, Chai HS, Wilson BS, Unal SS: Efficacy of omega-3 fatty acids in mood disorders – a systematic review and metaanalysis. Psychopharmacol Bull 42:39–54, 2009 [PubMed] [Google Scholar]
- Leonard B, Maes M: Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 36:764–785, 2012 [DOI] [PubMed] [Google Scholar]
- Lin P, Su K: A meta-analytic review of double-blind, placebo-controlled trails of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry 68:1056–1061, 2007 [DOI] [PubMed] [Google Scholar]
- Lundh A, Kowalski J, Sundberg CJ, Gumpert C, Landen M: Children's global assessment scale (CGAS) in a naturalistic clinical setting: Inter-rater reliability and comparison with expert ratings. Psychiatry Res 177:206–210, 2010 [DOI] [PubMed] [Google Scholar]
- Maes M, Christophe A, Bosmans E, Lin A, Neels H: In humans, serum polyunsaturated fatty acid levels predict the response of proinflammatory cytokines to psychologic stress. Biol Psychiatry 47:910–920, 2000 [DOI] [PubMed] [Google Scholar]
- Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY: Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res 85:275–291, 1999 [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, DeJong R, Van Gastrel A, Kenis G, Bosmans E, DeMeester I, Neels H, Janca A, Scharpe S, Smith RS: Immune and clinical correlates of psychological stress-induced production of interferon-γ and IL-10 in humans. In: Cytokines, Stress, and Immunity, edited by Plotnikoff N.P., Faith R.E., Murgo A.J., Good R.A. Boca Raton, FL: Raven Press, 1998. [Google Scholar]
- Martins JG: EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: Evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr 28:525–542, 2009 [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Nishi D, Yonemoto N, Hamazaki K, Hashimoto K, Hamazaki T: Omega-3 fatty acids for secondary prevention of post traumatic stress disorder after accidental injury: An open-label pilot study. J Clin Psychopharmacol 30:217–219, 2010 [DOI] [PubMed] [Google Scholar]
- Mintzer S, Boppana P, Toguri J, DeSantis A: Vitamin D levels and bone turnover in epilepsy patients taking carbamazepine or oxcarbazepine. Epilepsia 47:510–515, 2006 [DOI] [PubMed] [Google Scholar]
- Mossaheb N, Schafer MR, Schlogelhofer M, Klier CM, Cotton SM, McGorry PD, Amminger GP: Effect of omega-3 fatty acids for indicated prevention of young patients at risk for psychosis: When do they begin to be effective? Schizophr Res 148:163–167, 2013 [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Mason NS, Muldoon MF, Moghaddam B: Improved working memory but no effect on striatal vesicular monoamine transporter type 2 after omega-3 polyunsaturated fatty acid supplementation. PLos One 7:1–7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH: Omega-3 treatment of childhood depression: A controlled, double-blind pilot study. Am J Psychiatry 163:1098–1100, 2006 [DOI] [PubMed] [Google Scholar]
- Owen C, Rees A, Parker G: The role of fatty acids in the development and treatment of mood disorders. Curr Opin Psychiatry 21:19–24, 2008 [DOI] [PubMed] [Google Scholar]
- Sarris J, Mischoulon D, Schweitzer I: Omega-3 for bipolar disorder: Meta-analyses of use in mania and bipolar depression. J Clin Psychiatry 73:81–86, 2012 [DOI] [PubMed] [Google Scholar]
- Shaffer JA, Edmondson D, Wasson LT, Falzon L, Homma K, Ezeokoli N, Li P, Davidson KW: Vitamin D supplementation for depressive symptoms: A systematic review and meta-analysis of randomized controlled trials. Psychosom Med 76:190–196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F, Miraliakbari H. Omega-3 fatty acids in health and disease: Part 2 – health effects of omega-3 fatty acids in autoimmune diseases, mental health, and gene expression. J Med Food 8:133–148, 2005 [DOI] [PubMed] [Google Scholar]
- Simopoulos AP: Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 21:495–505, 2002 [DOI] [PubMed] [Google Scholar]
- Sinn N, Milte CM, Street SJ, Buckley JD, Coates AM, Petkov J, Howe PRC. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomized controlled trial. Br J Nutr 107:1682–1693, 2012 [DOI] [PubMed] [Google Scholar]
- Stonehouse W, Conlon CA, Podd J, Hill SR, Minihane AM, Haskell C, Kennedy D: DHA Supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am J Clin Nutr 97:1134–1143, 2013 [DOI] [PubMed] [Google Scholar]
- Tassoni D, Kaur G, Weisinger RS, Sinclair AJ: The role of eicosanoids in the brain. Asia Pac J Clin Nutr 17(S1):220–228, 2008 [PubMed] [Google Scholar]
- Wagner KD, Weller EB, Carlson GA, Sachs G, Biederman J, Frazier JA, Wozniak P, Tracy K, Weller RA, Bowden C: An open-label trial of divalproex in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 41:1224–1230, 2002 [DOI] [PubMed] [Google Scholar]
- Wozniak J, Biederman J, Mick E, Waxmonsky J, Hantsoo L, Best C, Cluette-Brown JE, Laposata M: Omega-3 fatty acid monotherapy for pediatric bipolar disorder: A prospective open-label trial. Eur Neuropsychopharmacology 17:440–447, 2007 [DOI] [PubMed] [Google Scholar]
- Zeev K, Michael M, Ram K, Hagit C: Possible deleterious effects of adjunctive omega-3 fatty acids in post-traumatic stress disorder patients. Neuropsychiatr Dis Treat 1:187–190, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer L, Durand G, Guilloteau D, Chalon S: n-3 polyunsaturated fatty acid deficiency and dopamine metabolism in the rat frontal cortex. Lipids 34 (Suppl):S251, 1999 [DOI] [PubMed] [Google Scholar]
- Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, Durand G, Chalon S. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am J Clin Nutr 75:662–667, 2002. [DOI] [PubMed] [Google Scholar]