Abstract
Chronic hepatitis C infection frequently coexists with human immunodeficiency virus (HIV) and together are associated with increased hepatic steatosis. Steatosis is a risk factor for progression of liver disease and may persist despite a sustained virologic response to hepatitis C treatment. Therefore, therapies to target hepatic steatosis are important for individuals with HIV and hepatitis C virus (HCV) coinfection. We completed a 48-week, randomized, double-blind, placebo-controlled trial of pioglitazone (45 mg/day) in 13 subjects with HIV/HCV coinfection. The primary outcome variable was hepatic fat content, measured by magnetic resonance spectroscopy (MRS) imaging. Individuals randomized to pioglitazone had a significant decrease in hepatic fat content measured by MRS from baseline (15.1 ± 7.0%) to week 48 (7.6 ± 3.9%), with a mean difference of −7.4% (p = 0.02, n = 5). There was no significant change in hepatic fat content with placebo. Glycemic control as measured by oral glucose challenge improved significantly with pioglitazone (p = 0.047). Though not statistically significant, there were trends toward improved alanine aminotransferase (ALT) and histopathologic grade of steatosis in subjects who received pioglitazone. Pioglitazone was well tolerated and no one discontinued due to side effects. This study demonstrates that 48 weeks of pioglitazone therapy, and not placebo, results in significant reductions in hepatic fat content as measured by MRS in subjects with HIV and HCV coinfection and hepatic steatosis. This small study shows that pioglitazone helps ameliorate steatosis in the context of HIV/HCV coinfection.
Introduction
Following the introduction of effective antiretroviral therapy for HIV, the management of comorbidities such as hepatitis C virus (HCV) has taken on increasing significance in the care and health maintenance of chronically infected patients.1 HCV coinfection is common in HIV with an estimated prevalence of 30% among HIV-infected adults in the United States.2 Furthermore, the reported prevalence of hepatic steatosis in HIV/HCV coinfection is between 40% and 67%.3–6
The presence of hepatic steatosis has important clinical implications in chronic HCV infection. In one report, steatosis is associated with the development of hepatocellular carcinoma (HCC), even in patients who showed a sustained virologic response (SVR) following interferon-based therapy for HCV.7 Several trials have demonstrated lower rates of SVR in HCV-infected patients with concomitant hepatic steatosis.8–10 Additionally, Kumar et al.11 found that the degree of steatosis did not change after achievement of SVR in those with Genotype 1 HCV infection. Together, these observations indicate that therapies to target steatosis remain important even with the availability of newer therapies for chronic HCV infection.
The thiazolidinedione pioglitazone, which was originally developed to treat diabetes, is a peroxisome proliferator-activated receptor-gamma (PPARγ) agonist that has been shown to be beneficial in treating hepatic steatosis in other populations. In patients with nonalcoholic steatohepatitis (NASH), pioglitazone led to significant reductions in hepatic steatosis and inflammation, and in some cases to improvements in fibrosis.12–15 Therefore, we conducted a 48-week, double-blind, randomized placebo-controlled pilot trial of pioglitazone (45 mg/day) in order to determine the potential benefit of pioglitazone on hepatic steatosis in patients coinfected with HIV and HCV.
Materials and Methods
Subjects
A total of 38 HIV/HCV-coinfected men and women were screened to determine eligibility between February 2009 and January 2011 at the National Institutes of Health (NIH) and Veterans Affairs Medical Center (VAMC) of Washington DC. Eligibility requirements included previously documented HIV and HCV infection; liver proton magnetic resonance spectroscopy (MRS) hepatic fat content >5% and confirmed steatosis on biopsy; no changes in antiretroviral therapy in the past 3 months; and no evidence of cirrhosis. Patients were excluded if they were considering initiation of HCV therapy within the coming year, had a fasting glucose level greater than 7.0 mmol/liter (>126 mg/dl); liver aminotransferase levels >4 times the upper limit of normal (ULN); hemoglobin level less than 9 g/dl; active drug or alcohol abuse; pregnancy; or any contraindications to MRI or liver biopsy. Of the 38 participants screened, 13 were found to be eligible and continued to randomization; among the 25 ineligible patients, 18 did not have evidence of steatosis, one had diabetes, one could not tolerate MRS, one declined a liver biopsy, one had ALT >4 times ULN, one had low hemoglobin, and two did not have evidence of chronic HCV infection. Patients were also characterized for the presence of the PNPLA3 allele (rs738409, minor allele G) that is associated with nonalcoholic steatohepatitis.16 Patients provided informed written consent, and the Institutional Review Boards at the NIH and the VAMC approved the study protocol. In January 2011, the study was closed prior to full enrollment due to slow accrual. All patients enrolled on study at that time were informed of the decision to close to enrollment and each opted to continue on study. The study was registered with Clinical Trials.gov: NCT00742326.
Design
The study design was a 48-week, double-blind, placebo-controlled trial of pioglitazone (45 mg/day) versus placebo in HIV/HCV-coinfected adults. Baseline and end of treatment assessments included liver MRS, liver biopsy, and a 120-min 75 g Oral Glucose Tolerance Test (OGTT). After baseline assessment, patients were randomly assigned to treatment stratified by age (<50 years vs. ≥50 years) and sex. A member of the NIH Clinical Center Pharmacy prepared and labeled the pioglitazone and placebo in identical capsules. Study drug was supplied by Takeda Pharmaceuticals, which had no role in the collection, analysis, or interpretation of the data, or in the decision to submit the manuscript for publication. The research physicians, nurses, and patients were blinded to treatment assignment during the entire study.
Participants were seen at week 2, week 8, and every 8 weeks thereafter until week 48 for assessment of side effects, medication adherence, and safety laboratoriess.
An MRS of the liver was performed using a 3T whole-body Philips scanner at baseline, week 24, and week 48. The technique used for quantification of hepatic fat content was validated in a previous publication, where it is described in detail.17 Liver biopsies were performed at the NIH Clinical Center using ultrasound guidance. The samples were scored by a single pathologist (D.E.K.) using a modified Ishak scoring system to assess inflammation and fibrosis18 and a previously standardized and validated grading system for steatosis.19 The pathologist was not aware of treatment assignment or MRS findings.
Statistical analysis
All between-group comparisons were made using nonparametric Wilcoxon rank sum tests or chi-square statistics where indicated. The primary outcome variable was hepatic fat content (measured by MRS imaging), and the effect of treatment (pioglitazone vs. placebo) was assessed at 48 weeks using within group paired t-test, comparing week 48 measures to baseline measures. The original design estimated 90% power to detect a 9% difference in change in hepatic fat content with data from 45 subjects. Despite limited enrollment, a Wilcoxon rank sum test comparing change in hepatic fat content between treatment groups, as originally planned for primary endpoint analysis, was performed and the results of this analysis are also reported. All values are presented as means (±SD), unless otherwise indicated. Statistical analysis was performed using SAS JMP version 9.0 (SAS Institute, Cary, NC).
Results
The demographic and baseline clinical characteristics of the 13 participants who entered the randomized portion of the study are summarized in Table 1. All subjects had HCV genotype 1. No subject reported taking a vitamin E supplement during the course of the study. With respect to HIV therapy, two subjects were not currently on antiretroviral therapy, 10 were receiving tenofovir–emtricitabine, and one zidovudine–lamivudine; four were on an nonnucleoside reverse transcriptase inhibitor (NNRTI) and seven on a protease inhibitor (PI)-containing regimen. There was no significant difference between subjects randomized to pioglitazone and placebo on any of the baseline characteristics or measurements except for hemoglobin; subjects randomized to pioglitazone had a higher hemoglobin concentration at baseline compared to placebo (pioglitazone 14 ± 1 g/dl vs. placebo 13 ± 1 g/dl, p = 0.03). Twelve subjects completed 48 weeks of double-blind randomized treatment; one subject was discontinued after 7 months of study drug due to development of renal insufficiency and a subsequent diagnosis of adenocarcinoma of the lung. In addition, one subject could not complete a follow-up MRI due to anxiety. Therefore, 11 subjects have pretreatment and posttreatment MRS data for analysis of the primary endpoint. Adherence data on both cohorts were collected at each study visit from pill counts. The mean study medication adherence was greater than 90% at each study visit for both placebo and pioglitazone, based on pill count.
Table 1.
Baseline Characteristics
| Pioglitazone (n = 6) | Placebo (n = 7) | p-value | |
|---|---|---|---|
| Age (years) | 52 (5) | 53 (6) | 0.72 |
| Gender (n): male/ female | 5/1 | 4/3 | 0.30 |
| Hispanic or Latino ethnicity | 0 | 1 | 0.25 |
| Race (n): black/white | 6/0 | 6/1 | 0.25 |
| BMI (kg/m2) | 29.2 (3.1) | 30.5 (5.0) | 0.52 |
| Systolic blood pressure (mm Hg) | 130 (14) | 129 (19) | 1.0 |
| Diastolic blood pressure (mm Hg) | 77 (8) | 77 (9) | 0.89 |
| Total CD4 count (cells/ml) | 485 (108) | 667 (221) | 0.18 |
| HIV viral load below detection limit (n) | 6 (0 viremic) | 5 (2 viremic) | 0.10 |
| Hepatitis C virus (IU/ml) | 11.5 × 106 (15.7 × 106) | 21.7 × 106 (27.9 × 106) | 0.52 |
| Hepatitis C genotype 1a/1b (n) | 3/3 | 4/3 | 0.79 |
| ALT (IU/liter) | 58 (20) | 53 (27) | 0.57 |
| AST (IU/liter) | 48 (26) | 43 (17) | 1.00 |
| Alcohol intake (g/day) | 1.6 (3.8) | 9.4 (22.8) | 0.68 |
| MRS hepatic fat estimate (%) | 15.5 (6.4) | 15.5 (9.0) | 0.94 |
| Fasting glucose (mg/dl) | 96 (14) | 89 (6) | 0.43 |
| Fasting insulin (IU/ml) | 64.2 (82.8) | 21.0 (11.3) | 0.13 |
| Total cholesterol (mg/dl) | 142 (25) | 151 (23) | 0.61 |
| Triglyceride (mg/dl) | 133 (55) | 165 (80) | 0.72 |
| LDL cholesterol (mg/dl) | 66 (14) | 81 (19) | 0.13 |
| HDL cholesterol (mg/dl) | 48 (11) | 41 (10) | 0.28 |
| Hemoglobin (g/dl) | 14 (1) | 13 (1) | 0.03 |
Values represent mean (standard deviation) unless otherwise indicated.
BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; MRS, magnetic resonance spectroscopy.
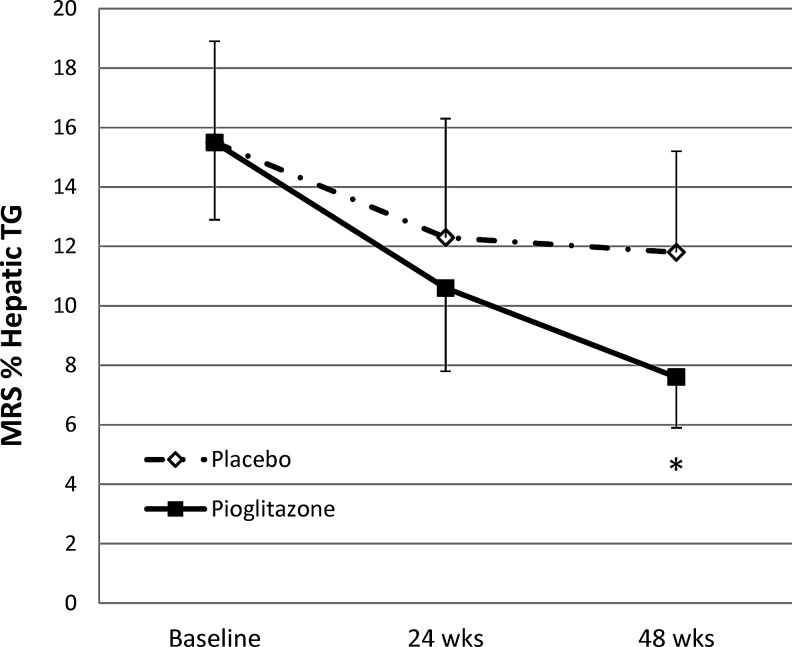
Individuals randomized to pioglitazone had a significant decrease in hepatic fat content measured by MRS between baseline (15.1 ± 7.0%) and week 48 (7.6 ± 3.9%), with a mean difference of −7.4 ± 4.6% (p = 0.02, n = 5). There was no significant change in hepatic fat content with placebo (baseline 14.0 ± 8.8% vs. 48 weeks 11.8 ± 8.3%; mean difference of −2.17 ± 9.9% p = 0.6, n = 6) (Table 2 and Fig. 1). The between group comparison of the change in hepatic fat content at 48 weeks was not statistically significant (p = 0.2). There was a significant reduction in glucose area under the curve (AUC) during OGTT in the group treated with pioglitazone, and a trend toward a significant reduction in alanine aminotransferase (ALT) after 48 weeks of pioglitazone (Table 2). There was a nonsignificant reduction in hemoglobin and nonsignificant increases in total cholesterol, LDL cholesterol, and body mass index (BMI) in subjects who received pioglitazone. There was no difference in body weight, waist-to-hip ratio, or daily caloric or alcohol intake from 3 day food records at baseline or 48 weeks between groups (data not shown).
Table 2.
Within Group Comparisons from Baseline to Week 48 for Pioglitazone and Placebo
| Pioglitazone (n = 6) | Placebo (n = 6)a | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 48 | Mean change | p-value | Baseline | Week 48 | Mean change | p-value | |
| Hepatic triglyceride content (%) | 15.1 (7.0) n = 5b | 7.6 (3.9) n = 5b | −7.4 | 0.02 | 14.0 (8.8) | 11.8 (8.3) | −2.1 | 0.6 |
| ALT (IU/liter) | 58 (20) | 49 (19) | −9.3 | 0.056 | 54 (30) | 58 (29) | +3.5 | 0.3 |
| Fasting glucose (mg/dl) | 96 (14) | 92 (4) | −4.0 | 0.4 | 90 (7) | 92 (10) | +2.0 | 0.6 |
| Glucose AUC (mg/dl/120 min) | 300 (53) | 269 (37) | −31 | 0.047 | 264 (51) | 275 (54) | +11 | 0.6 |
| HOMA-IR | 16.4 (22.2) | 7.7 (10.0) | −8.6 | 0.2 | 5.0 (2.9) | 5.2 (3.0) | +0.2 | 0.9 |
| Hemoglobin (g/dl) | 14 (1) | 14 (2) | −0.6 | 0.4 | 13 (1) | 13 (1) | −0.2 | 0.5 |
| BMI (kg/m2) | 29.2 (3.3) | 29.5 (3.1) | +0.4 | 0.4 | 31.8 (4.1) | 31.3 (3.3) | −0.5 | 0.4 |
| Total cholesterol (mg/dl) | 141 (25) | 154 (33) | +13 | 0.3 | 155 (22) | 157 (26) | +2 | 0.9 |
| LDL cholesterol (mg/dl) | 67 (16) n = 5 | 77 (28) n = 5 | +10 | 0.4 | 86 (15) | 94 (26) | +8 | 0.4 |
| Ishak total inflammationc | 5.8 (3.1) | 7.2 (4.1) | +1.4 | 0.1 | 8.0 (1.1) | 7.5 (1.6) | −0.5 | 0.4 |
| Ishak fibrosis stagec | 1.2 (0.4) | 1.6 (1.1) | +0.4 | 0.2 | 2.0 (1.7) | 1.5 (1.5) | −0.5 | 0.4 |
One subject assigned to placebo discontinued due to unrelated illness prior to week 48, had no week 48 values for comparison, and therefore is not included here.
One subject developed claustrophobia and could not complete a follow-up MRI, therefore only the five subjects with baseline and follow-up MRI data are included.
One subject randomized to pioglitazone did not have a week 48 liver biopsy due to an abnormal PT/PTT.
Values represent mean (standard deviation) unless otherwise indicated.
FIG. 1.
Effects of pioglitazone (n = 6) versus placebo (n = 7) on hepatic triglyceride (TG) content by MR spectroscopy. Within group comparison of baseline to week 48 is significant for pioglitazone (*p = 0.02), but not placebo (p = 0.6). Between group comparison of change in hepatic TG at 48 weeks is not significant (p = 0.2).
Pretreatment and 48 week posttreatment liver biopsy data were available on six subjects randomized to placebo and five subjects randomized to pioglitazone. Of those who received placebo, 0/6 had a reduction in steatosis grading whereas 2/5 on pioglitazone showed histologic improvement in steatosis (p = 0.05). There were no statistically significant changes in mean total Ishak inflammation or fibrosis histopathology scores between baseline and week 48 in either treatment group (Table 2). Two subjects randomized to placebo and three randomized to pioglitazone were heterozygous for the rs738409 G allele, the PNPLA3 genotype associated with hepatic steatosis. However, there was no difference in baseline hepatic fat content, histology scoring, or response to therapy between those with and those without the rs738409 G allele.
In general, study medication was well tolerated and no subject discontinued participation due to side effects. Common side effects and laboratory abnormalities are summarized in Table 3. As previously noted, one subject who was randomized to placebo developed renal insufficiency, believed to be related to their HIV-specific medications, and was later found to have advanced stage lung cancer and was withdrawn from further participation or follow-up.
Table 3.
Side Effects and Serious Adverse Event Summary Through Week 48
| Total (n = 13) | Pioglitazone (n = 6) | Placebo (n = 7) | |
|---|---|---|---|
| Description | |||
| Fatigue | 3 | 1 | 2 |
| Weight gain | 3 | 2 | 1 |
| Dizziness | 1 | 0 | 1 |
| Headache | 1 | 0 | 1 |
| Insomnia | 1 | 0 | 1 |
| Abdominal pain | 1 | 1 | 0 |
| Diarrhea | 1 | 0 | 1 |
| Sore throat | 1 | 0 | 1 |
| Weight loss | 1 | 0 | 1 |
| Conjunctivitis | 1 | 1 | 0 |
| Polydypsia | 1 | 0 | 1 |
| Laboratory abnormalities | |||
| Decreased serum albumin | 5 | 2 | 3 |
| Increased cholesterol | 3 | 1 | 2 |
| Hematuriaa | 1 | 0 | 1 |
| Elevated bilirubin | 1 | 0 | 1 |
| Proteinuria | 1 | 1 | 0 |
| Glycosuria | 1 | 1 | 0 |
| Serious adverse events | |||
| Dental abscess | 1 | 0 | 1 |
| Renal insufficiency | 1 | 0 | 1 |
| Adenocarcinoma, lung | 1 | 0 | 1 |
Subject diagnosed with renal calculi after presentation with hematuria.
Table includes all symptoms and laboratory abnormalities of grade 1 or higher identified as probably not, possibly, probably, and/or definitely related to study participation.
Discussion
This study demonstrates that 48 weeks of pioglitazone therapy results in significant reductions in hepatic fat content as measured by MRS in patients with HIV and HCV coinfection with hepatic steatosis. Pioglitazone therapy was also associated with improvements in glucose homeostasis and a trend toward improvements in liver transaminase levels, despite ongoing HCV infection. In general, pioglitazone was well tolerated and there was no apparent increase in side effects or laboratory abnormalities with pioglitazone compared to placebo during the 48 weeks of treatment.
There is increasing evidence that peroxisome PPARγ is an important modulator of steatohepatitis20 and may also modulate adipose tissue inflammation and resulting insulin resistance.21 A meta-analysis of four of the larger randomized placebo-controlled trials that used pioglitazone in NASH found that pioglitazone led to significant improvements in histopathologic parameters of steatosis and inflammation compared to placebo, but benefits with respect to fibrosis were not consistently identified.22 In a randomized double-blind placebo-controlled trial of men with HIV-associated lipodystrophy, Yki-Jarvinen et al.23 showed that the PPAR-γ agonist rosiglitazone (8 mg/day for 6 months) resulted in significant reductions in liver fat content, alanine aminotransferase, and fasting insulin levels. Despite the potential difference in the etiology of hepatic steatosis in the setting of HIV/HCV coinfection, we were able to identify improvements in hepatic steatosis similar to those seen in these earlier trials. Furthermore, Marks and colleagues also showed that 24 weeks of pioglitazone improved transaminase levels and metabolic parameters in HIV/HCV-coinfected patients seeking retreatment for HCV.24 We noted similar trends in improved ALT and glucose homeostasis with 48 weeks of pioglitazone treatment.
In an earlier investigation into the mechanisms of HIV-associated lipodystrophy, Lemoine et al.25 identified increased hepatic sterol regulatory element binding proteins (SREBP-1) expression in patients with steatosis and marked decreased expression of PPARγ1 and 2 in patients with hepatic fibrosis. In a rat model of NASH, hepatic expression of adiponectin mRNA and AMP-activated protein kinase protein was significantly increased following administration of pioglitazone and thereby inhibited hepatic stellate cells.26 In this model pioglitazone use was accompanied by reductions in hepatic steatosis and fibrosis. In the current study we did not find significant improvements in fibrosis. The inability to detect a change may be due to the very small sample of subjects with prehistology and posthistology data and to the relatively minimal fibrosis staging at baseline (stage 2 or lower in all but two subjects) or the continued exposure to HCV.
The recent development of novel, potent, and effective therapies to treat HCV has the field poised to enter a new era where the majority of chronic HCV-infected patients will be expected to achieve an SVR with interferon-sparing regimens of direct acting agents (DAAs). The focus of liver disease for HIV/HCV-coinfected patients will also shift to include evaluation and management of cirrhosis, fibrosis, and steatosis after HCV clearance.27 While SVR will convey significant benefits in morbidity and mortality, data from interferon-treated patients suggest that many will have persistent steatosis as well as ongoing increased risk for NASH and HCC, even after viral clearance.7,11 Therefore, identification of effective therapies to address hepatic steatosis in the context of HIV/HCV coinfection remains crucial and worthy of investigation.
The approval of HCV protease inhibitors and ongoing clinical investigations of interferon-free regimens that exclusively utilize DAAs contributed to difficulties in enrolling the originally targeted sample size. Despite the low enrollment, the study showed a significant result on the primary endpoint of reduction in hepatic triglyceride content as measured by MRS. The number of subjects completing the study is a limitation and a follow-up study with a larger number of subjects would be needed to confirm these observations. The mechanism for hepatic steatosis in this population is likely multifactorial and includes viral effects of HIV, HCV, and metabolic factors. Though not statistically different, higher lipid levels and alcohol intake in the group randomized to placebo may have influenced our findings. Furthermore, postmarketing findings have raised concern regarding the increased risk of bladder cancer with long-term exposure to pioglitazone, which must be taken into consideration. However, this small pilot study demonstrates that pioglitazone helps ameliorate steatosis in this setting, and may benefit patients with HIV/HCV coinfection.
Acknowledgments
The study team would like to thank the nursing staff in the NIAID and VAMC outpatient clinics for their ongoing dedication to patient care and the participants in this study for their time and contributions to this investigation.
This work was supported by resources from the National Institute of Allergy and Infectious Diseases Intramural Program through the National Institutes of Health, and the Washington DC VA Medical Center. This project was also funded in part by federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E. Takeda Pharmaceuticals U.S.A., Inc. provided study medication for the trial. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Jain MK, Skiest DJ, Cloud JW, et al. : Changes in mortality related to human immunodeficiency virus infection: Comparative analysis of inpatient deaths in 1995 and in 1999–2000. Clin Infect Dis 2003;36(8):1030–1038 [DOI] [PubMed] [Google Scholar]
- 2.Sulkowski MS, Mast EE, Seeff LB, and Thomas DL: Hepatitis C virus infection as an opportunistic disease in persons infected with human immunodeficiency virus. Clin Infect Dis 2000;30(Suppl 1):S77–84 [DOI] [PubMed] [Google Scholar]
- 3.Sulkowski MS, Mehta SH, Torbenson M, et al. : Hepatic steatosis and antiretroviral drug use among adults coinfected with HIV and hepatitis C virus. AIDS 2005;19(6):585–592 [DOI] [PubMed] [Google Scholar]
- 4.Marks KM, Petrovic LM, Talal AH, et al. : Histological findings and clinical characteristics associated with hepatic steatosis in patients coinfected with HIV and hepatitis C virus. J Infect Dis 2005;192(11):1943–1949 [DOI] [PubMed] [Google Scholar]
- 5.Bani-Sadr F, Carrat F, Bedossa P, et al. : Hepatic steatosis in HIV-HCV coinfected patients: Analysis of risk factors. AIDS 2006;20(4):525–531 [DOI] [PubMed] [Google Scholar]
- 6.Castera L, Loko MA, Le Bail B, et al. : Hepatic steatosis in HIV-HCV coinfected patients in France: Comparison with HCV monoinfected patients matched for body mass index and HCV genotype. Aliment Pharm Ther 2007;26(11–12):1489–1498 [DOI] [PubMed] [Google Scholar]
- 7.Tanaka A, Uegaki S, Kurihara H, et al. : Hepatic steatosis as a possible risk factor for the development of hepatocellular carcinoma after eradication of hepatitis C virus with antiviral therapy in patients with chronic hepatitis C. World J Gastroenterol 2007;13(39):5180–5187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patton HM, Patel K, Behling C, et al. : The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol 2004;40(3):484–490 [DOI] [PubMed] [Google Scholar]
- 9.Poynard T, Ratziu V, McHutchison J, et al. : Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology 2003;38(1):75–85 [DOI] [PubMed] [Google Scholar]
- 10.Lok AS, Everhart JE, Chung RT, et al. : Hepatic steatosis in hepatitis C: Comparison of diabetic and nondiabetic patients in the hepatitis C antiviral long-term treatment against cirrhosis trial. Clin Gastroenterol H 2007;5(2):245–254 [DOI] [PubMed] [Google Scholar]
- 11.Kumar D, Farrell GC, Fung C, and George J: Hepatitis C virus genotype 3 is cytopathic to hepatocytes: Reversal of hepatic steatosis after sustained therapeutic response. Hepatology 2002;36(5):1266–1272 [DOI] [PubMed] [Google Scholar]
- 12.Promrat K, Lutchman G, Uwaifo GI, et al. : A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 2004;39(1):188–196 [DOI] [PubMed] [Google Scholar]
- 13.Bajaj M, Suraamornkul S, Pratipanawatr T, et al. : Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes 2003;52(6):1364–1370 [DOI] [PubMed] [Google Scholar]
- 14.Belfort R, Harrison SA, Brown K, et al. : A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355(22):2297–2307 [DOI] [PubMed] [Google Scholar]
- 15.Sanyal AJ, Chalasani N, Kowdley KV, et al. : Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362(18):1675–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotman Y, Koh C, Zmuda JM, et al. : The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology 2010;52(3):894–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgoff P, Thomasson D, Louie A, et al. : Hydrogen-1 MR spectroscopy for measurement and diagnosis of hepatic steatosis. Am J Roentgenol 2012;199(1):2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishak K, Baptista A, Bianchi L, et al. : Histological grading and staging of chronic hepatitis. J Hepatol 1995;22(6):696–699 [DOI] [PubMed] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van Natta M, et al. : Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41(6):1313–1321 [DOI] [PubMed] [Google Scholar]
- 20.Nan YM, Han F, Kong LB, et al. : Adenovirus-mediated peroxisome proliferator activated receptor gamma overexpression prevents nutritional fibrotic steatohepatitis in mice. Scand J Gastroenterol 2011;46(3):358–369 [DOI] [PubMed] [Google Scholar]
- 21.Cipolletta D, Feuerer M, Li A, et al. : PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012;486(7404):549–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boettcher E, Csako G, Pucino F, et al. : Meta-analysis: Pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharm Ther 2012;35(1):66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yki-Jarvinen H, Sutinen J, Silveira A, et al. : Regulation of plasma PAI-1 concentrations in HAART-associated lipodystrophy during rosiglitazone therapy. Arterioscl Throm Vasc 2003;23(4):688–694 [DOI] [PubMed] [Google Scholar]
- 24.Marks KM, Kitch D, Chung RT, et al. : Pilot study of pioglitazone before HCV retreatment in HIV/HCV genotype 1-infected subjects with insulin resistance and previous nonresponse to peginterferon and ribavirin therapy: A5239. J Acquir Immun Defic Syndr 2014;65(3):345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemoine M, Barbu V, Girard PM, et al. : Altered hepatic expression of SREBP-1 and PPARgamma is associated with liver injury in insulin-resistant lipodystrophic HIV-infected patients. AIDS 2006;20(3):387–395 [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Wu R, Zhang F, et al. : Thiazolidinediones improve hepatic fibrosis in rats with non-alcoholic steatohepatitis by activating the adenosine monophosphate-activated protein kinase signalling pathway. Clin Exp Pharmacol P 2012;39(12):1026–1033 [DOI] [PubMed] [Google Scholar]
- 27.Zator ZA. and Chung RT: After the cure: Management of HCV after achievement of SVR. Curr HIV/AIDS Rep 2013;10(4):428–435 [DOI] [PubMed] [Google Scholar]