Abstract
The ex vivo mucosal explant model is frequently used to test the efficacy of microbicides that have the potential for preventing HIV-1 transmission. The conventional assessment of product efficacy has been the extent of HIV-1 p24 suppression in supernatant fluids sampled up to day 14 after HIV-1 challenge ex vivo. The purpose of this study was to determine if measurement of HIV-1 nucleic acids by real-time PCR and HIV-1 integration by Alu-gag PCR provides advantages with regard to monitoring HIV-1 infection in explants. Rectal biopsies from HIV-1-negative individuals were challenged with 1 × 105 virions/ml of HIV-1BaL or HIV-1CH077 ex vivo. HIV-1 RNA and HIV-1 p24 in supernatant fluids and HIV-1 nucleic acids and integrated provirus in individual biopsies were measured at days 1–14 after infection. HIV-1 RNA and proviral DNA were measured by quantitative real-time PCR (qRT-PCR) while integrated virus was detected by Alu-gag PCR. Real-time PCR assays detecting HIV-1 DNA and RNA performed similarly provided that the infecting virus sequences were a good match with the sequences of the assay primers and probes. Increased HIV-1 nucleic acid levels and DNA integration were measurable on days 11 and 14 after infection. The magnitude of explant infection was similar after challenge with HIV-1BaL and HIV-1CH077, although the trajectory of infection was delayed in the HIV-1CH077-infected biopsies. In the majority of experiments, qRT-PCR did not appreciably shorten the time necessary to detect evidence of HIV-1 infection.
Introduction
Explant culture of rectal and genital tissues has been used extensively to study the efficacy of drugs being developed for HIV-1 prevention as well as being used as a model for studying HIV-1 transmission.1–5 In this system, biopsies of the mucosal tissue are maintained in cell culture on collagen rafts at the air–liquid interface for a period of up to 2 weeks.6 An advantage of this system over cell culture is that, at time of viral challenge, cellular architecture is maintained, mimicking to a greater extent the environment found in vivo at the time of infection.7 Furthermore, rectal explant tissues do not need artificial stimulation with IL-2 or PMA for productive infection with HIV-1.8
Gut-associated lymphoid tissue (GALT) has many components of the lymphatic system including CD4+/CCR5+-activated T cells, which are targeted by HIV-1 infection.9 The gastrointestinal tract is thought to be the site most vulnerable to sexual transmission of HIV-1, exceeding that of the genital mucosa by 10- to 20-fold.10 It is also a very active site of HIV-1 replication11,12 and a major reservoir of HIV-1 in patients treated with antiretroviral therapy.13 Explants of colorectal tissue, because they recapitulate the environment encountered in vivo, have been used to study the efficacy of preclinical microbicides including TMC278, UC781, tenofovir, and maraviroc in preventing HIV-1 infection.14–17 Biopsies collected from individuals receiving antiretrovirals topically or orally are challenged with HIV-1 ex vivo and the extent of HIV-1 suppression is determined. The conventional means of assessing HIV-1 infection in these studies has been to use cumulative HIV-1 p24 antigen levels measured by ELISA at day 14 following infection.
In the present study, we hypothesized that quantitative real-time PCR (qRT-PCR) of HIV-1 nucleic acids could be used as an alternative endpoint to monitor HIV-1 infection in the ex vivo explant model and that PCR would be sensitive enough to enable detection of HIV-1 infection at time points earlier than day 14 after infection. Due to HIV-1 genetic diversity,18,19 detection of HIV-1 can be challenging. Nevertheless, there are many commercial and laboratory-developed assays that target highly conserved regions of the HIV-1 genome.20 Assays for determining HIV-1 viral load have targeted conserved regions of the long terminal repeat (LTR) domain and the gag and pol (integrase) genes of HIV-1.21–24 Two laboratory-developed probe-based qRT-PCR assays that detect overlapping regions of the HIV-1 LTR 21,24 were used in the present study to measure HIV-1 RNA levels in infected biopsy tissues and supernatant fluids. Assays to detect HIV-1 proviral load have targeted the LTR as well as the gag and pol (integrase) regions of the HIV-1 genome.22,23,25 An assay targeting the LTR/gag boundary and another assay targeting the HIV-1 gag gene were chosen to monitor HIV-1 provirus accumulation.22,25
Integration of HIV-1 into the host genome is essential for viral transmission as replication occurs mainly from integrated DNA.26–28 Therefore, the extent of HIV-1 integration was also measured. There are several methods of detecting integrated HIV-1 DNA,29–32 although Alu-gag PCR and Alu-LTR nested PCR methods are the most frequently used. The ability to detect integrated HIV-1 is influenced by the distance between the Alu sequences and the nearest integrated HIV-1 gag gene or LTR sequence.29–33 It is estimated that only a fraction of integrated provirus is detected rendering this assay qualitative.32
The aim of this study was to evaluate real-time PCR detection of viral nucleic acids as an alternative method to HIV-1 p24 protein measurement in monitoring HIV-1 infection in the ex vivo explant model. The kinetics of HIV-1 accumulation in tissue explants were evaluated following infection with two strains of HIV-1. The first strain, HIV-1BaL, is an R5, laboratory-adapted virus commonly used in studies of HIV-1 replication34 while the second strain, HIV-1CH077, is a transmitted/founder virus cloned from RNA obtained from the plasma of an individual acutely infected with HIV-1.35 As such, it is believed to more closely resemble HIV-1 species responsible for establishing acute infections.
Materials and Methods
Study population
Colorectal tissue was collected from healthy, HIV-uninfected male and female participants 18 to 45 years of age at time of screening. Individuals were free from abnormalities of the colorectal mucosa, had no significant gastrointestinal symptoms (such as a history of rectal bleeding), and tested negative for anorectal Chlamydia trachomatis (CT) and Neisseria gonorrhea (GC) infection. All participants signed informed consent documents and the study was approved by the Institutional Review Board at the University of Pittsburgh, Pittsburgh, PA.
Explant culture
A flexible sigmoidoscope was inserted into the rectum and 20 biopsies from each study participant were collected at 15 cm from the anal verge. The rectal biopsies (weighing between 10 and 20 mg each) were delivered to the laboratory within 2 h of collection in complete RPMI medium. Complete RPMI (cRPMI) consisted of Gibco RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 1% antibiotic-antimycotic (Invitrogen/Life Technologies, Carlsbad, CA), and 0.5 mg/ml Zosyn (Wyeth, Madison, NJ). Biopsies were handled under sterile conditions in a laminar flow biological safety cabinet (Baker Company, Inc., Sanford, ME) and placed in individual wells of a 24-well cell culture plate. Specimens were incubated with a final concentration of 1 × 105 virions/ml HIV-1BaL (Advanced Biotechnologies Inc., Columbia, MD) or transmitted/founder virus HIV-1CH077 (a generous gift of Dr. Charlene Dezzutti, Microbicide Trials Network, Pittsburgh, PA) in cRPMI.
There were four experiments in which biopsies were infected with HIV-1BaL and four experiments with HIV-1CH077 infection. Virus titer was determined using standard TCID50 methods.36,37 Biopsy tissues were incubated for 2 h with the virus at 37°C in a Binder incubator (Binder Inc., Bohemia, NY), purged with 5% CO2. After the incubation period, the biopsy tissues were washed four times with sterile D-PBS (Invitrogen/Life Technologies, Carlsbad, CA) and placed on Surgifoam (Ethicon, Inc., Somerville, NJ) rafts in individual wells of a sterile 24-well tissue culture plate containing cRPMI. The medium was sampled and replenished every 1–3 days for up to 14 days. Supernatant fluids were stored at −80°C until analysis of HIV-1 p24 antigen by an enzyme-linked immunosorbent assay (ELISA) (Perkin Elmer, Waltham, MA) and HIV-1 RNA by qRT-PCR. Biopsy specimens were harvested at several time points after virus exposure and placed in RNAlater stabilization solution (Life Technologies, Grand Island, NY) for 24 h prior to storage at −80°C.
Cell line controls
The ACH-2 human T cell line was obtained from the NIH AIDS Reagent Program (Division of AIDS, NIAID, NIH) and contains a single integrated copy of HIV-1 per cell.38 Cells were grown in RPMI-1640 supplemented with 10 mM HEPES, 2 mM l-glutamine, 10% heat-inactivated fetal bovine serum, and 1 × antibiotic-antimycotic (Life Technologies, Grand Island, NY). The 8E5 human lymphoblastoid cell line (ATCC CRL-8993) also contains a single integrated copy of HIV-1 (LAV strain) per cell39 and was obtained from ATCC (ATCC, Manassas, VA). Cells were grown in RPMI-1640, 10% heat-inactivated fetal bovine serum, and 1 × antibiotic-antimycotic (Life Technologies, Grand Island, NY). Both cell lines were grown in a humidified incubator (Binder, Inc., Bohemia, NY) at 37°C, purged with 5% CO2.
Viral protein determination
HIV-1 p24 levels were measured by AlphaLISA Immunodeficiency Virus Type 1 p24 protein (HIV p24) (high sensitivity kit; Perkin Elmer, Walton, MA) according to kit instructions. Ten-microliter aliquots of supernatant fluid were sampled for the assay, which has a dynamic range of 1.8–30,000 pg/ml.
Nucleic acid extraction
DNA and total RNA were extracted from biopsy tissue using the AllPrep DNA/RNA mini kit (Qiagen, Valencia, CA), which allows for simultaneous isolation of RNA and DNA from the same tissue. Prior to extraction, the vials containing biopsies were removed from the freezer and thawed. Individual biopsies were removed from the RNAlater reagent, placed in 1.5-ml Eppendorf tubes containing guanidinium lysis buffer (Qiagen, Valencia, CA) and 0.5 mM zirconium oxide beads (Next Advance, Averill, NY), and then homogenized with the Bullet Blender tissue disrupter for 5 min (Next Advance, Averill, NY). The homogenized lysates were placed on silica columns for isolation of RNA and DNA, according to the Qiagen AllPrep kit instructions (Qiagen, Valencia, CA). RNA (eluted in 50 μl) and DNA (eluted in 100 μl) concentrations were measured on a NanoVue Plus spectrophotometer (GE Healthcare Life Sciences, Piscataway, NJ). DNA concentrations ranged from 42 to 125 ng/μl while total RNA concentrations ranged from 13 to 66 ng/μl.
Viral RNA was isolated from 140 μl of supernatant fluid on silica columns using the QIAamp Viral RNA mini kit (Qiagen, Valencia, CA). RNA was eluted into 60 μl and 12 μl of the eluate was subsequently converted to complimentary DNA (cDNA) prior to quantification of RNA by real-time PCR.
HIV-1 assays
HIV-1 proviral DNA assay
HIV-1 proviral DNA was analyzed using two different assays. Assay 1 employed a conserved sequence of the HIV-1 LTR gag boundary.25 Assay 2 targeted a conserved region of the HIV-1 gag gene and made use of locked nucleic acid technology (LNA, Exiqon, Woburn, MA) for probe construction.22 Ten microliter PCR reactions consisted of primers and probe in 1 × QuantiTect probe master mix (Qiagen, Valencia, CA). Probes were labeled with 6-FAM at the 5′-end and Black Hole quencher 1 at the 3′-end (Eurofins Genomics, Huntsville, AL). Sequences of primers and probes and their concentrations are listed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/aid). β-Globin was also quantified by real-time PCR as a measure of DNA extraction efficiency and verification of the absence of PCR inhibitors in the DNA extract.40 Copies of HIV-1 DNA and β-globin DNA per aliquot were extrapolated from standard curves containing serial dilutions of known amounts of plasmid DNA. HIV-1 DNA and human β-globin plasmid standards were composed of the appropriate PCR fragments, subcloned into the pCR 2.1-TOPO cloning vector (Life Technologies, Grand Island, NY). Copies of HIV-1 provirus were normalized to 100,000 copies of β-globin.
HIV-1 RNA viral load
RNA from each extract (1 μg total RNA) was pretreated with DNase 1 prior to conversion to cDNA using the QuantiTect Reverse Transcription kit (Qiagen, Valencia, CA). One microgram total RNA was brought up to a volume of 12 μl in RNase-free water and 2 μl of DNase 1/buffer mix was added. The reaction mixture was incubated at 42°C for 2 min and then placed on ice. To each reaction was added 4 μl of buffer mix, 1 μl of proprietary reverse transcriptase/RNase inhibitor, and 1 μl of a primer mix consisting of random primers and oligo(dT). The reaction tubes were incubated at 42°C for 30 min, and then the reaction was deactivated by incubation at 95°C for 3 min. Positive controls were RNA from the HIV-1-positive 8E5 cell line, which constitutively expresses HIV-1 RNA.39 Negative controls consisting of all components of the reaction except for reverse transcriptase were included with each set of reactions to verify that the samples were free of contaminating DNA.
HIV-1 RNA was detected by two real-time PCR assays targeting conserved and overlapping regions of the LTR of the HIV-1 genome, referred to as Assays 324 and 4,21 respectively. The assays were performed using the QuantiTect Probe kit (Qiagen, Valencia, CA) and the appropriate primers and probes. Copies of HIV-1 were extrapolated from standard curves containing serial dilutions of known amounts of plasmid control. Sequences of primers and probes and their concentrations are listed in Supplementary Table S1.
cDNA samples were also analyzed for β-actin using Solaris primers and probe (GE Dharmacon, Lafayette, CO). A 134-bp fragment of the β-actin gene, subcloned into the pCR 2.1-TOPO cloning vector, served as a control plasmid (Life Technologies, Grand Island, NY). HIV-1 RNA levels in biopsy tissues were normalized to 100,000 copies of β-actin.
Real-time PCR reactions consisted of 1 μl of template DNA or cDNA in a total reaction volume of 10 μl with 1 × QuantiTect Probe PCR master mix (Qiagen, Valencia, CA) containing the appropriate primers and probe. PCR cycling conditions were as follows: one 10 min activation step at 95°C, 45 cycles of 15 s denaturation at 94°C, and 1 min annealing/extension at 60°C. Real-time PCR reactions were analyzed on the CFX96 Real-time PCR Detection System (Bio-Rad, Hercules, CA). Standard curves were constructed of serial dilutions of plasmid DNA with final concentrations ranging between 5 and 5 × 107 copies/μl target. Samples and standards were run in triplicate.
Construction of plasmid controls for real-time PCR assays
Plasmid controls for the two HIV-1 proviral assays,22,25 the two viral RNA real-time PCR assays,21,24 and assays for the β-globin and β-actin reference genes were constructed by amplifying gene fragments using the appropriate primers and AmpliTaq Gold DNA polymerase. For DNA assays, the PCR fragments were generated by amplifying regions of the HIV-1 genome using the 8E5 cell line DNA as a template (American Type Culture Collection, Manassa, VA). RNA from the 8E5 cell line, which constitutively expresses HIV-1 RNA,39 was also used to construct plasmid controls for the HIV-1 RNA assays. RNA from the 8E5 cell line was converted to cDNA and amplicons encompassing the real-time PCR assays were generated. PCR fragments for HIV-1 DNA and RNA assays as well as the β-globin and β-actin reference genes were subcloned into the pCR2.1TOPO cloning vector (Life Technologies, Grand Island, NY). All subcloned plasmid inserts were sequenced at the University of Pittsburgh Health Sciences Core Research Facility (Pittsburgh, PA) for verification of identity. Plasmids were expanded and concentrations were measured on a NanoVue spectrophotometer (GE Life Sciences, Piscataway, NJ). Concentrations were converted to copy number and serial dilutions of plasmid DNA ranging downward from 5 × 107copies/μl were used to construct standard curves for real-time PCR. Copy number for each sample was extrapolated from the standard curves.
HIV-1 DNA integration assay
HIV-1 DNA integration was estimated based on a qualitative nested Alu-gag PCR.33 In the first PCR reaction, Alu forward primer ( 5′ GCC TCC CAA AGT GCT GGG ATT ACA G-3′) and HIV-1 gag reverse primer (5′-GTT CCT GCT ATG TCA CTT CC-3′) were used to prime a PCR reaction amplifying integrated HIV-1 DNA and the nearest human Alu sequence. This was followed by quantification of the proviral DNA amplified in the Alu-gag PCR reaction by real-time PCR.25 Because the original gag reverse primer did not amplify HIV-1CH077 very efficiently, an alternate gag primer was designed that was a better match for the HIV-1CH077 proviral sequence (5′-GAT CCT GCT ATA TCA CTT CC-3′).
The final concentrations of reagents in the Alu-gag PCR reaction mixture were as follows: 100 nM Alu primer, 600 nM gag reverse primer, dNTP (0.4 μM each), 1.25 units TaKaRa LA Taq polymerase (Clontech, Mountain View, CA), 1 × reaction buffer, and up to 500 ng template DNA per 25 μl reaction. All reagents were kept on ice prior to PCR to minimize the amplification of nonspecific products. The amplification conditions were as follows: 1 cycle at 94°C for 1 min, 30 cycles at 98°C for 10 s, 68°C for 15 min, and 1 cycle at 72°C for 10 min. A parallel gag-only PCR reaction was set up to distinguish between integrated and unintegrated HIV-1 DNA. To calculate the amount of integrated DNA, the amount of HIV-1 DNA detected by real time PCR in the gag-only PCR reaction was subtracted from the amount of HIV-1 provirus detected in the Alu-gag preamplification reaction.
DNA from two cell lines was amplified with each run as controls. The ACH-2 T cell line contains a single integrated copy of HIV-1,38 which is in the proper orientation relative to the nearest Alu sequence for efficient amplification to occur.29 In the 8E5 cell line, the integrated HIV-1 gag sequence and the adjacent Alu sequences are in the same orientation and, thus, amplification does not proceed efficiently.29 This mimics to some extent the fact that HIV-1 integration into the human genome is a random event and therefore only a fraction of integrated virus is likely to be detected.32
Statistical methods
Statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC). HIV-1 infection patterns with HIV-1BaL and HIV-1CH077 were plotted using mean ± (SE) at days 1, 2, 4, 7, 11, and 14 for each of the tissue-based assays (Fig. 1) and supernatant-based assays (Fig. 2). To determine whether the increases over time were statistically significant, the results of repeated-measures analysis using linear mixed models (Table 1) are presented. These were necessary to account for the correlation between samples taken from the same participant. In the repeated-measures analysis, day 4 was used as the reference because that was the first time point with a sufficient number of replicates to obtain an accurate estimate of the standard error for each assay on that day. A scatterplot matrix (Fig. 4) and correlations (Table 2) were used to analyze the relationships between each combination of tissue-based assays and each combination of supernatant fluid-based assays.
FIG. 1.
Time course of HIV-1 nucleic acid accumulation in colorectal explants infected with HIV-1BaL (A) and HIV-1CH077 (B). Twenty biopsy tissues from each of four HIV-1-negative study participants were infected with 1 × 105 virions/ml of the R5, laboratory-adapted HIV-1 strain BaL or 1 × 105 virions/ml of the transmitted founder HIV-1 virus strain CH077. After exposure to virus for 2 h, virus was washed from the tissues and biopsies were returned to the cell culture incubator. Selected explant tissues were harvested at days 1, 2, 4, 7, 11, and 14 after infection. Viral nucleic acids were isolated and quantified by two quantitative real-time PCR (qRT-PCR) assays for DNA referred to as Assay 1 and Assay 222,25 and two qRT-PCR assays for RNA referred to as Assay 3 and Assay 4.21,24 DNA integration was estimated by a qualitative, nested Alu-gag PCR.33 Data are presented as means and standard error and samples assays were conducted in triplicate.
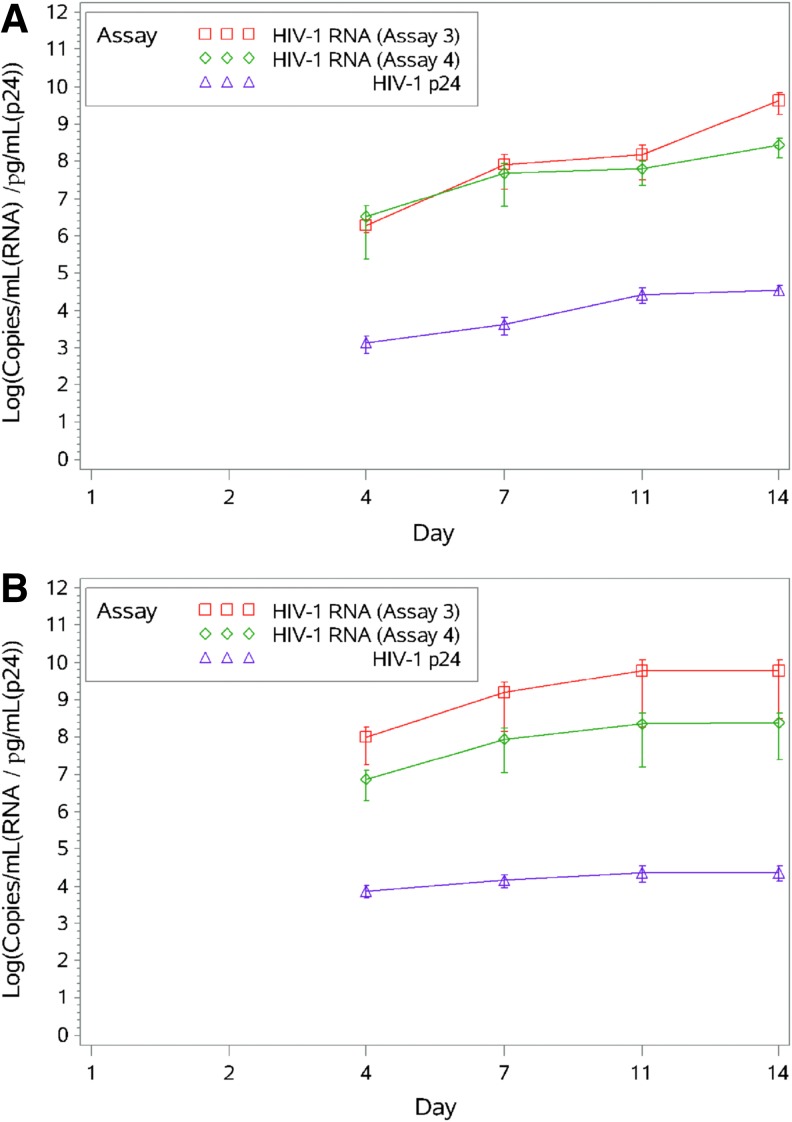
FIG. 2.
Time course of HIV-1 RNA and HIV-1 p24 accumulation in supernatant fluids from colorectal explants infected with HIV-1BaL (A) or HIV-1CH077 (B). Twenty biopsy tissues from each of four HIV-1-negative study participants were infected with HIV-1BaL or HIV-1CH077 as described in Materials and Methods. Supernatant fluids were collected from the explant tissues at days 1, 2, 4, 7, 11, and 14 after infection at which time the tissue culture medium was replenished. Viral RNA was extracted from supernatant fluid and measured by qRT-PCR using two assays (Assay 3 and Assay 4). HIV-1 p24 levels were quantified by ELISA. Data are presented as means and standard error and samples assays were conducted in triplicate.
Table 1A.
p-Values for Repeated Measures Analysis to Determine the First Day with Significant Increase in Viral Load (HIV-1BaL Virus)
| Assay | Day 7 (vs. day 4) | Day 11 (vs. day 4) | Day 14 (vs. day 4) |
|---|---|---|---|
| Assay 1 (T, DNA) | 0.042 | <0.001 | 0.001 |
| Assay 2 (T, DNA) | 0.076 | <0.001 | 0.001 |
| Integration (T, DNA) | 0.077 | <0.001 | 0.001 |
| Assay 3 (T, RNA) | 0.174 | 0.003 | 0.004 |
| Assay 4 (T, RNA) | 0.078 | <0.001 | <0.001 |
| Assay 3 (S, RNA) | 0.485 | 0.273 | 0.008 |
| Assay 4 (S, RNA) | 0.573 | 0.030 | 0.009 |
| p24 (S, HIV-1 p24) | 0.910 | 0.017 | 0.102 |
Real-time PCR assays and HIV-1 p24 ELISA assays were performed to measure levels of HIV-1 nucleic acid and antigen in tissues (T) and supernatant fluids (S) after infection of explant tissues with HIV-1BaL.
Table 1B.
p-Values for Repeated Measures Analysis to Determine the First Day with Significant Increase in Viral Load (HIV-1CHO77 Virus)
| Assay | Day 7 (vs. day 4) | Day 11 (vs. day 4) | Day 14 (vs. day 4) |
|---|---|---|---|
| Assay 1 (T, DNA) | 0.698 | 0.053 | 0.060 |
| Assay 2 (T, DNA) | 0.245 | 0.176 | 0.048 |
| Integration (T, DNA) | 0.847 | 0.002 | 0.002 |
| Assay 3 (T, RNA) | 0.277 | 0.059 | 0.178 |
| Assay 4 (T, RNA) | 0.731 | 0.067 | 0.040 |
| Assay 3 (S, RNA) | 0.251 | 0.348 | 0.432 |
| Assay 4 (S, RNA) | 0.635 | 0.800 | 0.577 |
| HIV-1 p24 (S, HIV-1 p24) | 0.349 | 0.044 | 0.012 |
Samples are compared to reference day = 4 because that is the first day at which we have a sufficient number of samples measured for each assay for comparison.
Real-time PCR assays and HIV-1 p24 ELISA were performed to measure levels of HIV-1 nucleic acid and antigen in tissues (T) and supernatant fluids (S) after infection with HIV-1CH077.
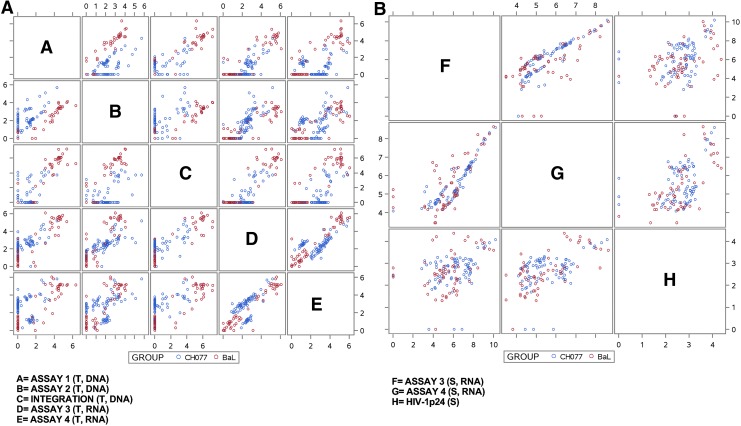
FIG. 4.
(A) Scatterplot matrix of tissue assays. A scatterplot depicting the relationships among all five qRT-PCR assays in tissue is shown. Two qRT-PCR assays for provirus were performed to assess levels of HIV-1 DNA (Assay 1 and Assay 2) and two qRT-PCR assays detected viral RNA (Assay 3 and Assay 4). HIV-1 DNA integration was detected by a qualitative, nested Alu-gag PCR assay. T-DNA, tissue HIV-1 DNA; T-RNA, tissue HIV-1 RNA; S-RNA, supernatant HIV-1 RNA. Assay units are the same as those presented in Figs. 1 and 2. (B) Scatterplot matrix of supernatant fluid assays. A scatterplot depicting the relationships among all three assays performed on supernatant fluid is shown. Two quantitative real-time assays detected HIV-1 RNA (Assay 3 and Assay 4) and HIV-1 p24 was determined by ELISA. S-RNA, supernatant HIV-1 RNA; S, supernatant HIV-1 p24.
Table 2A.
Correlations Between Each Tissue Assay Pairing
| Assay 1 (T, DNA) | Assay 2 (T, DNA) | Integration (T, DNA) | Assay 3 (T, RNA) | Assay 4 (T, RNA) | |
|---|---|---|---|---|---|
| HIV-1BaL | |||||
| Assay 1 (T, DNA) | — | — | — | — | — |
| Assay 2 (T, DNA) | 0.960 | — | — | — | — |
| Integration (T, DNA) | 0.956 | 0.952 | — | — | — |
| Assay 3 (T, RNA) | 0.917 | 0.897 | 0.926 | — | — |
| Assay 4 (T, RNA) | 0.921 | 0.902 | 0.923 | 0.965 | — |
| HIV-1CH077 | |||||
| Assay 1 (T, DNA) | — | — | — | — | — |
| Assay 2 (T, DNA) | 0.700 | — | — | — | — |
| Integration (T, DNA) | 0.548 | 0.538 | — | — | — |
| Assay 3 (T, RNA) | 0.674 | 0.772 | 0.597 | — | — |
| Assay 4 (T, RNA) | 0.175 | 0.378 | 0.569 | 0.518 | — |
Real-time PCR assays were performed to measure levels of HIV-1 nucleic acid in tissues (T) after infection with HIV-1BaL or HIV-1CH077.
Table 2B.
Correlations Between Each Supernatant Fluid Assay Pairing
| Assay 3 (S, RNA) | Assay 4 (S, RNA) | HIV-1 p24 (S, HIV-1 p24) | |
|---|---|---|---|
| HIV-1BaL | — | — | — |
| Assay 3 (S, RNA) | — | — | — |
| Assay 4 (S, RNA) | 0.879 | — | — |
| HIV-1 p24 (S, HIV-1 p24) | 0.362 | 0.428 | — |
| HIV-1CH077 | |||
| Assay 3 (S, RNA) | — | — | — |
| Assay 4 (S, RNA) | 0.674 | — | — |
| HIV-1 p24 (S, HIV-1 p24) | 0.362 | 0.682 | — |
Real-time PCR assays and HIV-1 p24 ELISA were performed to measure levels of HIV-1 nucleic acid and antigen in supernatant fluids (S) after infection with HIV-1BaL or HIV-1CH077.
Results
Kinetics of HIV-1 Infection in rectal mucosal explant tissues
Rectal mucosal explant tissues from each of eight participants were infected with either the laboratory-adapted HIV-1 strain HIV-1BaL or the transmitted/founder virus HIV-1CH077 at concentrations of 1 × 105 virions/ml. Explant tissues were incubated with virus for 2 h after which the excess virus was washed from the tissues. The explant tissues were placed on individual collagen rafts in the cell culture incubator and supernatant fluids and biopsies were collected at time points ranging from 1 to 14 days after infection. Viral nucleic acids were measured by quantitative real-time PCR using two different assays for HIV-1 RNA21,24 and two different assays for HIV-1 provirus.22,25 HIV-1 DNA integration was estimated by Alu-gag PCR33 while HIV-1 p24 was measured by AlphaLISA-HIV-1 p24 (Perkin Elmer, Waltham, MA).
Infection patterns over 14 days
As expected, HIV-1 nucleic acid levels in the infected mucosal explant tissue increased over time (Fig. 1). HIV-1 DNA levels, determined by qRT-PCR (Assays 1 and 2) rose in the same pattern, although Assay 1 detected higher levels of provirus in the HIV-1BaL-infected biopsies while Assay 2 detected higher levels in the HIV-1CH077-infected biopsies. The two RNA assays (Assays 3 and 4) detected comparable levels of HIV-1 RNA in tissues infected with either virus. The patterns of viral nucleic acid expression, however, were notably different for HIV-1BaL-infected and HIV-1CH077-infected biopsy tissues. Sharp increases in HIV-1 RNA and DNA were observed in samples infected with HIV-1BaL (Fig. 1A), while the increases in the HIV-1 RNA and DNA in HIV-1CH077-infected samples are much less pronounced (Fig. 1B).
Despite the less dramatic increase in HIV-1 nucleic acid levels in the HIV-1CH077-infected biopsies, viral RNA and DNA levels, measured by Assay 2, were higher (102 to 103 copies) at earlier time points in contrast to the undetectable levels observed in the HIV-1BaL-infected biopsies. By days 11 and 14, HIV-1 DNA and RNA levels in all infected biopsies were comparable, regardless of the infecting virus, and reached approximately 105 copies per 105 copies of the appropriate reference gene [(β-globin (DNA) or β-actin (RNA)]. Integrated provirus levels correlated with total HIV-1 provirus detected by qRT-PCR for explant tissues infected with either HIV-1BaL or HIV-1CH077. In the supernatant fluids, HIV-1 RNA determined by qRT-PCR reflected patterns of expression similar to HIV-1 p24 antigen, measured by ELISA. The HIV-1 p24 and HIV-1 RNA between-virus differences, however, were not as pronounced for the supernatant fluids (Fig. 2).
Figure 3A displays the trajectory of HIV-1 accumulation in explant tissues infected with HIV-1BaL for each of four individual study participants. There is a separate plot for each of the five tissue assays and three assays of supernatant fluid (Supplementary Fig. S1). In the HIV-1BaL-infected explants (Table 1A), Assay 1 for total HIV-1 DNA detected significant increases in provirus by day 7 after infection. The levels of total provirus and integrated provirus started to climb moderately at day 7 and registered statistically significant increases by day 11 after infection. The primer and probe sequences for Assay 1 matched perfectly with the HIV-1BaL viral sequences (GenBank accession number AB253432.1) unlike Assay 2 in which there was a single mismatch in the reverse primer relative to the HIV-1BaL sequence that compromised the ability of the assay to detect this proviral strain.
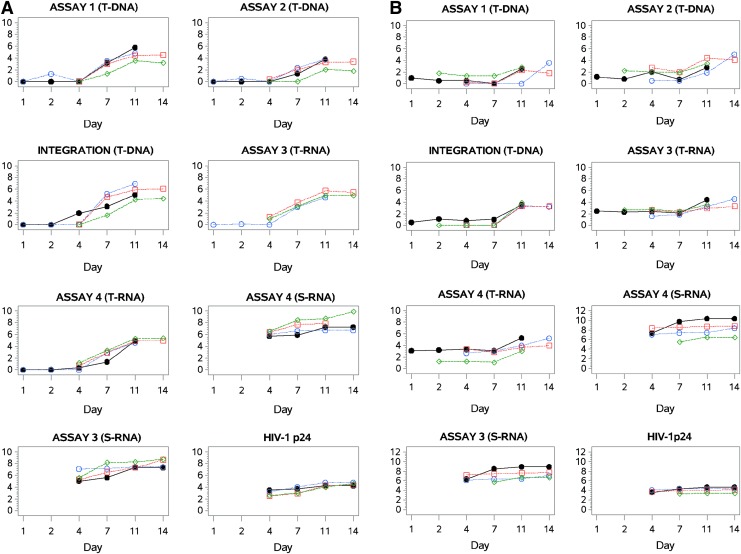
FIG. 3.
Time course of HIV-1 accumulation after infection with HIV-1BaL (A) or HIV-1CH077 (B) by assay. Twenty biopsy tissues from each of four HIV-1-negative study participants were infected with 1 × 105 HIV-1BaL or HIV-1CH077 as previously described. HIV-1 nucleic acid accumulation in tissue was measured by five different quantitative real-time PCR assays for HIV-1 nucleic acids and data are plotted according to assay type. Two assays were performed to detect HIV-1 provirus (Assay 1 and Assay 2) while two assays detected HIV-1 RNA (Assay 3 and Assay 4). A qualitative Alu-gag PCR method assessed HIV-1 DNA integration. The assays performed on collected supernatant fluids were qRT-PCR for HIV-1 RNA (Assay 3 and Assay 4) and HIV-1 p24, measured by ELISA. T-DNA, tissue HIV-1 DNA; T-RNA, tissue HIV-1 RNA; S-RNA, supernatant HIV-1 RNA. Assay units are the same as those presented in Figs. 1 and 2.
Figure 3B displays the trajectory of HIV-1 accumulation in explant tissues infected with HIV-1CH077 for each of four individual study participants. There is a separate plot for each of the five tissues assays and three assays of supernatant fluids. In the HIV-1CH077-infected explants (Table 1B), the increases in HIV-1 nucleic acid levels were generally not as pronounced before day 14 as those in the HIV-1BaL-infected biopsies. Statistically significant increases in total HIV-1 provirus were detected only at day 14 after infection, while integrated HIV-1 DNA was increased significantly by day 11. Assay 1 for HIV-1 proviral DNA has a single mismatch in the reverse primer relative to the HIV-1CH077 sequence (GenBank accession number JN944909.1) and, consequently, the sensitivity of the assay was diminished for detecting the transmitted/founder virus. While there were no statistically significant increases detected in viral RNA, the RNA levels at days 1 to 4 after infection were higher in HIV-1CH077 -infected tissues than those detected in the HIV-1BaL-infected biopsies at the same time points.
Between-assay correlations
The scatterplot matrix (Fig. 4A and B) shows the agreement between each pairing of the five assays that measured HIV-1 nucleic acid content in tissues and the three assays that measured HIV-1 RNA and p24 levels in supernatant fluids, complimented by correlation coefficients shown in Tables 2A and B. Between-assay correlations are stratified by infecting virus due to differences in the results observed between infections with HIV1BaL and infections with HIV-1CH077.
In samples infected with HIV-1BaL virus (Table 2A), all pairs of tissue-based assays generally showed strong correlations with one another. In particular, the tissue-based DNA assays (Assay 1, Assay 2, and HIV-1 DNA Integration) showed extremely strong correlations (correlations > 0.952 for all pairs), as did the pair of tissue-based RNA assays (Assays 3 and 4; correlation = 0.965). The pairwise correlations were still quite good when comparing tissue-based DNA assays against tissue-based RNA assays (correlations ranging from 0.897 to 0.926 for all pairings). The pattern of correlations between the respective assays was quite different for samples infected with HIV-1CH077 virus. The tissue-based DNA assays showed weaker agreement for samples infected with HIV-1CH077 virus (correlations from 0.538 to 0.700) than the HIV-1BaL-infected samples, as did the tissue-based RNA assays (correlation = 0.518), and their relationships with one another were also less consistent than those observed in the samples infected with HIV-1BaL virus.
There were somewhat more moderate correlations between the supernatant fluid-based assays (Assay 3, Assay 4, and HIV1 p24; correlations ranging from 0.362 to 0.684) for both HIV-1BaL and HIV-1CH077 viruses (Table 2B).
Discussion
Explants of mucosal tissue from the intestinal and genital tract have been used extensively as model systems to study the efficacy of microbicides used in the prevention of HIV-1 transmission.1–6 While cumulative HIV-1 p24 levels, determined by ELISA, are the conventional endpoint to assess evidence of infection, this method is not very sensitive and has a low dynamic range. A goal of the present study was to determine if HIV-1 nucleic acid detection by real-time PCR could serve as a more sensitive, alternative method in measuring the extent of HIV-1 infection in mucosal explant cultures. Specifically, we sought to determine if real-time PCR could detect evidence of HIV-1 infection in explant tissue at time points earlier than 14 days after infection, the time at which infection is most reliably detectable by HIV-1 p24 ELISA.
Comparisons were made between the kinetics of infection of mucosal explants exposed to two different HIV-1 viral strains. Explants were infected by either the R5, laboratory-adapted HIV-1 strain HIV-1BaL or the transmitted/founder virus, HIV-1CH077, derived by single genome amplification of HIV-1 RNA isolated from the serum of an individual at the early stages of infection.35,41 Cumulative supernatant HIV-1 p24 levels often serve as the conventional end point measurement for explant HIV-1 infection. While HIV-1 p24 ELISA is a straightforward, well-established method for which numerous commercial kits are available, a disadvantage has been the difficulty in detecting HIV-1 infection at early time points. This, coupled with the low dynamic range of the assay that may necessitate dilution of samples with high HIV-1 p24 levels, can compromise the quantitative aspects of the assay. A recent modification, the AlphaLISA (Perkin Elmer) p24 HIV-1 detection kit, has a greater dynamic range (1.8 pg/ml to 30,000 pg/ml), but samples may still need to be diluted to conform to the range of the standard curve. PCR, on the other hand, has a much greater linear dynamic range, from 10 copies to over 107 copies, and has the potential for being more sensitive than HIV-1 p24 ELISA.
Contrary to expectations, the majority of PCR assays did not offer any appreciable advantages over HIV-1 p24 ELISA with respect to early detection of HIV-1. This is in agreement with reports of a similar time course of accumulation of HIV-1 RNA and HIV-1 p24, determined by qRT-PCR and ELISA in Hut78 lymphoblastoid cells infected with HIV-1.42 In explants infected with either HIV-1BaL or HIV-1CH077, the highest HIV-1 nucleic acid levels were attained at days 11 and 14 after infection, levels that were significantly elevated above the (Table 1A, 1B) levels observed at day 4 after infection.
Assay 1, a real-time PCR assay for HIV-1 provirus, was the only method capable of detecting a significant increase over baseline in HIV-1 nucleic acids by day 7 after infection. This occurred in only those biopsies infected with HIV-1BaLwhere there was a perfect match between the assay primers and probe and the viral sequence (GenBank accession number AB253432.1). The other proviral assay tested had a single mismatch in the reverse primer relative to the HIV-1BaL sequence.22 While the mismatch did not occur in an ostensibly critical location such as the probe or near the 3′ end of a primer,43,44 it nevertheless decreased the sensitivity of the assay by several orders of magnitude. A similar phenomenon was observed for the transmitted/founder virus HIV-1CH077, in which Assay 222 matched perfectly with the viral sequence but Assay 125 had a single mismatch in the reverse primer relative to the viral sequence (GenBank accession number JN944909.1), which decreased assay sensitivity.
These results are consistent with the difficulty often encountered when attempting to quantify HIV-1 by PCR. HIV-1 has substantial genetic variability due to the high rate of mutation,45,46 error-prone reverse transcriptase,47 and frequent genetic recombination,48 and it is therefore challenging to design universal primers and probes that detect all clades and subtypes of HIV-1.49 It is well known that mismatches between primers, probes, and target sequences can have detrimental effects on the sensitivity of real-time PCR assays. Malnati et al. performed a systematic examination of the effects of mismatches between primers and the sequences of various HIV-1 isolates.25 While mismatches in the forward primer had only minor effects, single mismatches in the reverse primer decreased the sensitivity of the assay by 80% in some instances, an effect mirrored in the present study.
The two assays for HIV-1 provirus used in this study target the LTR-gag and the gag genes of HIV-1. While the HIV-1 gag gene is highly conserved among HIV-1 subtypes, the variability in this region is greater than previously thought.50,51 As a consequence, HIV-1 real-time PCR assays have also been developed that target conserved regions of the HIV-1 LTR. The two assays chosen to measure viral RNA in the present study were directed against this region21,24 and there was a complete concordance between the primer and probe sequences of these assays and the sequences of the two HIV-1 viral strains used. Reflecting a difference in the kinetics of infection between HIV-1BaL and HIV-1CH077, HIV-1 RNA was detectable at 2–4 days after infection in the HIV-1CH077-infected tissues and ranged from 100 to 1,000 copies/105 copies β-actin, whereas it was barely detectable at early time points in the HIV-1BaL-infected tissues. The RNA levels in tissues reached similar levels by day 11 after infection, however, in tissues infected with either of the viruses investigated.
Similar to HIV-1 RNA in tissues, differences in the kinetics of HIV-1 provirus accumulation were also observed between HIV-1BaL-infected and HIV-1CH077-infected explant tissues. Considering only the results of the HIV-1 DNA assay that best matched the sequence of the infecting virus (Assay 1), the highest levels of provirus were detected by day 11 after infection although higher levels of provirus were observed in the HIV-1BaL-infected biopsies. At early time points after infection, HIV-1CH077-infected biopsies exhibited higher levels of HIV-1 provirus, in agreement with the higher levels of HIV-1 RNA.
There are well-documented differences between transmitted/founder viruses and laboratory-adapted viruses or viruses isolated from chronically infected individuals. Transmitted/founder viruses have been cloned by single genome amplification from HIV-1 RNA isolated from the plasma of infected individuals at the early stages of infection.35,41,52 These viruses are known to have a high degree of infectivity, increased env (viral envelope) expression, more efficient binding to dendritic cells, and more resistance to interferon alpha.52–54 Dendritic cells are believed to be the initial targets of HIV-1 in the gastrointestinal tract and they are known to transmit virus to CD4+ lymphocytes.55 HIV-1 infects cells primarily through CD4+ receptors and one of two coreceptors, CCR5 or CXCR4.55–59 As most acute HIV-1 infections are transmitted preferentially through the CCR5 coreceptor,59 transmitted/founder viruses thus utilize CCR5 and only rarely CXCR4.35,41,60 HIV-1CH077, however, is one of the few transmitted/founder viruses that have been reported to have dual tropism.35,61 It is capable of infecting PBMCs from individuals homozygous for the CCR5Δ32 mutation, which display a truncated CCR5 receptor protein.62,63 Furthermore, it can infect cells in the presence of CCR5 receptor inhibitors and can infect NP2 CD4+/CXCR4+ cells.35,61
Laboratory-adapted HIV-1BaL, on the other hand, infects CD4+ T cells through CD4+ receptors and CCR5 coreceptors but does not utilize CXCR4.64 It infects monocyte-derived macrophages as well as CD4+ T cells whereas HIV-1CH077 infects primarily CD4+ T cells and macrophages only inefficiently.35 How this relates to the kinetics of viral replication observed in these studies is unclear; however, there may be some evidence of more efficient infection in the HIV-1CH077-infected biopsies due to the higher levels of HIV-1 RNA at early time points after infection. It is unresolved, however, whether the increased levels of HIV-1 RNA in the HIV-1CH077-infected biopsies are indicative of productive infection or merely virus that has adhered to target cells prior to the infection process. Furthermore, it is not known if the increased viral nucleic acids represent replication-competent virus as PCR does not distinguish between viable and defective virus.
The extent of HIV-1 DNA integration was also estimated in this study by Alu-gag PCR.33 Integration of HIV-1 DNA into the host genome is a critical component of the HIV-1 life cycle as virtually all viral replication occurs from integrated provirus.26–28 In the mucosal explants infected with HIV-1 ex vivo, the highest levels of integrated HIV-1 DNA were observed at day 11 in the HIV-1BaL-infected biopsies and day 14 in the HIV-1CH077-infected biopsies. The levels of integrated HIV-1 DNA correlated with levels of total provirus as well as HIV-1 RNA in tissues. A limitation of the assay in the detection of integrated HIV-1CH077 is a mismatch in the reverse primer in the real-time PCR assay relative to the HIV-1CH077 target sequence. Nevertheless, the pattern of accumulation of integrated HIV-1 in the transmitted/founder virus was the same as that observed for the HIV-1BaL-infected biopsies, with peak amounts detected at days 11 and 14 after infection.
This research study investigated the utility of qRT-PCR as a sensitive alternative endpoint to HIV-1 p24 ELISA for measuring the extent of HIV-1 infection in rectal mucosal biopsies infected with HIV-1 ex vivo. While qRT-PCR did not appreciably decrease the time at which HIV-1 infection could be reliably detected, it did provide additional information regarding the kinetics of HIV-1 nucleic acid accumulation in the tissues infected under these experimental conditions. It is particularly useful when measuring HIV-1 at later time points when HIV-1 nucleic acids are abundant. Sensitivity at earlier time points can be augmented by the use of methods such as droplet digital PCR, which was developed to identify rare events in experimental samples.65
Droplet digital PCR may also circumvent the problems encountered due to viral sequence heterogeneity. When performing ex vivo explant experiments and infecting with a well-characterized and sequenced virus, the assay can easily be tailored to the virus being investigated. When dealing with uncharacterized viruses, however, droplet digital PCR for HIV-1 detection may then be a better option as it reported to be less susceptible to mismatches between primers, probes, and the target sequence.65 Despite these potential difficulties with detection, qRT-PCR offers other advantages in that multiple stages of the HIV-1 life cycle can be monitored simultaneously, thereby providing valuable biological information regarding viral replication.
Supplementary Material
Acknowledgments
This study received support from the Microbicide Trials Network, which is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Abner SR, Guenthner PC, Guarner J, et al. : A human colorectal explant culture to evaluate topical microbicides for the prevention of HIV infection. J Infect Dis 2005;192(9):1545–1556 [DOI] [PubMed] [Google Scholar]
- 2.Autrup H, Barrett LA, Jackson FE, et al. : Explant culture of human colon. Gastroenterology 1978;74(6):1248–1257 [PubMed] [Google Scholar]
- 3.Fletcher PS, Elliott J, Grivel JC, et al. : Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS 2006;20(9):1237–1245 [DOI] [PubMed] [Google Scholar]
- 4.Cummins JE, Jr., Guarner J, Flowers L, et al. : Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother 2007;51(5):1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins KB, Patterson BK, Naus GJ, et al. : Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med 2000;6(4):475–479 [DOI] [PubMed] [Google Scholar]
- 6.Grivel JC. and Margolis L: Use of human tissue explants to study human infectious agents. Nat Protoc 2009;4(2):256–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merbah M, Introini A, Fitzgerald W, et al. : Cervico-vaginal tissue ex vivo as a model to study early events in HIV-1 infection. Am J Reprod Immunol 2011;65(3):268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dezzutti CS. and Hladik F: Use of human mucosal tissue to study HIV-1 pathogenesis and evaluate HIV-1 prevention modalities. Curr HIV/AIDS Rep 2013;10(1):12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guy-Grand D. and Vassalli P: Gut intraepithelial T lymphocytes. Curr Opin Immunol 1993;5(2):247–252 [DOI] [PubMed] [Google Scholar]
- 10.Boily MC, Baggaley RF, Wang L, et al. : Heterosexual risk of HIV-1 infection per sexual act: Systematic review and meta-analysis of observational studies. Lancet Infect Dis 2009;9(2):118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dandekar S: Pathogenesis of HIV in the gastrointestinal tract. Curr HIV/AIDS Rep 2007;4(1):10–15 [DOI] [PubMed] [Google Scholar]
- 12.Brenchley JM, Schacker TW, Ruff LE, et al. : CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004;200(6):749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yukl SA, Shergill AK, Ho T, et al. : The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: Implications for viral persistence. J Infect Dis 2013;208(8):1212–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesquita PM, Srinivasan P, Johnson TJ, et al. : Novel preclinical models of topical PrEP pharmacodynamics provide rationale for combination of drugs with complementary properties. Retrovirology 2013;10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anton PA, Cranston RD, Kashuba A, et al. : RMP-02/MTN-006: A Phase 1 rectal safety, acceptability, pharmacokinetic and pharmacodynamic study of tenofovir 1% gel compared to oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses 2012;11:1412–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher P, Kiselyeva Y, Wallace G, et al. : The nonnucleoside reverse transcriptase inhibitor UC-781 inhibits human immunodeficiency virus type 1 infection of human cervical tissue and dissemination by migratory cells. J Virol 2005;79(17):11179–11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollenhagen C. and Asin SN: Enhanced HIV-1 replication in ex vivo ectocervical tissues from post-menopausal women correlates with increased inflammatory responses. Mucosal Immunol 2011;4(6):671–681 [DOI] [PubMed] [Google Scholar]
- 18.Goodenow M, Huet T, Saurin W, et al. : HIV-1 isolates are rapidly evolving quasispecies: Evidence for viral mixtures and preferred nucleotide substitutions. J Acquir Immune Defic Syndr 1989;2(4):344–352 [PubMed] [Google Scholar]
- 19.Smyth RP, Davenport MP, and Mak J: The origin of genetic diversity in HIV-1. Virus Res 2012;169(2):415–429 [DOI] [PubMed] [Google Scholar]
- 20.Luft LM, Gill MJ, and Church DL: HIV-1 viral diversity and its implications for viral load testing: Review of current platforms. Int J Infect Dis 2011;15(10):e661–e670 [DOI] [PubMed] [Google Scholar]
- 21.Rouet F, Chaix ML, Nerrienet E, et al. : Impact of HIV-1 genetic diversity on plasma HIV-1 RNA quantification: Usefulness of the Agence Nationale de Recherches sur le SIDA second-generation long terminal repeat-based real-time reverse transcriptase polymerase chain reaction test. J Acquir Immune Defic Syndr 2007;45(4):380–388 [DOI] [PubMed] [Google Scholar]
- 22.Li P, Ruel T, Fujimoto K, et al. : Novel application of Locked Nucleic Acid chemistry for a Taqman assay for measuring diverse human immunodeficiency virus type 1 subtypes. J Virol Methods 2010;170(1–2):115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yun Z, Fredriksson E, and Sonnerborg A: Quantification of human immunodeficiency virus type 1 proviral DNA by the TaqMan real-time PCR assay. J Clin Microbiol 2002;40(10):3883–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drosten C, Panning M, Drexler JF, et al. : Ultrasensitive monitoring of HIV-1 viral load by a low-cost real-time reverse transcription-PCR assay with internal control for the 5' long terminal repeat domain. Clin Chem 2006;52(7):1258–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malnati MS, Scarlatti G, Gatto F, et al. : A universal real-time PCR assay for the quantification of group-M HIV-1 proviral load. Nat Protoc 2008;3(7):1240–1248 [DOI] [PubMed] [Google Scholar]
- 26.Englund G, Theodore TS, Freed EO, et al. : Integration is required for productive infection of monocyte-derived macrophages by human immunodeficiency virus type 1. J Virol 1995;69(5):3216–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaFemina RL, Schneider CL, Robbins HL, et al. : Requirement of active human immunodeficiency virus type 1 integrase enzyme for productive infection of human T-lymphoid cells. J Virol 1992;66(12):7414–7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakai H, Kawamura M, Sakuragi J, et al. : Integration is essential for efficient gene expression of human immunodeficiency virus type 1. J Virol 1993;67(3):1169–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandegraaff N, Kumar R, Burrell CJ, and Li P: Kinetics of human immunodeficiency virus type 1 (HIV) DNA integration in acutely infected cells as determined using a novel assay for detection of integrated HIV DNA. J Virol 2001;75(22):11253–11260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brussel A, Delelis O, and Sonigo P: Alu-LTR real-time nested PCR assay for quantifying integrated HIV-1 DNA. Methods Mol Biol 2005;304:139–154 [DOI] [PubMed] [Google Scholar]
- 31.Chun TW, Finzi D, Margolick J, et al. : In vivo fate of HIV-1-infected T cells: Quantitative analysis of the transition to stable latency. Nat Med 1995;1(12):1284–1290 [DOI] [PubMed] [Google Scholar]
- 32.Agosto LM, Yu JJ, Dai J, et al. : HIV-1 integrates into resting CD4+ T cells even at low inoculums as demonstrated with an improved assay for HIV-1 integration. Virology 2007;368(1):60–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu JJ, Wu TL, Liszewski MK, et al. : A more precise HIV integration assay designed to detect small differences finds lower levels of integrated DNA in HAART treated patients. Virology 2008;379(1):78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gartner S, Markovits P, Markovitz DM, et al. : The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 1986;233(4760):215–219 [DOI] [PubMed] [Google Scholar]
- 35.Ochsenbauer C, Edmonds TG, Ding H, et al. : Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol 2012;86(5):2715–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown WF: Variance estimation in the Reed-Muench fifity per cent end-point determination. Am J Hyg 1964;79:37–46 [DOI] [PubMed] [Google Scholar]
- 37.Ball SC, Abraha A, Collins KR, et al. : Comparing the ex vivo fitness of CCR5-tropic human immunodeficiency virus type 1 isolates of subtypes B and C. J Virol 2003;77(2):1021–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clouse KA, Powell D, Washington I, et al. : Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol 1989;142(2):431–438 [PubMed] [Google Scholar]
- 39.Folks TM, Powell D, Lightfoote M, et al. : Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med 1986;164(1):280–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitz M, Scheungraber C, Herrmann J, et al. : Quantitative multiplex PCR assay for the detection of the seven clinically most relevant high-risk HPV types. J Clin Virol 2009;44(4):302–307 [DOI] [PubMed] [Google Scholar]
- 41.Salazar-Gonzalez JF, Bailes E, Pham KT, et al. : Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J Virol 2008;82(8):3952–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein SA, Karsten S, Ruster B, et al. : Comparison of TaqMan real-time PCR and p24 Elisa for quantification of in vitro HIV-1 replication. J Virol Methods 2003;107(2):169–175 [DOI] [PubMed] [Google Scholar]
- 43.Damond F, Descamps D, Farfara I, et al. : Quantification of proviral load of human immunodeficiency virus type 2 subtypes A and B using real-time PCR. J Clin Microbiol 2001;39(12):4264–4268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stadhouders R, Pas SD, Anber J, et al. : The effect of primer-template mismatches on the detection and quantification of nucleic acids using the 5' nuclease assay. J Mol Diagn 2010;12(1):109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao F, Korber BT, Weaver E, et al. : Centralized immunogens as a vaccine strategy to overcome HIV-1 diversity. Expert Rev Vaccines 2004;3(4 Suppl):S161–S168 [DOI] [PubMed] [Google Scholar]
- 46.Peeters M. and Sharp PM: Genetic diversity of HIV-1: The moving target. AIDS 2000;14(Suppl 3):S129–S140 [PubMed] [Google Scholar]
- 47.Roberts JD, Bebenek K, and Kunkel TA: The accuracy of reverse transcriptase from HIV-1. Science 1988;242(4882):1171–1173 [DOI] [PubMed] [Google Scholar]
- 48.Hu WS. and Temin HM: Retroviral recombination and reverse transcription. Science 1990;250(4985):1227–1233 [DOI] [PubMed] [Google Scholar]
- 49.Althaus CF, Gianella S, Rieder P, et al. : Rational design of HIV-1 fluorescent hydrolysis probes considering phylogenetic variation and probe performance. J Virol Methods 2010;165(2):151–160 [DOI] [PubMed] [Google Scholar]
- 50.Drosten C, Seifried E, and Roth WK: TaqMan 5'-nuclease human immunodeficiency virus type 1 PCR assay with phage-packaged competitive internal control for high-throughput blood donor screening. J Clin Microbiol 2001;39(12):4302–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Louwagie J, McCutchan FE, Peeters M, et al. : Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS 1993;7(6):769–780 [DOI] [PubMed] [Google Scholar]
- 52.Parrish NF, Gao F, Li H, et al. : Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci USA 2013;110(17):6626–6633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. : Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA 2008;105(21):7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fenton-May AE, Dibben O, Emmerich T, et al. : Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology 2013;10:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen R, Smythies LE, Clements RH, et al. : Dendritic cells transmit HIV-1 through human small intestinal mucosa. J Leukoc Biol 2010;87(4):663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dragic T, Litwin V, Allaway GP, et al. : HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 1996;381(6584):667–673 [DOI] [PubMed] [Google Scholar]
- 57.Deng H, Liu R, Ellmeier W, et al. : Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996;381(6584):661–666 [DOI] [PubMed] [Google Scholar]
- 58.Feng Y, Broder CC, Kennedy PE, and Berger EA: HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. J Immunol 2011;186(11):6076–6081 [PMC free article] [PubMed] [Google Scholar]
- 59.Margolis L. and Shattock R: Selective transmission of CCR5-utilizing HIV-1: The 'gatekeeper' problem resolved? Nat Rev Microbiol 2006;4(4):312–317 [DOI] [PubMed] [Google Scholar]
- 60.Mlcochova P, Watters SA, Towers GJ, et al. : Vpx complementation of 'non-macrophage tropic' R5 viruses reveals robust entry of infectious HIV-1 cores into macrophages. Retrovirology 2014;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilen CB, Parrish NF, Pfaff JM, et al. : Phenotypic and immunologic comparison of clade B transmitted/founder and chronic HIV-1 envelope glycoproteins. J Virol 2011;85(17):8514–8527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dean M, Carrington M, Winkler C, et al. : Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 1996;273(5283):1856–1862 [DOI] [PubMed] [Google Scholar]
- 63.Samson M, Libert F, Doranz BJ, et al. : Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 1996;382(6593):722–725 [DOI] [PubMed] [Google Scholar]
- 64.King DF, Siddiqui AA, Buffa V, et al. : Mucosal tissue tropism and dissemination of HIV-1 subtype B acute envelope-expressing chimeric virus. J Virol 2013;87(2):890–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strain MC, Lada SM, Luong T, et al. : Highly precise measurement of HIV DNA by droplet digital PCR. PLoS One 2013;8(4):e55943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.