Abstract
Controlling receptor-mediated processes in cells is paramount in many research areas. The activity of small molecules and growth factors is difficult to control and can lead to off-target effects through the activation of nonspecific receptors as well as binding affinity to nonspecific cell types. In this study, we report the development of a molecular trigger in the form of a divalent nucleic acid aptamer assembly toward vascular endothelial growth factor receptor-2 (VEGFR2). The assembly binds to VEGFR2 and functions as a receptor agonist with targeted receptor binding, promoting receptor phosphorylation, activation of the downstream Akt pathway, upregulation of endothelial nitric oxide synthase, and endothelial cell capillary tube formation. The agonist action we report makes this aptamer construct a promising strategy to control VEGFR2-mediated cell signaling.
Introduction
Recent advances in tissue engineering, regenerative medicine, and cell-based therapies necessitate the development of novel strategies that allow for controlling cellular processes. Of interest are approaches that are targeted, specific, robust, and ultimately result in molecular pathway activation and the transduction of signals that direct cellular responses, such as stem cell differentiation or tissue matrix production. For receptor-mediated cell processes, the identification of stable receptor ligands showing precise binding, high specificity, and targeted activation represents an area of active research [1].
Current efforts aim to use receptor-specific growth factors and cytokines or their respective receptor-binding peptide sequences as these are responsible for native interactions that result in receptor activation. While inherently successful as receptor agonists, these agents, often being difficult to control spatially, can lead to off-target effects through the activation of nonspecific receptors and binding to nonspecific cell types [2,3]. Unfortunately, identification of therapeutic agents that selectively activate specific cell surface receptor targets has proven to be quite a challenge. Overcoming these limitations in the control of receptor-mediated processes will result in distinct improvements in directing cellular processes, such as proliferation, migration, and differentiation.
A promising approach to achieving this goal is through the use of nucleic acid aptamers, single-stranded oligonucleotides that exhibit highly specific binding to intracellular and extracellular target sites [4]. The high affinity and specificity of aptamer binding can be attributed to their ability to form complex three-dimensional structures [5]. Ever since their isolation using the systematic evolution of ligands by exponential enrichment (SELEX) process, which was developed in the laboratory of Larry Gold at the University of Colorado [6], aptamers have been studied extensively as diagnostic and therapeutic tools for a wide range of applications [7–9]. In addition, using this method, aptamers have been selected against a wide array of targets from small molecules, proteins, and bacteria to whole cells (ie, whole-cell SELEX) [10,11].
The major advantages in using aptamers over traditional antibodies and growth factors are their high affinity and targeting specificity, as well as their nonimmunogenic nature. Aptamers are also easily synthesized and can be modified to confer nuclease resistance and thus stability [12]. With regard to aptamer–receptor interactions, most work with aptamers exploits the ability of aptamers to inhibit protein–protein interactions, such as receptor–ligand interactions, thereby rendering them receptor antagonists [12]. This approach has been quite successful in the area of neovascularization and angiogenesis inhibition [13–15]. The higher affinity of aptamers to their targets compared with other receptor ligands, such as antibodies or growth factors, makes them excellent at blocking receptor activation.
As a departure from current strategies, we show the ability of an engineered aptamer assembly to act as a receptor agonist that activates vascular endothelial growth factor receptor-2 (VEGFR2) on human endothelial cells. The activation of VEGFRs plays a crucial role in many processes of vascular stem and mature cells, including cell proliferation, migration, differentiation, survival, tissue factor production, and nitric oxide production, as well as functions such as angiogenesis [16]. VEGFR2 is expressed on vascular endothelial cells and is involved in angiogenic activities and thus is used in this study [16]. With that in mind, herein, we report the use of a DNA oligonucleotide sequence (aptamer) that when assembled into a unique divalent assembly acts as a receptor agonist toward VEGFR2.
Materials and Methods
Aptamer design
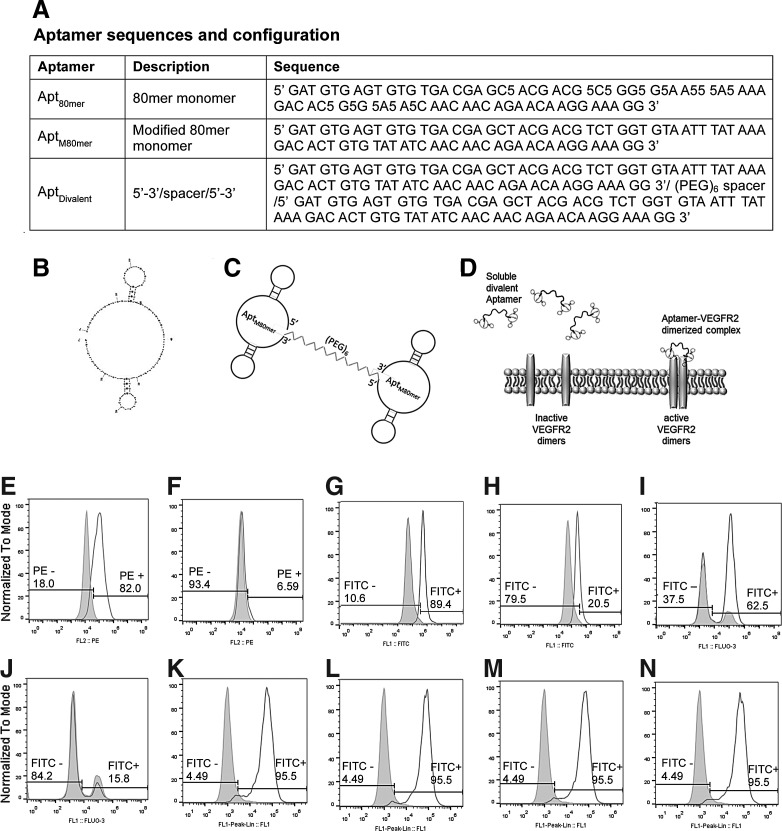
The aptamers used in our study include an 80mer VEGFR2-binding DNA aptamer with modified nucleic acids that was obtained from Aptamer Sciences (AptSci, Pohang, Gyeongbuk, South Korea) and used to confirm endothelial cell binding. The sequence (Apt80mer) is shown in Fig. 1A. Following validation of endothelial cell binding with the commercially available aptamer sequence, subsequent receptor agonist studies were conducted with a modified 80mer DNA aptamer of similar sequence, however, by replacing the benzyl-containing modified bases (BndU) (indicated as 5 in the sequence) with thymine (Integrated DNA Technologies, Coralville, IA). The modification of the bases results in slightly lower binding affinity; however, the binding specificity toward VEGFR2 is maintained. The modified sequence (AptM80mer) is also shown in Fig. 1A.
FIG. 1.
Aptamer design and binding to endothelial cells. (A) Table showing aptamer sequences and configuration. (B) The predicted secondary structure with the lowest free energy of the AptM80mer monomer (structure predicted by UNAfold). (C) Schematic representation of the divalent aptamer assembly (AptDivalent) showing two AptM80mer monomers tethered to the 3′ and 5′ ends to an 18-atom hexaethylene glycol spacer. (D) Schematic representation of the proposed method of action of the divalent aptamer assembly to target and activate through dimerization, membrane-bound vascular endothelial growth factor receptor-2 (VEGFR2). Images (E–J) show flow cytometry data of aptamer–endothelial cell binding. Unprobed human umbilical vein endothelial cells (HUVECs) are shown in each panel as the gray histogram. The white histogram shows (E) positive binding of CD309 (VEGFR2) antibody; (F) negative binding from the isotype control; (G) positive binding of fluorescein isothiocyanate (FITC)-conjugated Apt80mer aptamer; and (H) inhibition or decreased binding of the aptamer when blocked with soluble vascular endothelial growth factor (VEGF) before aptamer incubation. (I) Positive binding of FITC-conjugated modified AptM80mer aptamer and (J) inhibition or decreased binding of the aptamer when blocked with soluble VEGF before aptamer incubation. Images (K–N) show flow cytometry data of aptamer–endothelial cell binding in competition with soluble VEGF. Unprobed HUVECs are shown in each panel as the gray histogram. The white histogram in each figure shows positive binding of 150 nM of the fluorescent-tagged AptM80mer solution in competition with (K) 50 nM, (L) 75 nM, (M) 100 nM, and (N) 150 nM VEGF solutions.
Toward the goal of assessing aptamer agonist function, we fabricated an aptamer assembly comprising two identical AptM80mer monomers tethered to each end of an 18-atom hexaethylene glycol spacer, thus creating a divalent assembly (AptDivalent) (Integrated DNA Technologies). Polyethylene glycol (PEG) was chosen to be the spacer of the divalent aptamer assembly because it exhibits many qualities desirable for the functionality of our assembly. First, PEG shows favorable biological properties, in that it is nontoxic and nonimmunogenic. Additionally, the hydrophilic nature of PEG helps to support water solubility of the assembly. Furthermore, PEG is flexible and does not impose steric hindrance on aptamer molecules bound to it. Finally, PEG molecules have been shown extensively in the literature to prevent nonspecific binding and thus may prevent nonspecific aptamer binding [17].
As another selection criterion, we considered that the root-mean-square distance between the two subunits of VEGFR2 is reported as 3.5 Å [18]. Therefore, to make possible the binding of both receptor subunits, we selected the 18-atom hexaethylene glycol spacer with an end-to-end chain length of 21 Å and a calculated Flory radius of 10.26 Å [19] as this length would make such a dimerization mechanism possible. It should be noted that while we do not demonstrate this mechanism in this work, based upon the length of our spacer, it is possible with our AptDivalent assembly. The sequence, predicted secondary structure with the lowest free energy, and a schematic representation of the divalent aptamer assembly are shown in Fig. 1A–C.
Aptamer–endothelial cell binding
To determine the ability of the 80mer aptamer and the modified base 80mer, Apt80mer and AptM80mer, respectively, to bind to the VEGFR2, flow cytometric analysis was performed using human umbilical vein endothelial cells (HUVECs; Lonza, Baltimore, MD). HUVECs between passages 5 and 9 were cultured in endothelial cell basal media (EBM-2; Lonza), supplemented with human epidermal growth factor-2 (hEGF-2), hydrocortisone, human fibroblast growth factor-basic (hFGF-b), vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), ascorbic acid, and gentamycin/amphotericin-B, and 2% fetal bovine serum (FBS) at 37°C in a CO2 incubator.
The VEGFR2-binding aptamer (Apt80mer) and the modified base aptamer (AptM80mer) containing a terminal amine group were obtained to allow for fluorescein isothiocyanate (FITC) modification. The FITC tag was incubated with the amine-terminated aptamers separately in borate buffer (pH 8.5) for 1 h at room temperature with gentle mixing. The excess FITC was removed through the use of three consecutive Zeba desalting columns (Pierce Biotechnology, Rockford, IL) to ensure complete removal of the dye. The amine group on the aptamer was then linked to the isothiocyanate group, forming a thiourea-type bond between the FITC molecule and the aptamer. The FITC-conjugated aptamer solution was suspended in phosphate-buffered saline (PBS) (pH 7.4) and stored in the dark.
Control experiments included the use of VEGFR2 antibody (CD309) and an IgG isotype control antibody for testing nonspecific binding (BD Biosciences, San Jose, CA). In preparation for flow cytometry, HUVECs were detached from tissue culture polystyrene (TCPS) flasks using cell dissociation media, then split into separate microcentrifuge tubes, each containing a suspension of 300,000 cells. The FITC-conjugated aptamers, that is, Apt80mer and AptM80mer previously prepared, were then incubated with HUVECs for 1 h in suspension to allow for the aptamer binding to the cell surface VEGFR2. This was followed by a washing step (three centrifugal spins and washes) utilizing PBS containing 0.1% Tween-20 to ensure the removal of nonspecifically bound aptamer. The cells were suspended in PBS containing 4% FBS and run on an Eclipse EC800 flow cytometer (Sony Biotechnology, Champaign, IL). The data were analyzed using FlowJo (Treestar, Ashland, OR) and plotted as a histogram.
To confirm binding specificity of the Apt80mer and AptM80mer, in a separate experiment sample, HUVECs were treated as described, except the cells were incubated with 2 ng/mL soluble VEGF before incubation with the aptamer to block the target VEGFR2. In another experiment, we assessed AptM80mer binding when in competition with soluble VEGF. In this experiment, HUVECs were incubated simultaneously with 150 nm AptM80mer and 50, 75, 100, or 150 nm soluble VEGF. Flow cytometry was conducted to detect changes in aptamer binding affinity in the presence of soluble VEGF and the data were analyzed using FlowJo.
VEGFR2 phosphorylation
The activation of VEGFR2 by both the monomeric form and divalent aptamer assembly was tested by assessing phosphorylation events of VEGFR2 on HUVECs. Phospho-VEGFR2 was quantitatively measured spectrophotometrically using Human Phospho-VEGF R2/KDR DuoSet IC (R&D Systems, Minneapolis, MN). Briefly, HUVECs were grown in complete media previously described to nearly 100% confluence on TCPS. The cells were then serum starved overnight in basal media (BM) containing 0.2% FBS and 0.1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO). Immediately, before stimulation, the HUVECs were washed with PBS, and then stimulated for 10 min in unsupplemented BM, unsupplemented BM containing 100 ng/mL VEGF, unsupplemented BM containing 300 nM AptM80mer, or unsupplemented BM containing 150 nM AptDivalent.
Following stimulation, the cells were first rinsed with ice-cold PBS, and then incubated on ice for 15 min in ice-cold lysis buffer containing 10 μg/mL each of protease inhibitors, aprotinin and leupeptin (R&D Systems). The lysates were collected and assayed by following the standard enzyme-linked immunosorbent assay protocol provided by the manufacturer (R&D Systems). The optical density of the samples was immediately determined using a Synergy H1 microplate reader (Biotek, Winooski, VT) with the absorbance measured at 450 nm and wavelength correction set at 540 nm.
Signal pathway activation
Activation of the downstream Akt pathway by the divalent aptamer assembly was assessed through immunological labeling and flow cytometry. HUVECs were grown to 80% confluence on TCPS before stimulation, and then subsequently serum starved overnight in EBM-2 devoid of FBS and growth factors. Following culture overnight in serum-free media, the cells were stimulated for 20 min in unsupplemented serum-free BM (EBM-2), unsupplemented BM containing 2 ng/mL VEGF, or in unsupplemented BM containing 300 nM AptDivalent.
Following the stimulation, the cells were washed with PBS, trypsinized, and split into microcentrifuge tubes containing 300,000 cells. The stimulated cells were permeabilized using a cold 100% methanol solution at 4°C for 15 min, washed thrice to remove excess methanol, and fixed using fluorofix buffer (Sony Biotechnology) for 30 min at room temperature. The cells were again washed using PBS thrice to remove any excess fixative in preparation for staining. The cells were then probed using anti-phospho-Akt antibody (Ser473) (EMD Millipore, Billerica, MA) (250 μg/sample) for 1 h in the dark with occasional gentle mixing. The cells were subsequently washed thrice with PBS containing 0.05% Tween-20 to remove nonspecifically bound antibody. The cells, resuspended in PBS containing 4% FBS, were subsequently run on the Eclipse EC800 flow cytometer and the data were examined and plotted using FlowJo.
Endothelial nitric oxide synthase expression
Changes in expression of endothelial nitric oxide synthase (eNOS) were assessed using western blot analysis. Briefly, HUVECs were grown in complete media previously described to 80% confluence. Before stimulation, the HUVECs were washed with PBS, and then stimulated for 24 h in unsupplemented BM (EBM-2 devoid of serum and growth factors), unsupplemented BM containing 2 ng/mL VEGF, or in unsupplemented BM containing 300 nM AptDivalent. Following stimulation, cells were lysed on ice for 15 min in 0.1% Triton X in PBS containing 10 μg/mL protease inhibitor cocktail (Sigma-Aldrich). Cellular proteins were resolved by 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subsequently transferred to a polyvinylidene difluoride (PVDF) membrane (Fisher Scientific, Waltham, MA).
After transfer, the membranes were divided with the top portion being immunoblotted with anti-eNOS primary antibody (1:500; Abcam, Cambridge, United Kingdom) overnight at 4°C. The lower portion of the membrane was probed with anti-β-tubulin primary antibody to serve as a loading control (1:500; Abcam). eNOS and β-tubulin protein expression was visualized using a horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:5,000; Abcam) and colorimetrically detected using the Opti-4CN HRP substrate detection kit (Bio-Rad, Hercules, CA). The membrane was digitally imaged and the intensity of the resulting bands was quantified using ImageJ software version 1.46.
Capillary tube formation (angiogenesis)
To assess the function of the divalent aptamer assembly, we conducted a capillary tube formation assay. Recall that the divalent aptamer comprises two AptM80mer monomers tethered at both ends of a PEG spacer. Therefore, to confirm the effectiveness of our divalent assembly structure over the monomeric aptamer, we also included the AptM80mer.
Briefly, HUVECs were plated on a 48-well tissue culture plate precoated with 250 μL of Cultrex basement membrane extract (Trevigen, Gaithersburg, MD). The cells were then treated with 100 μL each of unsupplemented BM (EBM-2 devoid of serum and growth factors), unsupplemented BM containing 2 ng/mL VEGF, unsupplemented BM containing 300 nM AptM80mer, or unsupplemented BM containing 150 nM AptDivalent. After 12 h of incubation in a 5% CO2 humidified atmosphere at 37°C, the three-dimensional structures formed by the cells in this matrix were examined using an inverted phase-contrast microscope and digital images were recorded. Tube-like structures were further analyzed by measuring the sum of the lengths all tubules per field using the image analysis software, AxioVision (Zeiss, v.4.5; Carl Zeiss Microsystems, Thornwood, NY). Four randomly selected low-power fields were examined for each sample.
Results and Discussion
In this study, we report the design of a supramolecular aptamer assembly for targeted VEGFR2 binding and activation. The design of the aptamer assembly is based upon the native interaction of soluble VEGF with both subunits of surface-bound VEGFR2. When bound, soluble VEGF acts as a bridge between the two subunits and results in their dimerization. The rationale behind this approach has proven successful in other surface receptors, such as OX40, a receptor expressed on activated T cells [20]. In this work, an RNA aptamer scaffold was designed with agonist function by binding to the receptor subunits of the OX40 receptor. In a similar manner, we hypothesize that the aptamer assembly described herein allows for the VEGFR2-binding aptamer at each end of the 18-atom spacer to bind to each of the two subunits comprising the VEGFR2 and facilitate dimerization. A schematic demonstrating the proposed mode of action of the divalent aptamer assembly is shown in Fig. 1D.
Receptor agonist activity is predicated on the ability of the aptamer assembly to first bind the target receptor. The Apt80mer used in this work has a high VEGFR2-binding affinity with a Kd of 0.12 nM (AptSci). However, the divalent aptamer assembly comprises two identical monomeric units of AptM80mer tethered to each end of an 18-atom hexaethylene glycol spacer. To demonstrate specificity of the Apt80mer as well as the AptM80mer monomer toward HUVECs, known for high levels of VEGFR2 expression, flow cytometry was performed on HUVECs probed with FITC-conjugated Apt80mer (FITC-Apt80mer) and FITC-conjugated AptM80mer (FITC-AptM80mer). Controls included phycoerythrin (PE)-labeled VEGFR2-specific antibody and PE-labeled isotype control (BD Biosciences). Our results confirm VEGFR2 expression on HUVECs as evidenced by the rightward shift (∼82% positive) for the VEGFR2 antibody compared with ∼6% positive for the isotype control (Fig. 1E, F).
To demonstrate binding specificity of the Apt80mer and AptM80mer toward the VEGFR2 target receptor, HUVECs were incubated with Apt80mer and AptM80mer with and without pretreatment with soluble VEGF. The flow cytometry data demonstrate ∼89% positive targeted binding of Apt80mer to VEGFR2 compared with the diminished ∼20% positive Apt80mer binding when pretreated with soluble VEGF (Fig. 1G, H). Similarly, the data also showed ∼62.5% positive targeted binding of AptM80mer to VEGFR2, while the cells pretreated with soluble VEGF showed diminished binding (∼15.8% positive) (Fig. 1I, J). These data demonstrate successful receptor–ligand binding, which is the critical first step in the complex process of membrane receptor activation [21]. In addition, the cells preincubated with VEGF showed diminished aptamer binding, which indicates specificity of the binding aptamer to the VEGF receptor.
Finally, the assessment of competitive binding of the aptamer in the presence of VEGF at varying concentrations showed that VEGF did not interfere with the aptamer binding at concentrations ranging from 50 to 150 nM (Fig. 1K–N). In addition, our preliminary data (not shown), from a separate experiment conducted, indicate that over a period of 20 h the bound monomeric aptamer (AptM80mer) is internalized by the cell. This early finding is consistent with reports in the literature that showed internalization of aptamers by the target cell [22,23]. Further investigation is required to study the specific localization of the aptamer assembly within a cell.
The binding of soluble VEGF to VEGFR2 initiates a sequence of events resulting in receptor dimerization, kinase activation, and autophosphorylation of specific tyrosine kinase residues within the dimeric complex [24]. The activation of tyrosine kinase through transphosphorylation between receptor molecules is the first step leading to activation of intracellular signal transduction pathways [21]. Therefore, to assess the activation of a cell surface receptor, assessment of phosphorylation events is important. Toward this goal, HUVECs were treated with BM supplemented with the AptDivalent, AptM80mer, or soluble VEGF and assessed for VEGFR2 phosphorylation.
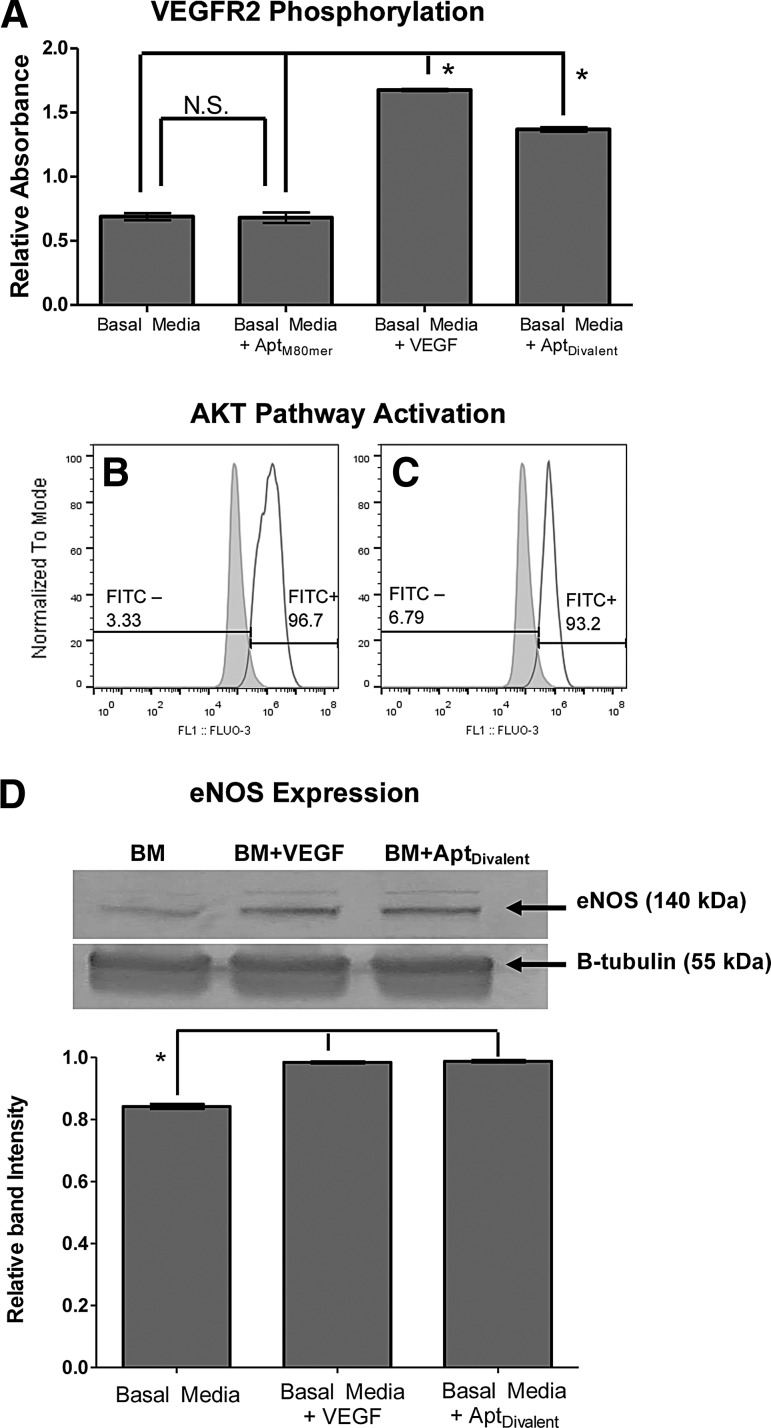
As expected, treatment of HUVECs with soluble VEGF results in activation of VEGF receptor-2 phosphorylation events (Fig. 2A). We also show that treatment of HUVECs with AptDivalent also results in phosphorylation of VEGFR2 (Fig. 2A). Furthermore, treatment of HUVECs with the divalent assembly results in significantly greater phosphorylation relative to the amount of phosphorylation from BM or BM containing monomeric AptM80mer (Fig. 2A; P<0.05). These data reaffirm the specificity of the divalent aptamer assembly and demonstrate the promotion of VEGFR2 phosphorylation events on HUVECs. More importantly, these data demonstrate the unique advanced ability of the divalent assembly in receptor activation that is not seen in the monomeric form. In fact, despite its ability to bind VEGFR2, the addition of the monomeric aptamer (AptM80mer), from which the divalent assembly is composed, resulted in no difference in phosphorylation over unsupplemented BM alone (Fig. 2A; P>0.05). The induction of receptor phosphorylation through an aptamer assemblage shown here represents one of the early and few examples in the literature of receptor activation through an aptamer complex.
FIG. 2.
Aptamer induced VEGFR2 activation and downstream molecular events. (A) Quantification of HUVEC VEGFR2 phosphorylation upon exposure to unsupplemented basal media (BM), BM supplemented with 100 ng/mL soluble VEGF, 150 nM soluble divalent aptamer (AptDivalent), or 300 nM monomer (AptM80mer). Data shown are mean±standard deviation (SD). Images (B, C) show activation of the Akt signal transduction pathway. HUVECs are shown in each panel as the gray histogram. The white histogram shows positive binding of anti-phospho-Akt (Ser473) antibody, thus indicating pathway activation upon stimulation with (B) 2 ng/mL soluble VEGF; and (C) 300 nM AptDivalent. Image (D) represents endothelial nitric oxide synthase (eNOS) protein expression from HUVECs treated with unstimulated BM or BM supplemented with 2 ng/mL soluble VEGF or 300 nM soluble divalent aptamer (AptDivalent). The top membrane shows eNOS-specific protein bands, and the bottom membrane shows β-tubulin-specific bands that were used as a loading control. eNOS protein band intensity was normalized to β-tubulin and quantified using ImageJ software version 1.46. In figures (A, D), based upon one-way analysis of variance (ANOVA) with Tukey's multiple comparison test, N.S. indicates no significant difference (P>0.05) and * indicates a statistically significant increase (P<0.05).
The downstream events in VEGF receptor activation are quite complex, involving multiple signal molecules and multiple molecular pathways, including the well-characterized Akt pathway [16]. To further assess the effect of aptamer–receptor binding, we examined the activation of this downstream molecular pathway. Akt pathway activation is involved in numerous diverse biological processes, such as cell survival/apoptosis, cell cycle control, angiogenesis, differentiation, and cell growth and proliferation [25,26]. In addition, Akt pathway activation has been associated with activation of VEGFR2 through treatment with soluble VEGF [27,28].
In this study, serum-starved HUVECs were treated with AptDivalent or soluble VEGF, then phosphorylation of serine 473 is detected with a phospho-Akt antibody and analyzed through flow cytometry. Data reveal that treatment with the AptDivalent results in activation of the Akt pathway in levels similar to that seen with soluble VEGF (Fig. 2B, C). The cells treated with either AptDivalent or soluble VEGF showed positive staining for phospho-Akt, ∼97% and 93%, respectively. Although the activation of the Akt pathway by soluble VEGF is expected and has been shown by others [16], there are currently no reports of Akt pathway activation triggered by treatment with a DNA aptamer or an aptamer assembly as we have fabricated. These promising results provide early evidence that the AptDivalent assembly, as designed, promotes activation of VEGFR2 and causes downstream activation of pathways that control critical cell functions.
eNOS is an important protein involved in the production of nitric oxide in endothelial cells and is critical for regulation of cardiac function and angiogenesis. Our protein expression experiments reveal that aptamer induced upregulation of eNOS to levels comparable with what is seen in VEGF-induced upregulation (Fig. 2D). Quantification of the protein levels shows a statistically relevant increase in eNOS expression in HUVECs stimulated with VEGF or our divalent aptamer assembly. These results are encouraging and indicate a potential angiogenic downstream effect of receptor activation.
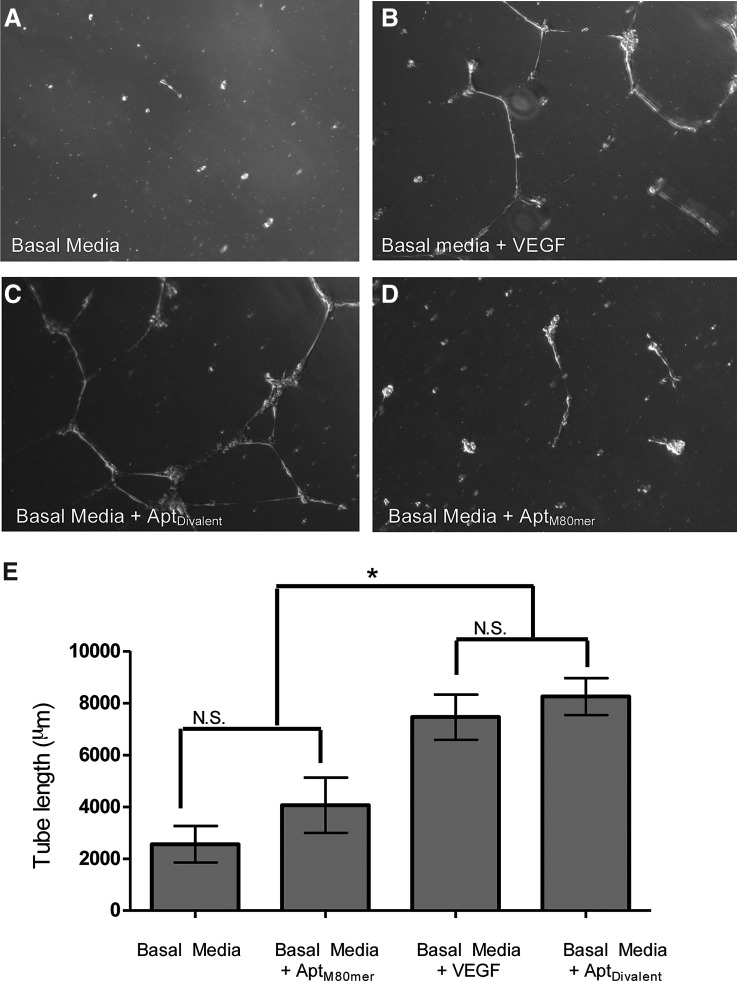
As a final functional assessment of the agonist behavior of the AptDivalent, we utilized a tube formation assay. The tube formation assay is well characterized and regarded as a powerful tool to screen for various factors that promote or inhibit in vitro angiogenesis. In addition, angiogenesis, as measured in the tube formation assay, is mediated by VEGF signaling through activation of VEGFR2 [29]. We demonstrate that soluble AptDivalent promotes HUVEC migration and capillary-like tube formation (Fig. 3A–D). When compared with HUVECs stimulated with soluble VEGF, cells stimulated with AptDivalent show statistically similar tube length measurements (Fig. 3B, C; P>0.05). The data also indicate that the AptDivalent is more effective in inducing capillary tube formation than the AptM80mer (Fig. 3E; P<0.05).
FIG. 3.
Functional assessment of VEGFR2–aptamer binding. Representative phase-contrast images of HUVECs cultured on Matrigel treated with (A) unsupplemented BM; (B) BM supplemented with 2 ng/mL soluble VEGF; (C) 150 nM soluble divalent aptamer (AptDivalent); or (D) 300 nM monomer (AptM80mer). Images in each panel show tube-like structures formed. Quantification of the tube length in micrometers was taken from four separate low-powered magnification images using AxioVision v.4.5 software. Data represent the mean±SD of between 18 and 75 measurements taken from each of four images. Based upon one-way ANOVA with Tukey's multiple comparison test, N.S. indicates no significant difference (P>0.05) and * indicates a statistically significant increase (P<0.05).
These data are particularly impactful as this is the first report of a divalent aptamer assembly activating cellular processes involved in promoting angiogenesis. In addition, these data also show the potency of the divalent assembly to act as a receptor agonist, while the monomeric form (AptM80mer) shows very little or no receptor phosphorylation and downstream angiogenic effect, despite exhibiting excellent binding affinity toward VEGFR2. These data reaffirm the rationale behind the design of the divalent aptamer assembly.
The data presented here demonstrate the ability of an aptamer assembly to act as a receptor agonist for VEGFR2 on human endothelial cells. Furthermore, we demonstrate the function of the aptamer to promote endothelial cell capillary-like tube formation, an important criterion demonstrating functional agonist activity. Our mechanism utilizing an aptamer agonist to modulate receptor response and activate downstream signaling pathways in endothelial cells has not been shown previously in the literature. This work can further be extended to develop aptamer-based receptor agonists to control other cell-mediated processes, such as differentiation, proliferation, and apoptosis.
Future work will utilize structural biology techniques to elucidate the binding specificity and localization of the divalent aptamer toward the target VEGF receptor. Questions remain about the structural mechanism by which the aptamer assembly binds the receptor target and facilitates activation. It is currently unknown where the aptamer binds the receptor subunits and if, as we hypothesize, the aptamer assembly acts as a bridge between subunits. In addition, questions remain about the fate and downstream effects of the aptamer postreceptor binding. Indeed, this work opens the door to further research into the structural or architectural requirements that promote not only aptamer–target binding but agonist behavior as well. With expanding interest, this novel strategy has tremendous potential for the development of novel pharmaceuticals in the treatment of a wide range of diseases, including inflammation, autoimmune diseases, and vascular disease. This work also has implications in tissue engineering and regenerative medicine, where controlling cell processes is critical for success.
Acknowledgment
This work was supported in part by the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1TR000064 and by the National Science Foundation-CBET CAREER award (1453098) both provided to J. Allen. In addition, this work was also supported in part by a NIH/NIGMS grant P50 GM111152 to M. Segal.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gestwicki JE, Cairo CW, Strong LE, Oetjen KA. and Kiessling LL. (2002). Influencing receptor-ligand binding mechanisms with multivalent ligand architecture. J Am Chem Soc 124:14922–14933 [DOI] [PubMed] [Google Scholar]
- 2.Lee K, Silva EA. and Mooney DJ. (2011). Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 8:153–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglas MR, Morrison KC, Jacques SJ, Leadbeater WE, Gonzalez AM, Berry M, Logan A. and Ahmed Z. (2009). Off-target effects of epidermal growth factor receptor antagonists mediate retinal ganglion cell disinhibited axon growth. Brain 132:3102–3121 [DOI] [PubMed] [Google Scholar]
- 4.Jayasena SD. (1999). Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem 45:1628–1650 [PubMed] [Google Scholar]
- 5.Hermann T. and Patel DJ. (2000). Biochemistry—adaptive recognition by nucleic acid aptamers. Science 287:820–825 [DOI] [PubMed] [Google Scholar]
- 6.Tuerk C. and Gold L. (1990). Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510 [DOI] [PubMed] [Google Scholar]
- 7.Brody EN. and Gold L. (2000). Aptamers as therapeutic and diagnostic agents. J Biotechnol 74:5–13 [DOI] [PubMed] [Google Scholar]
- 8.Ellington AD. and Szostak JW. (1990). In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822 [DOI] [PubMed] [Google Scholar]
- 9.White RR, Sullenger BA. and Rusconi CP. (2000). Developing aptamers into therapeutics. J Clin Invest 106:929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris KN, Jensen KB, Julin CM, Weil M. and Gold L. (1998). High affinity ligands from in vitro selection: complex targets. Proc Natl Acad Sci U S A 95:2902–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Zhang M, Yang G, Zhang D, Ding H, Wang H, Fan M, Shen B. and Shao N. (2003). Single-stranded DNA aptamers that bind differentiated but not parental cells: subtractive systematic evolution of ligands by exponential enrichment. J Biotechnol 102:15–22 [DOI] [PubMed] [Google Scholar]
- 12.Keefe AD, Pai S. and Ellington A. (2010). Aptamers as therapeutics. Nat Rev Drug Discov 9:537–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng EW, Shima DT, Calias P, Cunningham ET, Jr., Guyer DR. and Adamis AP. (2006). Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 5:123–132 [DOI] [PubMed] [Google Scholar]
- 14.Ruckman J, Green LS, Beeson J, Waugh S, Gillette WL, Henninger DD, Claesson-Welsh L. and Janjic N. (1998). 2′-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem 273:20556–20567 [DOI] [PubMed] [Google Scholar]
- 15.White RR, Shan S, Rusconi CP, Shetty G, Dewhirst MW, Kontos CD. and Sullenger BA. (2003). Inhibition of rat corneal angiogenesis by a nuclease-resistant RNA aptamer specific for angiopoietin-2. Proc Natl Acad Sci U S A 100:5028–5033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrara N, Gerber HP. and LeCouter J. (2003). The biology of VEGF and its receptors. Nat Med 9:669–676 [DOI] [PubMed] [Google Scholar]
- 17.Mori Y, Nagaoka S, Takiuchi H, Kikuchi T, Noguchi N, Tanzawa H. and Noishiki Y. (1982). A new antithrombogenic material with long polyethyleneoxide chains. ASAIO J 28:459–463 [PubMed] [Google Scholar]
- 18.Leppänen V-M, Prota AE, Jeltsch M, Anisimov A, Kalkkinen N, Strandin T, Lankinen H, Goldman A, Ballmer-Hofer K. and Alitalo K. (2010). Structural determinants of growth factor binding and specificity by VEGF receptor 2. Proc Natl Acad Sci U S A 107:2425–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzaga F, D'Souza R. and Brook MA. (2011). Oriented crystallization of ultra-thin (2 nm) gold nanoplatelets inside a reactive hydrophobic polymeric matrix. Soft Matter 7:722–729 [Google Scholar]
- 20.Dollins CM, Nair S, Boczkowski D, Lee J, Layzer JM, Gilboa E. and Sullenger BA. (2008). Assembling OX40 aptamers on a molecular scaffold to create a receptor-activating aptamer. Chem Biol 15:675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ullrich A. and Schlessinger J. (1990). Signal transduction by receptors with tyrosine kinase activity. Cell 61:203–212 [DOI] [PubMed] [Google Scholar]
- 22.Xiao Z, Shangguan D, Cao Z, Fang X. and Tan W. (2008). Cell-specific internalization study of an aptamer from whole cell selection. Chemistry 14:1769–1775 [DOI] [PubMed] [Google Scholar]
- 23.Zhang K, Sefah K, Tang L, Zhao Z, Zhu G, Ye M, Sun W, Goodison S. and Tan W. (2012). A novel aptamer developed for breast cancer cell internalization. ChemMedChem 7:79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch S. and Claesson-Welsh L. (2012). Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med 2:a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manning BD. and Cantley LC. (2007). AKT/PKB signaling: navigating downstream. Cell 129:1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hers I, Vincent EE. and Tavaré JM. (2011). Akt signalling in health and disease. Cell Signal 23:1515–1527 [DOI] [PubMed] [Google Scholar]
- 27.Fujio Y. and Walsh K. (1999). Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem 274:16349–16354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V. and Ferrara N. (1998). Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273:30336–30343 [DOI] [PubMed] [Google Scholar]
- 29.Koolwijk P, Peters E, van der Vecht B, Hornig C, Weich HA, Alitalo K, Hicklin DJ, Wu Y, Witte L. and van Hinsbergh VW. (2001). Involvement of VEGFR-2 (kdr/flk-1) but not VEGFR-1 (flt-1) in VEGF-A and VEGF-C-induced tube formation by human microvascular endothelial cells in fibrin matrices in vitro. Angiogenesis 4:53–60 [DOI] [PubMed] [Google Scholar]