Abstract
We conducted a prospective monitoring study to determine whether antiretroviral (ARV) levels in hair of Asian children on second-line protease inhibitor-based ARV therapy (ART) are associated with virologic failure (VF), compared to plasma drug levels and self-reported adherence. HIV-infected Asian children on second-line ART regimens were enrolled into a longitudinal cohort. Traditional adherence measures, plasma, and hair samples were collected 24 weeks after study enrollment. Hair ARV levels were determined via liquid chromatography/tandem mass spectrometry. Among 149 children on lopinavir/ritonavir-based regimens, 47% were female; the median [interquartile range (IQR)] age was 10.3 (7.9–13.3) years. The median CD4% was 26% (IQR 21.7–32.1%) and the median CD4 cell count 754 (IQR 596–1,013) cells/mm3. The median duration of lopinavir-based ART prior to week 24 of the study was 2.9 (IQR 1.6–4.2) years. Adherence was >95% in 91% (135/148) by visual analogue scale and 89% (129/145) by pill count. The median lopinavir hair concentrations were 5.43 (IQR 3.21–9.01) ng/mg in children with HIV RNA >1,000 copies/ml and 9.96 (IQR 6.51–12.31) ng/mg in children with HIV RNA <1,000 copies/ml (p = 0.003). Plasma trough and lopinavir hair concentrations were not statistically significantly correlated (Pearson's correlation coefficient 0.20; p = 0.13). Increasing lopinavir hair concentrations in quartiles were strongly associated with virologic success (odds ratios ≥4.0, overall p = 0.02), while self-reported adherence, pill count, and plasma lopinavir levels were not. Based on this first report of hair ARV concentrations and virologic outcomes in children, ARV hair concentrations, representing longer-term adherence, may be useful to identify children at risk for VF.
Introduction
First-line antiretroviral (ARV) therapy (ART) regimens currently used in HIV-infected children <15 years of age in resource-limited settings usually include a nonnucleoside reverse transcriptase inhibitor (NNRTI).1 In those who fail first-line drugs, protease inhibitors (PI) are recommended in second-line regimens.2 The limited global access to third-line ARVs makes preventing second-line failure an urgent priority.
Measuring and supporting adherence is critical in perinatally infected children who require virologic suppression over a lifetime. Self-report, pill counts, and therapeutic drug monitoring (TDM) using random or timed plasma levels have been used to assess adherence. However, these methods have well-known limitations,3 and all reflect short-term adherence; measures reflecting long-term exposure could be useful in children to complement existing approaches for monitoring treatment outcomes.
Hair levels of ARVs reflect drug uptake from the systemic circulation over preceding weeks to months4,5 and have been shown to be strong and independent predictors of long-term adherence and associated treatment outcomes in adults,6–10 and have been compared to other adherence measures in African children.11 No prior study has examined associations between hair ARV levels and virologic outcomes in children. Moreover, ARV concentrations in hair have not previously been evaluated in Asians, where pharmacogenetic variations may influence drug exposure. We examine for the first time the relationship between ARV hair concentrations and virologic responses in an Asian cohort of HIV-infected children receiving second-line ritonavir-boosted lopinavir (LPV)-containing regimens.
Materials and Methods
The TREAT Asia Pediatric HIV network initiated a prospective monitoring study of second-line ART failure and resistance in HIV-infected children in eight participating sites in three Southeast Asian countries: Vietnam, Thailand, and Indonesia. Children <18 years old who experienced first-line treatment failure and had already switched or were switching to second-line ART were enrolled. Clinical and laboratory assessments to evaluate treatment outcomes and safety were performed every 6 months and according to local HIV treatment guidelines. Adherence assessments using the 30-day visual analogue scale (VAS) and pill counts, as well as plasma and hair sampling for LPV concentrations, were collected at week 24 and analyzed in relationship to HIV viral load.
Hair collection
Small samples of hair were collected at week 24 according to previously described methods.6,12 Approximately 20 strands of hair (1–3 mg) are required to measure the concentration of most ARVs.13,14 Hair samples were cut from underneath the top layer of hair from the occiput8,9 and then subsequently placed in aluminum foil, stored at room temperature, and shipped to the University of California, San Francisco (UCSF) for analysis.
Analyzing ARV concentrations in plasma and hair samples
Plasma concentrations of LPV were determined by validated high-performance liquid chromatography (HPLC) using an ultraviolet detection method,15 with a lower limit of quantification of 0.105 mg/liter. The within-run and between-run percent variation (precision) was <10%, and the accuracy of the ARV measurements was 100% ± 5%.15
The Hair Analysis Laboratory (HAL) at UCSF has developed, validated, and published the methods for analyzing lopinavir/ritonavir in hair using liquid chromatography/tandem mass spectrometry (LC/MS-MS). Briefly, the lopinavir and ritonavir in each hair specimen are extracted with methanol (MeOH):trifluoroacetic acid (TFA) at 9:1. The extracted samples are separated by reversed phase chromatography and detected by tandem mass spectrometry in electrospray positive ionization with multiple reaction monitoring (MRM) mode. The hair assays have been validated from 0.05 ng/mg to 20 ng/mg hair for lopinavir and 0.01 ng/mg to 4 ng/mg for ritonavir, with good linearity (R2>0.99) and reproducibility (coefficient of variation <15%) for both assays.
Statistical analysis
Pearson's correlation coefficients were calculated to assess the linear correlation between hair and plasma LPV concentrations. The Wilcoxon rank sum test was used to compare the distribution of LPV concentrations by whether or not children had virologic failure (i.e., HIV RNA ≥1,000 copies/ml at 24 weeks). Logistic regression was used to estimate the odds of virologic success. Predictors assessed in the models included age, sex, body surface area, self-reported adherence by 30-day VAS or pill count categorized as either ≥95% or <95%, and LPV concentrations in hair. Linearity of continuous covariates against the logit was assessed, and in the case of nonlinearity, the covariate was grouped into quartiles. Covariates found to be statistically significant at p-values < 0.2 in univariate analyses were included in a multivariate model.
Ethical considerations
Study protocols and consent forms were reviewed and approved by local institutional review boards (IRBs) of all participating sites and the coordinating center. Parents or legally recognized guardians provided informed consent. The requirement for patient assent was deferred to each site's IRBs and obtained if required.
Results
A total of 299 children who had been switched or were being switched to second-line ART were enrolled in the overall study. At the time of this analysis, 149 children receiving LPV-based regimens had reached their 24-week follow-up visit and had hair samples available for testing.
Demographics
Of 149 children, 47% were female; the median [interquartile range (IQR)] age was 10.3 (7.9–13.3) years and the median weight was 25.7 (IQR 19.9–35.4) kg. The median CD4% was 26 (IQR 21.7–32.1) and the median CD4 cell count was 754 (IQR 596–1,013) cells/mm3. All children were exposed to first-line NNRTI-based ART before switching to second-line LPV-based ART. The median duration of LPV-based ART prior to week 24 was 2.9 (IQR 1.6–4.2) years, at which time most children (85%) had achieved virologic suppression. Although 17 (11%) children had virologic failure by the study definition, an additional 22 (15%) had viral loads above the lower limit of detection (>400 copies/ml). Adherence to LPV-based ART was >95% in 135/148 (91%) children as assessed by VAS and in 129/145 (89%) determined via pill count. The median (IQR) self-reported adherence was 100 (100–100)%.
LPV concentrations in plasma and hair
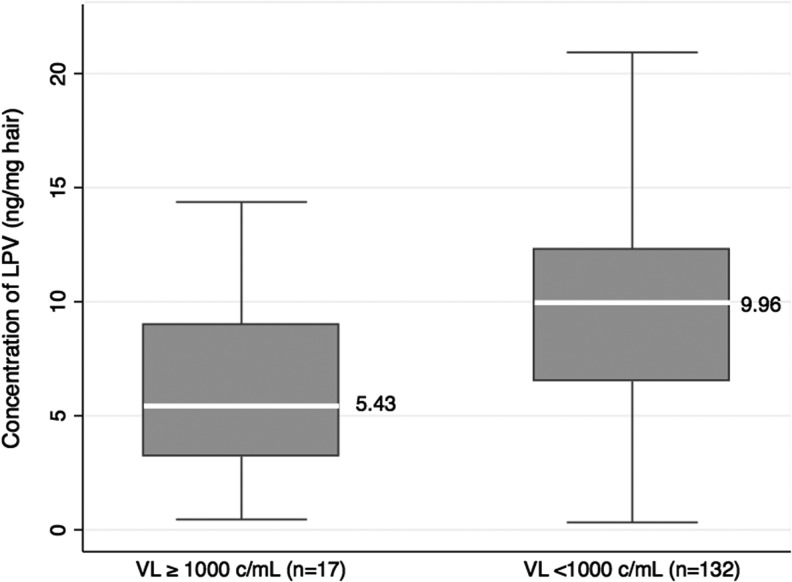
LPV concentrations were measured in 149 available week 24 plasma samples. Only 61 of 149 children had plasma concentrations representing true trough values as determined by the time of last dose taken; the remainder had only random plasma sample concentrations available. The median trough plasma LPV concentration for those with available tests was 6.7 (IQR 4.1–9.6) mg/liter. Figure 1 shows the median and range LPV concentrations in hair in children with sustained virologic success versus levels in those with virologic failure. The median LPV hair concentrations were 5.43 (3.21–9.01) ng/mg in children with HIV RNA levels >1,000 copies/ml and 9.96 (6.51–12.31) ng/mg in children with HIV RNA levels <1,000 copies/ml (p = 0.003). Plasma trough and hair concentrations of LPV were weakly, but not statistically significantly, correlated (Pearson's correlation coefficient 0.20; p = 0.13).
FIG. 1.
Lopinavir hair concentrations by virologic suppression category. Lopinavir (LPV) hair concentrations (ng/mg of hair) in children who had viral load (VL) ≥1,000 (left) and <1,000 copies/ml (right). The box shows the interquartile range (IQR) and the white line in the center shows the median. Whiskers show the lowest and highest points within 1.5 times the IQR.
Predictors of virologic success
In univariate models, age, sex, body surface area, weight, and adherence by VAS and pill count were not statistically significantly associated with virologic outcomes. LPV concentrations in hair categorized into quartiles were statistically significantly associated with virologic success (p = 0.02; Table 1). Compared to children with LPV hair concentrations in the lowest quartile (≤6.11 ng/mg of hair), the odds of virologic success were increased by 4-fold (OR 4.05; 95% CI 1.01–16.15) in those with LPV hair concentrations in the second quartile (LPV concentrations 6.36–9.56 ng/mg). The odds of virologic success were identical or higher (OR 6.25; 95% CI 1.27–30.88) in children with LPV concentrations in either the third (9.75–12.13 ng/mg) or highest quartile (12.15–22.10 ng/mg). The group with hair concentrations in the lowest quartile contained the highest percentage of children with virologic failure (26%); the percentage of virologic failures among those in the second quartile was 8% and was 5% each in the highest quartiles. Similar relationships between hair levels and virologic suppression were seen when adjustment was made for potential confounders including age, self-reported adherence, weight, and body surface area (BSA). When we additionally evaluated a model including both hair level and BSA as predictors, the effects of hair level were similar to the univariate results and remained statistically significant (p = 0.03), while those for BSA were also similar to univariate results and remained statistically nonsignificant (p = 0.40).
Table 1.
Univariate Logistic Regression Analysis of Virologic Success
| Covariate | n/N (%) with virologic success | OR (95% CI) for virologic failure | p |
|---|---|---|---|
| Sex | 0.99 | ||
| Male | 70/79 (89) | 1 (ref) | |
| Female | 62/70 (89) | 1.00 (0.36–2.76) | |
| Age (years) | 0.50 | ||
| 2–7.8 | 33/38 (87) | 1 (ref) | |
| 7.9–10.3 | 31/37 (84) | 0.78 (0.22–2.83) | |
| 10.4–13.3 | 33/37 (89) | 1.25 (0.31–5.07) | |
| 13.4–18.5 | 35/37 (95) | 2.65 (0.48–14.62) | |
| Body surface area (m2) | 0.31 | ||
| 0.49–0.80 | 31/37 (84) | 1 (ref) | |
| 0.81–0.95 | 31/37 (84) | 1.00 (0.29–3.44) | |
| 0.96–1.19 | 34/37 (92) | 2.19 (0.50–9.53) | |
| ≥1.20 | 25/37 (95) | 0.3 (0.64–18.02) | |
| Adherence by 30-day visual analogue scale | 0.48 | ||
| ≥95% | 119/135 (88) | 1 (ref) | |
| <95% | 12/13 (92) | 2.13 (0.26–17.19) | |
| Adherence by pill count | 0.66 | ||
| ≥95% | 113/129 (88) | 1 (ref) | |
| <95% | 15/16 (94) | 1.61 (0.20–13.25) | |
| Lopinavir hair concentration (ng/mg of hair) | 0.02 | ||
| 1st quartile: ≤6.11 | 28/38 (74) | 1 (ref) | |
| 2nd quartile: 6.36–9.56 | 34/37 (92) | 4.05 (1.01–16.15) | |
| 3rd quartile: 9.75–12.13 | 35/37 (95) | 6.25 (1.27–30.88) | |
| 4th quartile: 12.15–22.10 | 35/37 (95) | 6.25 (1.27–30.88) |
Discussion
Our study is the first to examine the association of ARV hair concentrations with virologic responses in HIV-infected children. We show that LPV concentrations in hair are strongly associated with virologic outcomes among children on second-line ART, with a higher odds of virologic success with increasing LPV in hair. We have previously demonstrated similar associations between ARV hair concentrations and viral suppression in adults,6,7,16 but the current study has implications for monitoring treatment outcomes among children, where adherence can be difficult to measure and sustain. Moreover, hair levels of ARVs serve not only as a marker of adherence, but of drug exposure, and since childhood is a period of pharmacokinetic flux, quantitative measurement of drug levels in pediatric populations may be important for prolonging the duration of effective treatment, especially when alternative regimen options are limited.
The limitations of self-report and pill counts to assess adherence are well described.3,17–20 Compared to hair drug levels, our study found that measuring adherence using 30-day self-report with VAS, pill count, or even TDM via single plasma samples was less sensitive in identifying chronically poor adherence (as adjudicated by virologic failure). Moreover, self-reported adherence was high (median 100%), despite variability in hair concentrations. Plasma drug levels can exhibit high rates of intraindividual variability,21 and reflect only those doses administered within the previous few days.22 “White-coat” effects, in which adherence transiently improves prior to clinic visits,23 can limit the ability of a single level to reflect typical adherence patterns. In this particular study, only a subset of children (61/149) had true plasma trough concentrations available, and, of those, 60 demonstrated virologic suppression, implying a possible selection bias in the plasma samples available for analysis. Therefore, we could not meaningfully explore the relationship between trough plasma concentrations and virologic suppression rates further in this study.
Second- and third-line ARV options are limited in resource-limited settings, creating challenges to providing prolonged potent therapy to perinatally infected children. Being able to accurately measure long-term adherence and exposure is essential to help predict and prevent treatment failure. Drug concentrations in hair represent an average level of exposure over time, which may be useful to monitor in pediatric populations. In our study, the acceptability of hair collection was high (100%), which is consistent with prior studies by our group in Africa showing high rates of feasibility and acceptability (>95%) in hair collection.8,12 Hair collection for exposure monitoring may be particularly desirable in pediatric populations, since hair sampling avoids phlebotomy.24 However, we have shown that early implementation of community information campaigns and field staff training are important to enhance hair collection rates.8 A qualitative study from South Africa on the acceptability of hair collection in HIV-infected women underscored this point.25 HIV studies/cohorts planning on incorporating hair collection should provide clear explanations of the risks and benefits of hair sampling to field staff and participants in order to enhance acceptability.
Our study had several limitations. Over half of the children with hair samples did not have trough plasma samples available so we could not perform a meaningful analysis of the relationship between trough plasma level and virologic suppression. Moreover, only children with both trough LPV plasma levels and LPV hair concentrations could be included in the correlation analysis. Another limitation was that there were few children with virologic failure at week 24, which may have impacted our ability to fully characterize the associations between the various adherence measurements included in the study. Furthermore, we did not have information on the protein content of the hair samples, which could be influenced (along with rate of hair growth) by nutritional status. Finally, hair testing for ARV levels has yet to be implemented within Asia, and requires shipment of hair samples to another region, limiting its feasibility outside of the research context. The plasma and hair assays used for this study have been standardized and validated using high-performance, but expensive chromatography platforms. Widespread applicability of hair assays in clinical settings will rely on the availability of low-cost lower-technology hair assays, currently under development.26
In conclusion, this is the first study to demonstrate an association between ARV concentrations in hair and virologic outcomes among HIV-infected children. In our cohort, self-reported adherence and pill counts were not associated with virologic outcomes, reflecting well-described limitations of these measures. Measuring drug exposure in pediatric populations, when adherence and pharmacokinetic parameters can vary, may be important for predicting and influencing outcomes. Earlier detection of those at risk for treatment failure using this innovative and noninvasive monitoring method could facilitate targeted interventions for those most at risk for nonadherence in settings with limited second- and third-line ARV options, but would require expansion of testing access for further implementation.
Appendix
The TASER-Pediatrics Study Group: N. Kurniati* and P. Wicaksana, Cipto Mangunkusumo General Hospital, Jakarta, Indonesia. T. Sudjaritruk,* V. Sirisanthana, and L. Aurpibul, Chiang Mai University, Chiang Mai, Thailand. P. Kosalaraksa,* P. Lumbiganon, and C. Sopharak, Khon Kaen University, Khon Kaen, Thailand. W. Prasitsuebsai,* S. Kerr,* T. Bunupuradah, S. Teeraananchai, T. Jupimai, J. Intasan, N. Thammajaruk, and C. Ruengpanyathip, The HIV Netherlands, Australia, Thailand Research Collaboration (HIV-NAT), Bangkok, Thailand. K. Chokephaibulkit,* S. Sricharoenchai, W. Phongsamart, and N. Kongstan, Siriraj Hospital, Mahidol University, Bangkok, Thailand. K.H. Truong* and T.P.K. Le, Children's Hospital 1, Ho Chi Minh City, Vietnam. V.C. Do,* T.M. Ha, and V.T. An, Children's Hospital 2, Ho Chi Minh City, Vietnam. L.V. Nguyen,* K.D.T. Khu, and T.T.T. Giang, National Hospital of Pediatrics, Hanoi, Vietnam. A.H. Sohn,* N. Durier,* and T. Singtoroj, TREAT Asia, amfAR–The Foundation for AIDS Research, Bangkok, Thailand. *TASER-P Steering Committee member.
Acknowledgments
This study was funded by TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from ViiV Healthcare, the U.S. National Institutes of Health's National Institute of Allergy and Infectious Diseases (M. Gandhi and P. Bacchetti), Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907), and the Austrian AIDS Life Association. Partial support for hair assays came from the National Institutes of Allergy and Infectious Diseases (NIAID)/NIH R01 AI098472 (M. Gandhi) and the Women's Interagency HIV Study (U01AI034989); we would like to thank Dr. Leslie Z. Benet and Alexander Louie in the laboratory for work on the hair assays. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above. The authors would like to thank the participants and their families for taking part in this study.
These data were presented in part at the 5th International Workshop on HIV Pediatrics, June 28–29, 2013, Kuala Lumpur, Malaysia.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chavanet P, Lopez J, Grappin M, et al. : Cross-sectional study of the susceptibility of Candida isolates to antifungal drugs and in vitro-in vivo correlation in HIV-infected patients. AIDS 1994;8(7):945–950 [DOI] [PubMed] [Google Scholar]
- 2.Pittet D, Monod M, Suter P, Frenk E, and Auckenthaler R: Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg 1994;220(6):751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg KM. and Arnsten JH: Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr 2006;43(Suppl 1):S79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beumer JH, Bosman IJ, and Maes RA: Hair as a biological specimen for therapeutic drug monitoring. Int J Clin Pract 2001;55(6):353–357 [PubMed] [Google Scholar]
- 5.Hegstad S, Khiabani HZ, Kristoffersen L, et al. : Drug screening of hair by liquid chromatography-tandem mass spectrometry. J Anal Toxicol 2008;32(5):364–372 [DOI] [PubMed] [Google Scholar]
- 6.Gandhi M, Ameli N, Bacchetti P, et al. : Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis 2011;52(10):1267–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi M, Ameli N, Bacchetti P, et al. : Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS 2009;23(4):471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hickey MD, Salmen CR, Tessler RA, et al. : Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr 2014;66(3):311–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Zyl GU, van Mens TE, McIlleron H, et al. : Low lopinavir plasma or hair concentrations explain second-line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr 2011;56(4):333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koss C, Natureeba P, Mwesigwa J, et al. : Hair concentrations of antiretrovirals predict viral suppression in HIV-infected pregnant and breastfeeding Ugandan women. AIDS 2015;29:825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olds PK, Kiwanuka JP, Nansera D, et al. : Assessment of HIV antiretroviral therapy adherence by measuring drug concentrations in hair among children in rural Uganda. AIDS Care 2015;27(3):327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baxi SM, Liu A, Bacchetti P, et al. : Comparing the novel method of assessing PrEP adherence/exposure using hair samples to other pharmacologic and traditional measures. J Acquir Immune Defic Syndr 2015;68(1):13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassani M, Da Re N, Giuliani L, and Sesana F: Experience with hair testing in the clinical biochemistry laboratory of Ca’ Granda Niguarda Hospital, Milan, Italy. Forensic Sci Int 1997;84(1–3):17–24 [DOI] [PubMed] [Google Scholar]
- 14.Kosuge K, Uematsu T, Araki SI, et al. : Comparative dispositions of ofloxacin in human head, axillary, and pubic hairs. Antimicrob Agents Chemother 1998;42(5):1298–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Droste JA, Verweij-Van Wissen CP, and Burger DM: Simultaneous determination of the HIV drugs indinavir, amprenavir, saquinavir, ritonavir, lopinavir, nelfinavir, the nelfinavir hydroxymetabolite M8, and nevirapine in human plasma by reversed-phase high-performance liquid chromatography. Ther Drug Monit 2003;25(3):393–399 [DOI] [PubMed] [Google Scholar]
- 16.Gandhi M, Ameli N, Bacchetti P, et al. : Concentrations of Efavirenz in Hair Are Strongly Correlated with Virologic Response. 16th Conference on Retroviruses and Opportunistic Infections (CROI) Montreal, Canada, 2009 [Google Scholar]
- 17.Biressaw S, Abegaz WE, Abebe M, et al. : Adherence to antiretroviral therapy and associated factors among HIV infected children in Ethiopia: Unannounced home-based pill count versus caregivers' report. BMC Pediatr 2013;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naar-King S, Frey M, Harris M, and Arfken C: Measuring adherence to treatment of paediatric HIV/AIDS. AIDS Care 2005;17(3):345–349 [DOI] [PubMed] [Google Scholar]
- 19.Simoni JM, Kurth AE, Pearson CR, et al. : Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav 2006;10(3):227–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marrazzo JM, Ramjee G, Richardson BA, et al. : Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015;372(6):509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nettles RE, Kieffer TL, Parsons T, et al. : Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis 2006;42(8):1189–1196 [DOI] [PubMed] [Google Scholar]
- 22.Duong M, Piroth L, Peytavin G, et al. : Value of patient self-report and plasma human immunodeficiency virus protease inhibitor level as markers of adherence to antiretroviral therapy: Relationship to virologic response. Clin Infect Dis 2001;33(3):386–392 [DOI] [PubMed] [Google Scholar]
- 23.Podsadecki TJ, Vrijens BC, Tousset EP, et al. : “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials 2008;9(4):238–246 [DOI] [PubMed] [Google Scholar]
- 24.ter Heine R, Beijnen JH, and Huitema AD: Bioanalytical issues in patient-friendly sampling methods for therapeutic drug monitoring: Focus on antiretroviral drugs. Bioanalysis 2009;1(7):1329–1338 [DOI] [PubMed] [Google Scholar]
- 25.Coetzee B, Kagee A, Tomlinson M, et al. : Reactions, beliefs and concerns associated with providing hair specimens for medical research among a South African sample: A qualitative approach. Future Virol 2012;7(11):1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandhi M, Yang Q, Bacchetti P, and Huang Y: Short communication: A low-cost method for analyzing nevirapine levels in hair as a marker of adherence in resource-limited settings. AIDS Res Hum Retroviruses 2014;30(1):25–28 [DOI] [PMC free article] [PubMed] [Google Scholar]