Abstract
Endoplasmic Reticulum (ER) is an organelle where most secretory and membrane proteins are synthesized, folded, and undergo further maturation. As numerous conditions can perturb such ER function, eukaryotic cells are equipped with responsive signaling pathways, widely referred to as the Unfolded Protein Response (UPR). Chronic conditions of ER stress that cannot be fully resolved by UPR, or conditions that impair UPR signaling itself, are associated with many metabolic and degenerative diseases. In recent years, Drosophila has been actively employed to study such connections between UPR and disease. Notably, the UPR pathways are largely conserved between Drosophila and humans, and the mediating genes are essential for development in both organisms, indicating their requirement to resolve inherent stress. By now, many Drosophila mutations are known to impose stress in the ER, and a number of these appear similar to those that underlie human diseases. In addition, studies have employed the strategy of overexpressing human mutations in Drosophila tissues to perform genetic modifier screens. The fact that the basic UPR pathways are conserved, together with the availability of many human disease models in this organism, makes Drosophila a powerful tool for studying human disease mechanisms. [BMB Reports 2015; 48(8): 445-453]
Keywords: Drosophila, Endoplasmic reticulum, IRE1, PERK, Unfolded protein response
INTRODUCTION
It is estimated that approximately a third of all genes in eukaryotes encode secretory or membrane proteins that are synthesized on the rough endoplasmic reticulum (ER) (1, 2). Peptides that are synthesized into this organelle first undergo chaperone-assisted folding, and a subset is further modified through glycosylation or oxidation to form disulfide bonds (3). Proteins that fail to undergo proper folding and maturation can be toxic to cells, and underlie many metabolic and degenerative diseases that include diabetes and various forms of neurodegeneration (4, 5). Eukaryotic cells have evolved a robust Unfolded Protein Response (UPR), which specifically refers to signaling pathways that regulate gene expression in response to ER stress (6, 7). Naturally, the regulation of the UPR has been a topic that has drawn significant interest in the field.
Cells can suffer from ER stress for a variety of reasons. Perhaps most obvious are mutations that impair the inherent folding properties of an encoded protein (6). These proteins can cause aggregates, and also have the effect of overwhelming the protein folding machinery. ER is also an organelle that stores high concentrations of Ca2+, which in turn is essential for proper ER function. In fact, the Ca2+ pump inhibitor thapsigargin is frequently used among researchers to impose stress in the ER (8). Calnexin and Calreticulin are examples of Ca2+ binding proteins that are specifically involved in the folding of glycosylated proteins in the ER, and inhibition of glycosylation with tunicamycin similarly interferes with protein folding in this organelle (9). In addition, ER has an oxidizing environment that promotes the formation of disulfide bonds between cysteine residues (10). Many proteins in the ER make stable domain structures only when certain disulfide bonds are formed, and inhibition of cysteine oxidation with reducing agents such as DTT also imposes severe stress in the ER. The rapid elucidation of the UPR pathways was possible, in part, due to the facile ER stress assays based on tunicamycin, thapsigargin and DTT treatment on cultured cells.
The term, UPR, was first coined to describe the transcriptional response to mutant viral protein expression in cultured mammalian cells (6). Such transcriptional response is also observed in Drosophila, as documented in detail in more than a hundred inbred Drosophila species that were fed tunicamycin (11). In recent years, there have been significant efforts to go beyond drug treatment experiments, and determine the physiological role of the UPR in animal development, tissue homeostasis, disease models and lifespan regulation. Drosophila has emerged as a popular model organism for those studies, and here I will discuss the recent advances in this area.
IRE1/XBP1 PATHWAY OF THE UPR
The UPR pathway was initially dissected in the baker’s yeast, Saccharomyces cerevisiae (12). It was first found that IRE1 is an essential mediator of ER chaperone induction after ER stress, as such conditions prompt the activation of this transmembrane signaling protein by forcing oligomer formation (13, 14). X-ray crystallography studies revealed that IRE1 has an RNase domain (15). Once activated, IRE1 cleaves the HAC1 mRNA to initiate mRNA splicing. This splicing reaction is completed after the two cleaved mRNA pieces are put together by a tRNA ligase (16). Once the spliced HAC1 mRNA is translated, the encoded protein serves as a transcription factor that induces the expression of ER quality control genes (17).
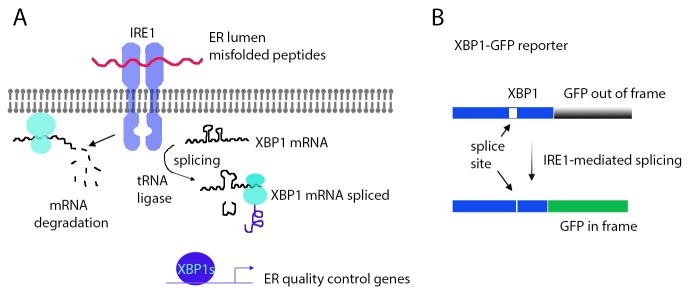
Mammals have two IRE1 homologs, IRE1alpha and beta, which respond to ER stress by cutting XBP1 mRNA in two specific positions, similar to how yeast IRE1 cuts HAC1 mRNA (18, 19) (Fig. 1A). The splicing reaction is completed by the ligation of the two cleaved pieces, and recently, the responsible RNA ligase was identified in mammals and C. elegans (20-22). Drosophila has a single IRE1 gene that cleaves a conserved XBP1 mRNA (23, 24), and an uncharacterized homolog of the newly identified mammalian ligase, annotated as CG9987. Both in mammals and Drosophila, BAX Inhibitor-1 overexpression reportedly suppresses IRE1 activity (25). Once the spliced isoform (also known as the RB isoform) of XBP1 is generated, this active transcription factor induces many ER quality genes that help to restore homeostasis (23).
Fig. 1. Regulation and detection of the IRE1/XBP1 pathway. (A) A schematic diagram of the IRE1/XBP1 pathway in Drosophila. IRE1 is an ER stress sensor that directly binds to misfolded peptides in the ER lumen. Upon detecting ER stress, IRE1 activates its RNase domain on the cytoplasmic side. IRE1 works together with a tRNA ligase to catalyze the splicing of XBP1 mRNA. The product of this spliced isoform acts as a transcription factor that induces ER quality control genes. In addition, IRE1 promotes the decay of many mRNAs associated with the ER. (B) The XBP1-GFP reporter used to detect IRE1 activity in vivo. As XBP1 mRNA splicing by IRE1 shifts the reading frame, an XBP1-GFP fusion transgene was designed to have GFP expressed in frame only when IRE1-mediated splicing occurs.
In S. cerevisiae, a systematic approach has failed to identify any IRE1 substrates other than the HAC1 mRNA (26). On the other hand, a study based on Drosophila S2 cells revealed that IRE1 cleaves other mRNAs (23), so as to degrade them upon ER stress. More recent work has found that, in general, mRNAs that are normally translated on the rough ER, and therefore accessible to IRE1, are substrates for degradation (27) (Fig. 1A). The initial observation made in S2 cells have now been validated in vivo: Whereas XBP1 mutant mosaic clones did not show obvious developmental defects in the Drosophila eye, IRE1 mutants had defects in the secretion of extracellular materials, which gave rise to a shorter inter-ommatidial distance. One of the targets that contributed to this IRE1-specific phenotype was the mRNA of FATP, which is a target of IRE1-mediated degradation (28). The idea that IRE1 degrades mRNAs associated with the ER has now been validated in other organisms as well, including mammals (29, 30). Interestingly, the fission yeast S. pombe does not encode an ortholog of HAC1, and a recent study has found that IRE1 helps that organism withstand ER stress primarily by degrading mRNAs associated with the ER (31).
PHYSIOLOGICAL ROLES OF THE IRE1/XBP1 PATHWAY IN Drosophila
Our understanding of the IRE1/XBP1 pathway is largely based on experiments with cells that were exposed to ER stress causing chemicals, or conditions that express high levels of mutant proteins. Less well understood is the role of IRE1 and XBP1 in vivo, particularly in tissues that are not exposed to exogenous sources of stress. It is clear that IRE1 and XBP1 play important roles even in normally developing tissues, as these are developmentally essential genes. In Drosophila, IRE1 mutants survive only up to the 1st instar stage (32), and XBP1 mutants up to the 2nd instar stage (24).
The precise role of these genes in the developing tissues is only beginning to be understood. In the larval fat body, it was found that XBP1 mutant cells activate autophagy, indicating that XBP1 is normally active in that tissue to maintain cellular homeostasis (33). The expression pattern and activity of IRE1 and XBP1 suggest possible roles in a number of other tissues. One of those is the gastro-intestinal system. In situ hybridization of xbp1 most prominently shows high transcript levels in the salivary gland and the intestines, and this has been further confirmed with a reporter under the control of XBP1’s upstream sequence (24, 32). Aside from the expression pattern, IRE1 activity can be visualized with another reporter, XBP1-GFP (24, 34). This reporter takes advantage of the fact that the splicing of XBP1 results in a reading frame shift of that transcript. By placing GFP after the XBP1 sequence in a specific reading frame, GFP is expressed in frame specifically when IRE1-mediated mRNA splicing occurs (24, 34) (Fig. 1B). In addition to detecting IRE1 activation in pathological conditions, this reporter can detect ER stress in various tissues, including the larval intestine, fat body, glia, certain neurons and developing photoreceptors (28, 34).
A number of adult tissues also show signs of IRE1 activity, including the male reproductive system and the aging adult intestinal stem cells (34, 35). The accessory gland normally secretes many seminal fluid proteins, and correlating with this, XBP1-based reporters are active in this tissue (32, 34). Further aggravation of such inherent ER stress, either by misexpressing mutant proteins, or by knocking down the ER chaperone BiP, leads to excessive activation of XBP1 splicing and infertility (36). In the adult intestine, XBP1 is required for the proper homeostasis of the epithelial cells, and in its absence the cells build up high levels of reactive oxygen species (ROS), which in turn, signal to promote stem cell hyperproliferation and epithelial dysplasia in the aging fly intestine. Conversely, hyper-activation of the IRE1/XBP1 branch by overexpressing the spliced isoform of XBP1 (XBP1-RB, also known as XBP1s) suppresses ER-stress related phenotypes in the intestinal stem cells (35, 37).
DEVELOPMENTAL DEFECTS ASSOCIATED WITH IRE1/XBP1
Major signaling pathways involve membrane receptors and ligands that are synthesized in the ER, and therefore, dysfunction of the ER may have a broad effect on those pathways. Intriguingly, the Notch signaling pathway in Drosophila appears to be particularly sensitive to the protein-folding environment in the ER. The connection with UPR was first noticed when a genetic screen for a Notch-like phenotype in the fly identified mutations in ero1L, whose normal function is to stimulate disulfide bond formation in the ER. Based on yeast genetic studies, it was assumed at the time that all disulfide bond formation in the ER should be impaired in ero1L mutants, and therefore, loss of this gene would result in a pleiotropic phenotype. However, ero1L mutant cells specifically showed a Notch-like phenotype in Drosophila, with Notch protein accumulation in the ER, and activation of the XBP1-GFP reporter (38). Since that study in Drosophila, it has been also determined in mammals that disulfide bonds can form without ero1L, indicating that this gene has assumed more specific roles in metazoans (39).
Other mutations that impair Notch maturation in the ER include mutations in the Catsup gene, a Drosophila homolog of ZIP7 zinc transporter (40), Rumi that encodes an O-glucosyltransferase (41) and pecanex (42). Overexpression of the spliced isoform of XBP1 suppressed the pecanex phenotype (42).
THE PERK/ATF4 PATHWAY IN Drosophila
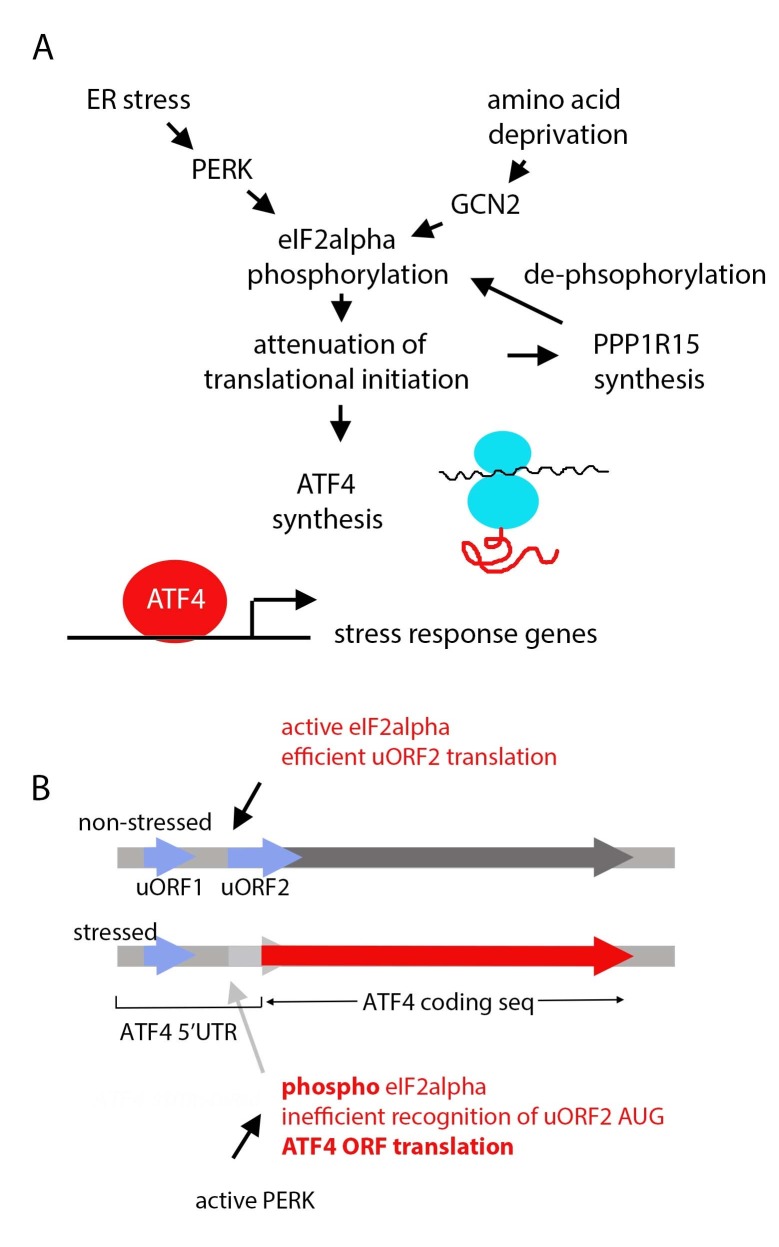
In parallel to the IRE1/XBP1 branch, the ER tran smembrane kinase PERK is activated in response to ER stress and phosphorylates the translational initiation factor eIF2alpha (43) (Fig. 2). The normal role of eIF2alpha is to help charge 40S ribosomal subunits with initiator methionyl tRNAs, which is essential for translational initiation. Therefore, the inhibitory phosphorylation by PERK attenuates the overall rate of translational initiation. It is generally understood that such reduction in translation helps to alleviate the protein-folding burden of cells, but excessive activation of PERK by gene overexpression in Drosophila tissues can also cause toxicity (44). In addition, such conditions activate downstream signaling pathways. One of the best characterized is that of ATF4, whose synthesis paradoxically increases when eIF2alpha is phosphorylated. The underlying mechanism of this intriguing phenomenon has been described in detail elsewhere (43, 45). In brief, the unique induction of ATF4 synthesis is possible due to a number of small upstream Open Reading Frames (uORFs) in the 5’ UTR (Fig. 2B). The last uORF overlaps with ATF4 in a different reading frame, and therefore, inhibits ATF4 translation in unstressed cells. eIF2alpha phosphorylation makes the recognition of this uORF by the 40S ribosome inefficient, thereby allowing the opportunity for the main ATF4 ORF to be translated. Once synthesized, ATF4 induces many targets that are involved in stress response. The Drosophila genome encodes single orthologs of PERK and ATF4 (44, 46). The latter transcript has uORFs in its 5’ UTR, similar to the homologs in other species. While the ATF4 transcript is widely distributed, the translation only occurs in response to stress (46, 47). A Drosophila ATF4 reporter was recently made by fusing the ATF4 5’ UTR with the coding sequence of dsRed. This reporter is activated in response to mutant membrane protein expression, and also detects stress in a number of normally developing tissues, including the pupal stage photoreceptors (48).
Fig. 2. Regulation of the PERK/ATF4 pathway in Drosophila. (A) A schematic diagram of the pathway initiated by the eIF2alpha kinases, PERK and GCN2. These kinases are activated by distinct types of stress and phosphorylate eIF2alpha. This results in the overall translation attenuation, but at least two transcripts in Drosophila enhance their translation during such conditions: ATF4 is a transcription factor that induces stress response genes, and PPP1R15 is a phosphatase subunit that helps to de-phosphorylate eIF2alpha as a feedback mechanism. (B) uORFs in the 5’ UTR allow enhanced ATF4 synthesis under conditions of eIF2alpha phosphorylation. ATF4 5’ UTR has multiple uORFs, and only two are shown for simplicity. eIF2alpha helps to charge 40S ribosomes with initiator methionyl tRNA after the synthesis of uORF1. When eIF2alpha is active, 40S ribosomes efficiently recognize uORF2 for translation. uORF2 overlaps with ATF4 ORF, and interferes with ATF4 ORF translation. When eIF2alpha is phosphorylated, 40S ribosome’s ability to recognize the uORF2 is compromised, bypassing its AUG to allow the recognition of the ATF4 ORF.
If ATF4 induces many transcripts, only to have their translation blocked by phosphorylated eIF2alpha, a robust gene expression response cannot be mounted. In reality, eIF2alpha phosphorylation occurs only for a few hours, before being de-phosphorylated to allow mRNA translational initiation. In mammals, such negative feedback occurs through ATF4, and its downstream transcription factor CHOP, which transcriptionally induces a phosphatase subunit PPP1R15 (also referred to as GADD34) to dephosphorylate eIF2lapha. A recent study shows that Drosophila PPP1R15 is also induced by ER stress, but not through a transcriptional mechanism. Instead, the Drosophila transcript also has a 5’ UTR with uORFs that translationally activates this gene upon eIF2alpha phosphorylation (Fig. 2A). Once synthesized, PPP1R15 opposes PERK’s effect on eIF2alpha (49).
Independent studies have reported the presence of ATF4-like 5’ UTRs in a number of other transcripts. These include ATF5 and CHOP (C/EBP homologous protein), transcription factors that contribute to the UPR (50), as well as a kinase of unclear function (51, 52). As there have not been any systematic efforts to identify such transcripts in Drosophila, it is likely that additional transcripts specifically translated during UPR remain at large.
ROLE OF THE PERK/ATF4 PATHWAY IN Drosophila DEVELOPMENT
A hypomorphic allele of ATF4, cryptocephal1, was first described in the 1940s as a mutant with heads that fail to emerge from the thorax during the pupal stage of development (53). Null alleles show defects in larval molting and pupariation (46). Consistent with this idea, Drosophila ATF4 transcriptionally induces Ecdysis triggering hormone (ETH) in the endocrine cells, which in turn promote molting. Intriguingly, a recent study reported that ATF4 works as a coactivator of the Ecdysone receptor, which controls numerous target genes involved in metamorphosis (54). As ATF4 protein cannot be synthesized without stress-induced eIF2alpha phosphorylation, these observations provide a tantalizing link between metamorphosis and ER stress, which remains to be further characterized.
Consistent with the developmentally essential role of ATF4, we recently reported that the PERK allele e01744, with a Piggybac element inserted in the first intron, causes developmental lethality. Moreover, PERK is active in healthy adult intestinal stem cells, and without PERK, intestinal stem cell proliferation is reduced. Intriguingly, PERK activity in the stem cells has negative consequences on the intestinal epithelium, and knock down of PERK in that tissue prolongs lifespan of Drosophila (37). These observations show that the PERK/ATF4 pathway is also active in healthy tissues. While it plays positive roles in certain tissues as judged by their developmental requirement, the pathway can have negative effects in others.
In mammals, there are a few additional layers of complexity to this pathway. One is the fact that there are four eIF2alpha kinases, each mediating distinct stress response. Two of those, PERK and GCN2, are conserved in Drosophila (49) (Fig. 2A). GCN2 is specifically activated by amino acid deprivation, and consistent with this, ATF4’s transcriptional targets include amino acid transporters and other metabolic genes (55-57). Adding to the complexity are non-canonical downstream effectors of PERK. Specifically, studies have reported the transcription factors Nrf2 and NF-kappaB to lie downstream of PERK (58-60). Whether these non-canonical axes of PERK signaling also exist in Drosophila, and whether they play physiologically significant roles in vivo remains to be determined.
THE ATF6 PATHWAY OF THE UPR
In vertebrates, ATF6 also plays an important role in the UPR. This protein has a DNA binding domain in addition to a transmembrane domain that tethers the protein at the ER membrane under unstressed conditions. Upon stress, ATF6 is released from the ER and traffics to the Golgi, where it is cleaved by membrane associated proteases to release the cytoplasmic portion (61, 62). Such conditions allow ATF6 to translocate to the nucleus, where it induces many ER quality control genes including XBP1 and ER chaperones (63, 64). There are two ATF6 genes in vertebrates, referred to as ATF6alpha and beta. Single knockout mice are viable, but double knockouts are embryonic lethal, indicative of redundancy of function in the two genes (64).
The Drosophila genome encodes a single ATF6 homolog, with conservation not only within the DNA binding domain, but also in the transmembrane and luminal domains. A Piggybac insertion line within the coding sequence of ATF6, PBac LL0743, is available from public stock centers. This insertion line is viable and fertile as homozygotes (unpublished data), indicating that Drosophila atf6 does not play a developmentally essential role as in mammals. Whether this gene is required for a proper ER stress response in adult tissues remain to be validated.
THE UPR IN Drosophila DISEASE MODELS
The role of IRE1 and XBP1 in vivo has been studied most extensively in the context of mutations that impair the folding property of cells in the ER. A well-characterized example is the Drosophila ninaE mutant alleles that cause age-related photoreceptor degeneration (65, 66). Drosophila ninaE encodes a light detecting protein, Rhodopsin-1, and certain missense mutant alleles are similar in nature with the human rhodopsin alleles that underlie Autosomal Dominant Retinitis Pigmentosa (ADRP) (67). Although the human alleles have been speculated to impair the encoded protein’s folding property, the link between the UPR and retinal degeneration by rhodopsin mutants was first established through the study of the Drosophila ninaEG69D mutant. Specifically, the XBP1-GFP reporter was used to show that the IRE1/XBP1 pathway is activated in these mutant photoreceptors, and that the loss of XBP1 accelerated the course of degeneration in this disease model (24). Conversely, enhancing the degradation of misfolded Rhodopsin-1, by overexpressing the HRD1 ubiquitin ligase, suppressed the course of retinal degeneration in Drosophila (68). In human ADRP, P23H substitution is the most common mutation, and the equivalent mutant P37H was generated in the fly to establish similar UPR activation (69).
It appears that Drosophila Rhodopsin-1 is particularly sensitive to the protein-folding environment in the ER. Drosophila genome encodes two Calnexins. Mutation in one of those, Calnexin 99A, gives rise to viable adults with significantly reduced reduced Rhodopsin-1 levels in their photoreceptors (70). A number of other genetic conditions impair proper rhodopsin folding in the ER. Most recently, it has been found that a complex of proteins with previously unknown function, termed the ER membrane protein complex (EMC), are required for multipass transmembrane domain protein folding. In photoreceptors, loss of EMC subunits resulted in Rhodopsin-1 misfolding (71). Although excessive ER stress is a cause of cellular dysfunction and cell death, it has been reported that there are milder conditions of rhodopsin misfolding can protect cells from other kinds of stress. Such effect was observed while examining p53-induced photoreceptor cell death. The authors found that a mutation in ninaA, a gene that is normally required for proper Rhodopsin-1 folding, protects against p53-induced cell death (72). The degree of rhodopsin misfolding is probably mild enough to avoid photoreceptor degeneration under these conditions, while activating UPR’s ability to attenuate translation and enhance anti-oxidant response to enhance general stress resistance of cells.
UPR is associated with a number of other neurodegenerative diseases. Research in Drosophila established a link between IRE1/XBP1 and a VapB mutation that underlies amyotrophic lateral sclerosis. VapB is a transmembrane protein with an immunoglobulin fold domain, MSB. Drosophila encodes a homologous gene, VAP, whose loss results in the disruption of neuromuscular junctions (73). Interestingly, a point mutation in the human gene, P56S, underlies the dominant effects in the motor neuron degeneration of amyotrophic lateral sclerosis (74), but the underlying reason for pathogenesis had remained unclear, before Drosophila was employed as a model system. Studies have determined that the P56S equivalent mutation in Drosophila, P58S, forms aggregates in the ER and activates the IRE1/XBP1 pathway of the UPR (75, 76). Defects in the ER quality control in this model are partly attributed to a failure of mutant VapB to bind and retain Oxysterol binding protein in the ER (76). In addition, this mutant allele shows a non-autonomous effect, as wild type VAP has an MSB domain that is cleaved off for secretion to bind to Ephrin reeceptors in neighboring cells, which does not occur in the mutants (75).
Another example of disease associated with UPR is Hereditary spastic paraplegias. These are neurological disorders that show progressive stiffness and spasticity in the lower limbs, due to damages or dysfunction of nerve fibers. A mutation in a reticulon family protein, RTN2, underlies an autosomal dominant form of this disease. Drosophila encodes a single reticulon, Rtn1. A recent study has found that the protein product is enriched in axons and is essential for proper organization of smooth ER in the distal parts of axons. Rtn1 mutants activate the UPR, as evidenced by XBP1-GFP expression (77).
Mutations in the cytoplasmic aminoacyl-tRNA synthetases are associated with Charcot-Marie-Tooth diseases, a common form of neurological disorder. A recent study has examined the physiological defects associated with phenylalanyl-tRNA synthetase mutations that underlie this disease, and found that, among others, the mutant protein expression triggers the activation of XBP1-GFP, providing a link between Charcot-Marie-Tooth disease and UPR (78). Very recently, a genetic screen for genes involved in axonal regeneration after injury identified regulators of XBP1 mRNA splicing. The study found that loss of XBP1 reduces axonal regeneration, whereas conditions that enhance XBP1 splicing stimulates it (79).
In a number of genetic conditions, it has been shown that hyper-stimulation of the IRE1/XBP1 pathway helps to suppress phenotypes. An interesting examples have been reported in a Drosophila model for Alzheimer’s disease, in which the amyloid-beta 1-42 peptide or mutant tau were overexpressed in the developing eye (80, 81). Such conditions not only activated the XBP1-GFP reporter, but overexpressing the spliced isoform of XBP1 suppressed the phenotype caused by amyloid-beta expression (81). Spliced XBP1 overexpression can also suppress a distinct phenotype associated with the disruption of the ER-mitochondria interface, caused by the knock down of mitofusin (82).
Drosophila has been actively employed to express human gene alleles that underlie diseases. Among those associated that activate the UPR include mutations in Pro-insulin that underlie diabetes (83), alpha-one antitrypsin mutations that underlie lung emphesyma (68, 84), the GBA gene that underlies Gaucher disease (85).
OTHER UPR PATHWAYS
Most studies in the field focus on the three UPR branches, mediated by IRE1, PERK and ATF6, respectively. These pathways are fully activated by ER stress within hours, and quickly become inactivated through feedback mechanisms. Although a number of studies have implicated their role in degenerative diseases, it is difficult to imagine how these pathways can be responsible for age-related degenerative diseases that manifest only after decades of chronic ER stress. Studies from Drosophila point to the role of distinct pathways that contribute to ER-stress inducible cell death, possibly involving Ca2+. Notably, the ER is a major storage site for Ca2+, and excessive leakage into the cytoplasm can trigger cell death.
Interestingly, an RNAi screen for genes that are required for mutant Rhodopsin-1 induced cell death identified stress-activated kinases, CDK5 and MEKK1, which had not been previously implicated in the canonical UPR pathways (47). CDK5 is a kinase that can be activated by ROS or excessive Ca2+ in the cytoplasm, and implicated in other forms of neuronal cell death (86). Interestingly, excessive Ca2+ release into the cytoplasm also occurs in a Drosophila model for Alzheimer’s disease, where amyloid beta peptide overexpression imposes stress in the ER. The amyloid beta overexpression phenotype is suppressed in the mutant background of Ryanodine Receptor, whose normal role is to release Ca2+ from the ER to the cytoplasm (81). Based on this, one can put together a working hypothesis that chronic stress in the ER causes Ca2+ to leak out into the cytoplasm, and initiate a distinct signaling pathway mediated by CDK5 and MEKK1. This particular signaling pathway may better explain gene expression changes that occur after decades of chronic ER stress, as breakdown of ER Ca2+ homeostasis may occur possibly years after chronic exposure to ER stress, whereas PERK, IRE1 and ATF6 mediate acute UPR responses within hours.
CONCLUSION
UPR research in Drosophila has accelerated in recent years, in part due to the availability of new genetic tools. This model organism nicely complements the existing approaches, particularly in the investigation of the normal physiological role of the UPR, and also regarding specific disease mechanisms. Regarding the molecular mechanism of UPR, much progress has been made in the IRE1/XBP1 branch of signaling. Our understanding of the PERK/ATF4 pathway in Drosophila has been gradually improving, but research on the other branches still lag behind. Unbiased genetic approaches in Drosophila may help elucidate other branches of the UPR that may remain at large.
Acknowledgments
I thank Brian Brown for comments on the manuscript. This work was supported by the NIH grant R01 EY020866, and the March of Dimes grant FY13-204.
References
- 1.Ghaemmaghami S, Huh WK, Bower K, et al. Global analysis of protein expression in yeast. Nature. (2003);425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 2.Kanapin A, Batalov S, Davis MJ, et al. Mouse proteome analysis. Genome Res. (2003);13:1335–1344. doi: 10.1101/gr.978703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braakman I, Hebert DN. Protein folding in the endoplasmic reticulum. Cold Spring Harb Perspect Biol. (2013);5:a013201. doi: 10.1101/cshperspect.a013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. (2006);86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 5.Hetz C, Mollereau B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci. (2014);15:233–249. doi: 10.1038/nrn3689. [DOI] [PubMed] [Google Scholar]
- 6.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. (1988);332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 7.Walter P, Ron D. The unfolded protein response: from stress pathway to hemeostatic regulation. Science. (2011);334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 8.Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. (1991);266:17067–17071. [PubMed] [Google Scholar]
- 9.Trombetta ES, Helenius A. Lectins as chaperones in glycoprotein folding. Curr Opin Struct Biol. (1998);8:587–592. doi: 10.1016/S0959-440X(98)80148-6. [DOI] [PubMed] [Google Scholar]
- 10.Bulleid NJ, van Lith M. Redox regulation in the endoplasmic reticulum. Biochem Soc Trans. (2014);42:905–908. doi: 10.1042/BST20140065. [DOI] [PubMed] [Google Scholar]
- 11.Chow CY, Wolfner MF, Clark AG. Using natural variation in Drosophila to discover previously unknown endoplasmic reticulum stress genes. Proc Natl Acad Sci U S A. (2013);110:9013–9018. doi: 10.1073/pnas.1307125110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman R, Sidrauski C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus. Annu Rev Cell Dev Biol. (1998);14:459–485. doi: 10.1146/annurev.cellbio.14.1.459. [DOI] [PubMed] [Google Scholar]
- 13.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. (1993);73:1197–1206. doi: 10.1016/0092-8674(93)90648-A. [DOI] [PubMed] [Google Scholar]
- 14.Korennykh AV, Egea PF, Korostelev AA, et al. The unfolded protein response signals through higher-order assembly of Ire1. Nature. (2009);457:687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KP, Dey M, Neculai D, Cao C, Dever TE, Sicheri F. Structure of the dual enzyme ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell. (2008);132:89–100. doi: 10.1016/j.cell.2007.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidrauski C, Cox JS, Walter P. tRNA ligase is required for regulated mRNA splicing in the unfolded protein response. Cell. (1996);87:405–413. doi: 10.1016/S0092-8674(00)81361-6. [DOI] [PubMed] [Google Scholar]
- 17.Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. (2000);101:249–258. doi: 10.1016/S0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 18.Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. (1998);17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. (1998);12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y, Liang FX, Wang X. A synthetic biology approach identifies the mammalian UPR RNA ligase RtcB. Mol Cell. (2014);55:758–770. doi: 10.1016/j.molcel.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurkin J, Henkel T, Nielsen AF, et al. The mammalian tRNA ligase complex mediates splicing of XBP1 mRNA and controls antibody secretion in plasma cells. EMBO J. (2014);33:2922–2936. doi: 10.15252/embj.201490332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosmaczewski SG, Edwards TJ, Han SM, et al. The RtcB RNA ligase is an essential component of the metazoan unfolded protein response. EMBO Rep. (2014);15:1278–1285. doi: 10.15252/embr.201439531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. (2006);313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 24.Ryoo HD, Domingos PM, Kang MJ, Steller H. Unfolded protein response in a Drosophila model for retinal degeneration. EMBO J. (2007);26:242–252. doi: 10.1038/sj.emboj.7601477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lisbona F, Rojas-Rivera D, Thielen P, et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. (2009);33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niwa M, Patil CK, DeRisi J, Walter P. Genome-scale approaches for discovering novel nonconventional splicing substrates of the Ire1 nuclease. Genome Biol. (2005);6:R3. doi: 10.1186/gb-2004-6-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaddam D, Stevens N, Hollien J. Comparison of mRNA localization and regulation during endoplasmic reticulum stress in Drosophila cells. Mol Biol Cell. (2013);24:14–20. doi: 10.1091/mbc.E12-06-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coelho DS, Cairrão F, Zeng X, et al. Xbp1-independent Ire1 signaling is required for photoreceptor differentiation and rhabdomere morphogenesis in Drosophila. Cell Rep. (2013);14:791–801. doi: 10.1016/j.celrep.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han D, Lerner AG, Vande Walle L, et al. IIRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. (2009);138:562–572. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. (2009);186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimmig P, Diaz M, Zheng J, et al. The unfolded protein response in fission yeast modulates stability of select mRNAs to maintain protein homeostasis. Elife. (2012);1:e00048. doi: 10.7554/eLife.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryoo HD, Li J, Kang MJ. Drosophila XBP1 expression reporter marks cells under endoplasmic reticulum stress and with high protein secretory load. PLoS One. (2013);8:e75774. doi: 10.1371/journal.pone.0075774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arsham AM, Neufeld TP. A genetic screen in Drosophila reveals novel cytoprotective functions of the autophagy-lysosome pathway. PLoS One. (2009);4:e6068. doi: 10.1371/journal.pone.0006068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sone M, Zeng X, Larese J, Ryoo HD. A modified UPR stress sensing system reveals a novel tissue distribution of IRE1/XBP1 activity during normal Drosophila development. Cell Stress Chaperones. (2013);18:307–319. doi: 10.1007/s12192-012-0383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Zeng X, Ryoo HD, Jasper H. Integration of UPRER and oxidative stress signaling in the control of intestinal stem cell proliferation. PLoS Genet. (2014);10:e1004568. doi: 10.1371/journal.pgen.1004568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow CY, Avila FW, Clark AG, Wolfner MF. Induction of excessive endoplasmic reticulum stress in the Drosophila male accessory gland results in infertility. PLoS One. (2015);10:e0119386. doi: 10.1371/journal.pone.0119386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Ryoo HD, Qi Y, Jasper H. PERK limits Drosophila lifespan by promoting intestinal stem cell proliferation in response to systemic and local ER stress. PLoS Genet. (2015);11:e1005220. doi: 10.1371/journal.pgen.1005220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tien AC, Rajan A, Schulze KL, et al. Ero1L, a thiol oxidase, is required for Notch signaling through cysteine bridge formation of the Lin12-Notch repeats in Drosophila melanogaster. J Cell Biol. (2008);182:1113–1125. doi: 10.1083/jcb.200805001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zito E, Chin KT, Blais J, Harding HP, Ron D. ERO1-beta, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J Cell Biol. (2010);188:821–832. doi: 10.1083/jcb.200911086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groth C, Sasamura T, Khanna MR, Whitley M, Fortini ME. Protein trafficking abnormalities in Drosophila tissues with impaired activity of the ZIP7 zinc transporter Catsup. Development. (2013);140:3018–3027. doi: 10.1242/dev.088336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acar M, Jafar-Nejad H, Takeuchi H, et al. Rumi is a CAP10 domain glycosyltransferase that modifies Notch and is required for Notch signaling. Cell. (2008);132:247–258. doi: 10.1016/j.cell.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamakawa T, Yamada K, Sasamura T, et al. Deficient Notch signaling associated with neurogenic pecanex is compensated for by the unfolded protein response in Drosophila. Development. (2012);139:558–567. doi: 10.1242/dev.073858. [DOI] [PubMed] [Google Scholar]
- 43.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. (1999);397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 44.Malzer E, Daly ML, Moloney A, et al. Impaired tissue growth is mediated by checkpoint kinase 1 (CHK1) in the integrated stress response. J Cell Sci. (2010);123:2892–2900. doi: 10.1242/jcs.070078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. (1992);68:585–596. doi: 10.1016/0092-8674(92)90193-G. [DOI] [PubMed] [Google Scholar]
- 46.Hewes RS, Schaefer AM, Taghert PH. The cryptocephal gene (ATF4) encodes multiple basic-leucine zipper proteins controlling molting and metamorphosis in Drosophila. Genetics. (2000);155:1711–1723. doi: 10.1093/genetics/155.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang MJ, Chung J, Ryoo HD. CDK5 and MEKK1 mediate pro-apoptotic signalling following endoplasmic reticulum stress in an autosomal dominant retinitis pigmentosa model. Nat Cell Biol. (2012);14:409–415. doi: 10.1038/ncb2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang K, Ryoo HD, Park JE, Yoon JH, Kang MJ. A Drosophila reporter for the translational activation of ATF4 marks stressed cells during development. PLoS One. (2015);10:e0126795. doi: 10.1371/journal.pone.0126795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malzer E, Szajewska-Skuta M, Dalton LE, et al. Coordinate regulation of eIF2alpha phosphorylation by PPP1R15 and GCN2 is required during Drosophila development. J Cell Sci. (2013);126:1406–1415. doi: 10.1242/jcs.117614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. (2011);286:10939–10949. doi: 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watatani Y, Ichikawa K, Nakanishi N, et al. Stress-induced translation of ATF5 mRNA is regulated by the 5' untranslated region. J Biol Chem. (2008);283:2543–2553. doi: 10.1074/jbc.M707781200. [DOI] [PubMed] [Google Scholar]
- 52.Baird TD, Palam LR, Fusakio ME, et al. Selective mRNA translation during eIF2 phosphorylation induces expression of IBTKalpha. Mol Biol Cell. (2014);25:1686–1697. doi: 10.1091/mbc.E14-02-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hadorn E, Gloor H. Cryptocephal, ein spat wirkender Leftalfaktor bei Drosophila melanogaster. Rev Suisse Zool. (1943);50:256–261. [Google Scholar]
- 54.Gaithier SA, VanHaaften E, Cherbas L, Cherbas P, Hewes RS. Cryptocephal, the Drosophila melanogaster ATF4, is a specific coactivator for ecdysone receptor isoform B2. PLoS Genet. (2012);8:e1002883. doi: 10.1371/journal.pgen.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. (2003);11:619–633. doi: 10.1016/S1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 56.Han J, Back SH, Hur J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. (2013);15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JE, Oney M, Frizzell K, Phadnis N, Hollien J. Drosophila melanogaster Activating Transcription Factor 4 regulates glycolysis during endoplasmic reticulum stress. G3 (Bethesda) (2015);5:667–675. doi: 10.1534/g3.115.017269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. (2003);23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang HY, Wek SA, McGrath BC, et al. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol. (2003);23:5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deng J, Lu PD, Zhang Y, et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translational initiation factor 2. Mol Cell Biol. (2004);24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ye J, Rawson RB, Komuro R, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. (2000);6:1355–1364. doi: 10.1016/S1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 62.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. (2002);3:99–111. doi: 10.1016/S1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida H, Okada T, Haze K, et al. Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6alpha and 6beta that activates the mammalian unfolded protein response. Mol Cell Biol. (2001);21:1239–1248. doi: 10.1128/MCB.21.4.1239-1248.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamamoto K, Sato T, Matsui T, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single, or combined action of ATF6alpha and xbp1. Dev Cell. (2007);13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 65.Kumar JP, Ready DF. Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development. (1995);121:4359–4370. doi: 10.1242/dev.121.12.4359. [DOI] [PubMed] [Google Scholar]
- 66.Colley NJ, Cassill JA, Baker EK, Zuker CS. Defective intracellular transport is the molecular basis of rhodopsin-dependent dominant retinal degeneration. Proc Natl Acad Sci U S A. (1995);92:3070–3074. doi: 10.1073/pnas.92.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sung CH, Davenport CM, Hennessey JC, et al. Rhdopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. (1991);88:6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang MJ, Ryoo HD. Suppression of retinal degeneration in Drosophila by stimulation of ER-Associated Degradation. Proc Natl Acad Sci U S A. (2009);106:17043–17048. doi: 10.1073/pnas.0905566106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Griciuc A, Aron L, Roux MJ, Klein R, Giangrande A, Ueffing M. Inactivation of VCP/ter94 suppresses retinal pathology caused by misfolded rhodopsin in Drosophila. PLoS Genet. (2010);6:e1001075. doi: 10.1371/journal.pgen.1001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenbaum EE, Hardie RC, Colley NJ. Calnexin is essential for Rhodopsin maturation, Ca2+ regulation, and photoreceptor cell survival. Neuron. (2006);49:229–241. doi: 10.1016/j.neuron.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Satoh T, Ohba A, Liu Z, Inagaki T, Satoh AK. dPob/EMC is essential for biosynthesis of rhodopsin and other multi-pass membrane proteins in Drosophila photoreceptors. Elife. (2015);4:e06306. doi: 10.7554/eLife.06306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mendes CS, Levet C, Chatelain G, et al. ER stress protects from retinal degeneration. EMBO J. (2009);28:1296–1307. doi: 10.1038/emboj.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pannetta G, Hiesinger PR, Fabian-Fine R, Meinertzhagen IA, Bellen HJ. Drosophila VAP-33A directs bouton formation at neuromuscular junctions in a dosage-dependent manner. Neuron. (2002);35:291–306. doi: 10.1016/S0896-6273(02)00769-9. [DOI] [PubMed] [Google Scholar]
- 74.Nishimura AL, Mitne-Neto M, Silva HC, et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet. (2004);75:822–831. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsuda H, Han SM, Yang Y, et al. The amyotriphic lateral sclerosis 8 protein VAPB is cleaved, secreted, and acts as a ligand for Eph receptors. Cell. (2008);133:963–977. doi: 10.1016/j.cell.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moustaqim-Barrette A, Lin YQ, Pradhan S, Neely GG, Bellen HJ, Tsuda H. The amyotrophic lateral sclerosis 8 protein, VAP, is required for ER protein quality control. Hum Mol Genet. (2014);23:1975–1989. doi: 10.1093/hmg/ddt594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Sullivan NC, Jahn TR, Reid E, O’Kane CJ. Reticulon-like-1, the Drosophila orthologue of the hereditary spastic paraplegia gene reticulon 2, is required for organization of endoplasmic reticulum and of distal motor axons. Hum Mol Genet. (2012);21:3356–3365. doi: 10.1093/hmg/dds167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu J, Bergert M, Walther A, Suter B. Double-sieving-defective aminoacyl-tRNA synthetase causes protein mistranslation and affects cellular physiology and development. Nat Commun. (2014);5:5650. doi: 10.1038/ncomms6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song Y, Stretavan D, Salegio EA, et al. Regulation of axon regeneration by the RNA repair and splicing pathway. Nat Neurosci. (2015);18:817–825. doi: 10.1038/nn.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loewen CA, Feany MB. The unfolded protein response protects from tau neurotoxicity in vivo. PLoS One. (2010);5:e13084. doi: 10.1371/journal.pone.0013084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Casas-Tinto S, Zhang Y, Sanchez-Garcia J, Gomez-Velazquez M, Rincon-Limas DE, Fernandez-Funez P. The ER stress factor XBP1s prevents amyloid-beta neurotoxicity. Hum Mol Genet. (2011);20:2144–2160. doi: 10.1093/hmg/ddr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Debattisti V, Pendin D, Ziviani E, Daga A, Scorrano L. Reduction of endoplasmic reticulum stress attenuates the defects caused by Drosophila mitofusin depletion. J Cell Biol. (2014);204:303–312. doi: 10.1083/jcb.201306121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park SY, Ludwig MZ, Tamarina NA, et al. Genetic complexity in a Drosophila model of diabetes-associated misfolded human proinsulin. Genetics. (2014);196:539–555. doi: 10.1534/genetics.113.157602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jang BY, Ryoo HD, Son J, et al. Role of Drosophila EDEMs in the degradation of the alpha-1-antitrypsin Z variant. Int J Mol Med. (2015);35:870–876. doi: 10.3892/ijmm.2015.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suzuki T, Shimoda M, Ito K, et al. Expression of human gaucher disease gene GBA generates neurodevelopmental defects and ER stress in Drosophila eye. PLoS One. (2013);8:e69147. doi: 10.1371/journal.pone.0069147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qu D, Rashidian J, Mount MP, et al. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson's disease. Neuron. (2007);55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]