Abstract
Objective
To determine the incidence rate and risk factors of tuberculosis (TB) among HIV-infected adults accessing antiretroviral therapy (ART) in Tanzania.
Design
A prospective observational study among HIV-infected adults attending 47 HIV clinics in Dar es Salaam.
Methods
We estimated TB incidence rates among HIV-infected patients prior to and after ART initiation. We used Cox proportional hazard regressions to determine the predictors of incident TB among HIV-infected adults enrolled in the HIV care and treatment program.
Results
We assessed 67,686 patients for a median follow-up period of 24 (interquartile range: 8–49) months; 7,602 patients were diagnosed with active TB. The TB incidence rate was 7.9 (95% Confidence Interval (CI), 7.6–8.2)/100 person-years prior to ART initiation, and 4.4(95%CI, 4.2–4.4)/100 person-years for patients receiving ART. In multivariate analyses, patients on ART in the first 3 months had a 57% higher risk of TB (Hazard Ratio:1.57, 95%CI:1.47–1.68) compared to those not on ART, but the risk significantly decreased with increasing duration of ART. Risk factors for incident TB included being male, having low body mass index or middle upper arm circumference, lower CD4 cell count, and advanced WHO disease stage. There was seasonal variation for incident TB, with higher risk observed following the rainy seasons (May, June, and November).
Conclusion
In TB endemic regions, HIV-infected patients initiating ART, particularly males and those with poor nutritional status, should be closely monitored for active TB in the months following ART initiation. In addition to increasing the access to ART, interventions should be considered to improve nutritional status among HIV-infected patients.
Keywords: Antiretroviral therapy, tuberculosis, HIV/AIDS, Tanzania, Nutrition
Introduction
Tuberculosis (TB) continues to be a major public health threat globally as an estimated 9.0 million new cases and 1.5 million TB deaths occurred worldwide in 2013[1]. World Health Organization (WHO) estimates that one third of the world’s population is infected with the latent form of TB and therefore at risk of progressing to active TB. For most individuals, the initial infection is contained by host defenses, and the infection remains latent. However among HIV-infected population, the risk of progression from latent TB to active TB is significantly higher. In 2013, globally people living with HIV are 29 times more likely to develop active TB disease than those who are HIV-negative[1]. It is also estimated that 1.1 million (13%) of the 9.0 million people who developed TB worldwide in 2013 were HIV-positive[1].
Approximately 25 million people are living with HIV/AIDS in sub-Saharan Africa[2]. The high HIV prevalence in this region has propagated a resurgence of both drug-susceptible and drug-resistant TB. According to a recent report by UNAIDS, over 75% of all estimated HIV-TB co-infected individuals live in just 10 countries, and nine of those countries are in sub-Saharan Africa[2]. The world has made substantial gains toward achieving the Millennium Development Goals (MDGs) of halving TB-related deaths among people living with HIV by 2015. From 2004 to 2012, TB-related deaths among people living with HIV declined by 36% worldwide[2]. However, TB continues to be the leading cause of death among people living with HIV, accounting for 25% of global HIV/AIDS-related deaths[1].
Antiretroviral therapy (ART) reduces the risk of active TB among people living with HIV[3, 4]. But longitudinal studies that include data on TB incidence in both pre-ART to post-ART initiation periods are limited. In a large, prospective cohort of HIV-infected patients attending HIV care and treatment in Dar es Salaam, Tanzania, we estimated TB incidence rates by ART status and determined risk factors associated with TB incidence.
Methods
Study Population
We conducted a prospective observational cohort study of HIV-infected individuals enrolled in the Management and Development for Health (MDH)-supported HIV care and treatment clinics in Tanzania. With financial support from the Presidents’ Emergency Plan for AIDS Relief (PEPFAR), the MDH Program was established in 2004 as a joint partnership between Muhimbili University of Health and Allied Sciences, Dar es Salaam City Council, and Harvard University. The MDH program provided infrastructure, laboratory and technical support to HIV care and treatment, integrated prevention of mother-to-child transmission of HIV, and TB services in the three municipalities of Dar es Salaam: Temeke, Ilala, and Kinondoni. From November 2004 to September 2012, 108,554 HIV-infected adults (≥15 years of age) were enrolled in 50 MDH-supported HIV clinical sites. All patients received clinical care and treatment following national and WHO guidelines[5, 6].
We excluded patients who had previously taken ART (n=8,912), been diagnosed with TB or TB status was not recorded thereby missing within 30 days after enrollment (n=16,391), or did not have any follow-up visits (n=15,565). After these exclusions, our final sample size was 67,686 patients (Fig. 1). Among them, 53,056 (78%) were found eligible for and initiated ART during follow-up, the other 14,630 (22%) patients remained on care and monitoring services (ART naive).
Fig. 1.
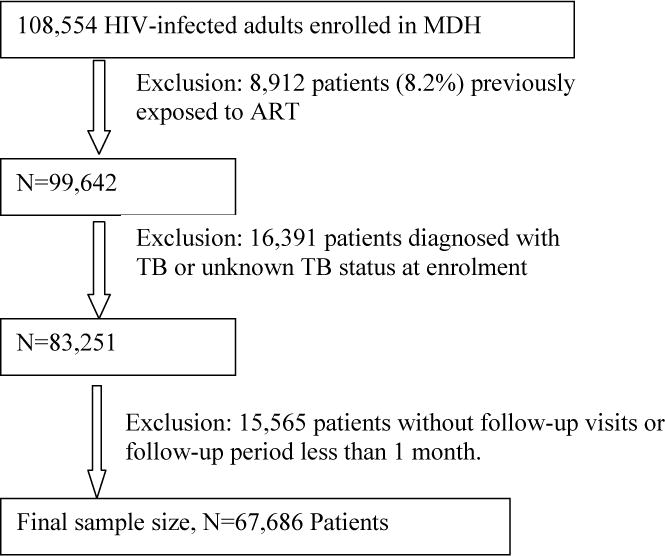
Flow diagram for selection of study participants (MDH, Management and Development for Health; ART, antiretroviral therapy; TB, tuberculosis.)
The institutional review boards for human research at the Harvard School of Public Health and the ethics review committees at Muhimbili University of Health and Allied Sciences in Dar es Salaam approved the study.
ART Eligibility
Upon enrollment, each patient was assessed for eligibility for ART initiation. During the study period, ART initiation criteria for adults included the following: WHO stage IV, WHO stage III with CD4+ cell count <350 cells/uL, or CD4+ cell count <200 cells/uL. Antiretroviral drugs were provided free of charge by the Tanzanian government. Standard first-line ART regimens included 2 nucleoside reverse-transcriptase inhibitors (NRTI) [lamivudine (3TC) or emtricitabine (FTC), plus stavudine (d4T) or zidovudine (AZT) or Tenofovir (TDF)], and 1 nonnucleoside reverse-transcriptase inhibitor (NNRTI) [efavirenz (EFV) or nevirapine (NVP)]. To avoid drug interactions, EFV was substituted for NVP in patients receiving rifampin therapy for the treatment of TB. Patients who were not immediately eligible to initiate ART continued to receive routine care and monitoring service in the HIV clinics, and were started on ART once they met eligibility criteria[5, 6].
Participant Assessments
In general, clinic visits were scheduled at 2 weeks of ART, then monthly thereafter for patients on ART, and 3 to 6-montly for patients not receiving ART. Patients could come to clinics without an appointment during weekdays if necessary to seek extra care and treatment. At each clinic visit, patients were screened for TB based on clinical symptoms including cough, fever, night sweats, weight loss, and hemo rifampcin ptysis. Sputum smears and chest X-rays were administered to all TB suspected patients to assist with the diagnosis. Physicians and nurses completed standardized forms to capture demographic and clinical information. Nurses used standardized techniques to obtain anthropometric measurements, including height, weight, middle upper arm circumference (MUAC). Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters. Laboratory tests for CD4+ cell counts, hemoglobin and alanine aminotransferase (ALT) concentrations were performed at enrollment, ART initiation, and every 6 months during follow-up. Viral load testing was not routinely performed.
Study variables
Incident TB cases were defined as patients who were free of TB at enrollment and newly diagnosed with TB by sputum smear or chest X ray, or prescribed anti-TB medications during follow-up. Demographic, clinical and nutritional characteristics were considered as independent variables, including age (<25, 25 to <35, 35 to <45, ≥45 years), sex (male/female), district of residence (Ilala, Kinondoni, Temeke), BMI (<17, 17 to <18.5, 18.5 to <25, 25 to <30, ≥30 kg/m2), MUAC (<20, 20 to <22, 22 to <25, 25 to <27, ≥27 cm), Anemia (no, mild, moderate, severe), CD4+ cell counts (<50, 50 to <100, 100 to <200, ≥200 cells/μL), WHO stage (I, II, III, IV), ALT (≤40, >40 U/L), pregnancy (no/yes), cotrimoxazole use (no/yes), Isoniazid preventive therapy (no/yes), ART (no/yes). ART regimens were grouped according to their NRTI (d4T, AZT or TDF) and NNRTI (EFV or NVP) agents. The use of INH has increased from 1.5% to 4.5% among patients over the 8 years of study period, and 2,079 (3.1%) of the 67,689 patients in this study have received Isoniazid (INH) preventive therapy during the follow-up period. We grouped hemoglobin concentrations into the following categories according to World Health Organization (WHO) guidelines: severe anemia (hemoglobin, <8.0 g/dl), moderate anemia (8.0 to <11.0 g/dl), mild anemia (11.0 to <13.0 g/dl for men and 11.0 to 12.0 g/dl for women), non-anemia (≥13.0 g/dl for men and ≥12.0 g/dl for women)[7]. Seasons of the clinical visit were categorized into long rainy (April-May), long dry (June-September), and short rainy (October-November), and Short dry (December-March). Nonadherence was defined as >5% noncompliance with scheduled visits.
Statistical analysis
Incidence rates were calculated by number of new incident TB diagnosed divided by person-time followed up during no-ART and ART periods, respectively. Patients who have started ART during follow-up could contribute follow-up time to both non-ART and ART periods. Rates were presented per 100 person-years of follow-up, and 95% confidence intervals were calculated using exact Poisson probabilities. The primary outcome was time to first TB diagnosis after enrollment. The Andersen-Gill formulation of the Cox proportional hazard model was applied to evaluate the association between each baseline characteristic measured at enrolment and selected time-varying parameters with time to first TB event after enrollment[8]. Patients who did not experience incident TB were censored at death or loss to follow-up or the last visit date by September 30, 2012. Missing indicator variables were created for covariates with incomplete information. Multivariate models were fit including all variables that were associated with the primary outcome with P value less than 0.20 in the univariate analysis. Likelihood ratio tests were used to calculate the P values for nominal variables including district, season, and calendar months. The possibility of nonlinear relations between continuous covariates and the risk of incident TB was examined nonparametrically with restricted cubic splines[9]. All statistical tests were two-sided, with P less than 0.05 considered significant, and conducted using SAS 9.3 (Cary, North Caroline, USA).
Results
At enrolment, the mean (±Standard Deviation, SD) age was 35.6(±9.7) years with 9% ≥50 years; approximately 25% were male. The mean BMI was 22.3(±4.9) kg/m2, and 21% of the patients were underweight (defined by BMI<18.5 kg/m2). The mean CD4 was 268(±239) cells/uL, and 51% were WHO HIV stage III or IV. These and other characteristics at enrollment are presented in Table 1.
Table 1.
Patient Characteristics at Enrolment (n=67,686)
| Variables | n (%) |
|---|---|
| Age at enrollment (yrs),% | |
| <25 | 6,398(9.5) |
| 25–<35 | 27,609(40.8) |
| 35–<45 | 22,157(32.7) |
| 45+ | 11,497(17.0) |
| Male sex, % | 17,053(25.2) |
| Family size >3 members, % | 8,615(14.6) |
| District of Dar es Salaam,% | |
| Ilala | 26,784(39.6) |
| Kinondoni | 22,979(34.0) |
| Temeke | 17,803(26.4) |
| Year of enrolment,% | |
| 2004 or 2005 | 3,658(5.4) |
| 2006 | 7,349(10.9) |
| 2007 | 10,279(15.2) |
| 2008 | 11,577(17.1) |
| 2009 | 11,068(16.3) |
| 2010 | 9,419(13.9) |
| 2011 | 8,101(2.0) |
| 2012 | 6,235(9.2) |
| Season of enrollment,% | |
| Dec.–Mar. (Short dry) | 21,784(32.2%) |
| Apr.–May (Long rainy) | 10,835(16.0) |
| Jun.–Sept. (Long dry) | 23,895(35.3) |
| Oct.–Nov. (Short rainy) | 111,72(16.5) |
| BMI at enrollment (kg/m2),% | |
| <17 | 6,332(10.2) |
| 17–<18.5 | 6749(10.9) |
| 18.5–<25.0 | 33,784(54.7) |
| 25–<30.0 | 10,627(17.2) |
| 30.0+ | 4,321(7.0) |
| MUAC at enrollment (cm),% | |
| <20 | 2,581(4.6) |
| 20–<22 | 5,137(9.1) |
| 22–<25 | 15,771(28.1) |
| 25–<27 | 12,061(21.4) |
| 27+ | 20,671(36.8) |
| Anemia status,% | |
| Severe anemia | 5,614(12.7) |
| Moderate anemia | 19,796(44.8) |
| Mild anemia | 9,292(21.0) |
| Non-anemia | 9,467(21.4) |
| CD4+ cell counts at enrolment (cells/uL) | |
| <50 | 8,643(15.6) |
| 50–<100 | 6,346(11.4) |
| 100–<200 | 11,449(20.6) |
| 200–<350 | 13,297(23.9) |
| 350+ | 15,836(28.5) |
| WHO Stage at enrollment,% | |
| I | 17,627(26.6) |
| II | 14,941(22.6) |
| III | 25,138(38.0) |
| IV | 8,478(12.8) |
| ALT>40u/L at enrolment, % | 4,832(11.3) |
| Cotrimoxazole use at enrollment, % | 21,087(33.2) |
| ART regimensa | |
| D4T+3TC+NVP | 13,816(37.7) |
| AZT+3TC+NVP | 5,965(16.3) |
| TDF+FTC+NVP | 32(0.1) |
| D4T+3TC+EFV | 2,489(6.8) |
| AZT+3TC+EFV | 13,351(36.5) |
| TDF+FTC+EFV | 940(2.6) |
Values are means (standard deviation) unless specified;
Notes: BMI, body mass index; MUAC, middle upper arm circumference; ALT, alanine aminotransferase; ART, antiretroviral therapy.
Distribution of ART regimens among patients who started ART during the follow-up. (d4T, stavudine; AZT, zidovudine; TDF, tenofovir; EFV, efavirenz; NVP, nevirapine; 3TC, lamivudine; FTC, emtricitabine)
During the study period, 53,056 of 67,686 patients initiated ART, and the median time between enrollment and ART initiation was 31 (Inter Quartile Range (IQR), 16–158) days. During a median follow-up of 24 (IQR, 8–49) months, 7,602 of 67,686 patients developed incident TB. The incidence rate was 7.9 (95% CI, 7.6–8.2)/100 person-years for patients on care and monitoring (ART naïve), and 4.4 (95%CI, 4.2–4.4)/100 person-years for patients on ART.
Table 2 presents univariate and multivariate analyses for potential risk factors associated with incident TB. In multivariate analyses, male patients had a 53% significantly higher risk of developing TB than females [Hazard Ratio (HR), 1.53; 95%CI, 1.45–1.60]. Patients enrolled in the Kinondoni (HR, 1.22; 95%CI, 1.15–1.29) and Temeke (HR, 1.06; 95%CI, 1.00–1.13) districts had a higher risk of developing TB than those enrolled in Ilala district. Patients enrolled in later calendar years tended to have a lower risk of TB than those enrolled in the earliest years of 2004–2005 when the HIV care and treatment program just started. Patients tended to have a relative higher risk of TB in the short rainy season (October–November). To further explore the seasonal pattern in TB risk, we examined the association of TB risk with calendar months, and found that higher risk TB was observed in months of May, June, and November, around the ends of the two rainy seasons in Dar es Salaam (Fig.2, P<0.001).
Table 2.
Risk factor for tuberculosis incidence among HIV-infected adults (n=67,686)
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years, time-varying) | ||||
| <25 | 0.62(0.59–0.67) | <0.001 | 0.77(0.69–0.87) | 0.09 |
| 25–<35 | Ref. | Ref. | ||
| 35–<45 | 1.17(1.11–1.23) | 1.11(1.05–1.17) | ||
| 45+ | 1.05(0.97–1.13) | 0.99(0.92–1.05) | ||
| Male | 1.90(1.81–1.99) | <0.001 | 1.53(1.45–1.60) | <0.001 |
| Pregnant womena | 0.18(0.15–0.23) | <0.001 | 0.43(0.34–0.53) | <0.001 |
| District | ||||
| Ilala | Ref. | <0.001 | Ref. | 0.02 |
| Kinondoni | 1.12(1.07–1.19) | 1.22(1.15–1.29) | ||
| Temeke | 1.14(1.07–1.20) | 1.06(1.00–1.13) | ||
| Family size (members) | ||||
| ≤3 | Ref. | <0.001 | Ref. | 0.74 |
| >3 | 0.91(0.85–0.98) | 0.99(0.92–1.06) | ||
| Year of enrollment | ||||
| 2004 or 2005 | Ref. | <0.001 | Ref. | <0.001 |
| 2006 | 0.97(0.88–1.07) | 0.98(0.89–1.08) | ||
| 2007 | 0.91(0.83–1.00) | 0.94(0.86–1.03) | ||
| 2008 | 0.78(0.71–0.85) | 0.80(0.73–0.88) | ||
| 2009 | 0.74(0.67–0.82) | 0.76(0.69–0.85) | ||
| 2010 | 0.68(0.62–0.76) | 0.74(0.67–0.83) | ||
| 2011 | 0.66(0.59–0.74) | 0.85(0.75–0.97) | ||
| 2012 | 0.71(0.62–0.81) | 1.12(0.88–1.44) | ||
| Season of visit (time-varying) | ||||
| Dec.–Mar. (Short dry) | Ref. | <0.001 | Ref. | 0.13 |
| Apr.–May (Long rainy) | 1.06(0.99–1.14) | 0.99(0.93–1.06) | ||
| Jun.–Sept. (Long dry) | 1.13(1.07–1.20) | 1.00(0.95–1.06) | ||
| Oct.–Nov. (Short rainy) | 1.11(1.04–1.19) | 1.06(0.98–1.13) | ||
| BMI (kg/m2,time-varing)b | ||||
| <17.0 | 3.07(2.88–3.27) | <0.001 | 1.96(1.83–2.09) | <0.001 |
| 17.0–<18.5 | 2.25(2.11–2.40) | 1.69(1.58–1.80) | ||
| 18.5–<25.0 | Ref. | Ref. | ||
| 25.0–<30.0 | 0.48(0.44–0.52) | 0.64(0.59–0.70) | ||
| 30+ | 0.36(0.31–0.41) | 0.55(0.48–0.62) | ||
| MUAC (cm, time-varying)b | ||||
| <20.0 | 5.99(5.48–6.54) | <0.001 | 3.15(2.86–3.46) | <0.001 |
| 20.0–<22.0 | 4.53(4.19–4.90) | 2.68(2.47–2.91) | ||
| 22.0–<25.0 | 2.48(2.33–2.64) | 1.81(1.69–1.93) | ||
| 25.0–<27.0 | 1.59(1.48–1.71) | 1.35(1.25–1.45) | ||
| 27.0+ | Ref. | Ref. | ||
| Anemia status (time-varying) | ||||
| Severe anemia | 3.57(3.28–3.89) | <0.001 | 2.03(1.86–2.22) | <0.001 |
| Moderate anemia | 2.08(1.93–2.23) | 1.66(1.55–1.79) | ||
| Mild anemia | 1.47(1.35–1.59) | 1.22(1.13–1.33) | ||
| No anemia | Ref. | Ref. | ||
| CD4+ cell counts (cells/uL, time-varying) | ||||
| <50 | 3.36(3.02–3.74) | <0.001 | 1.04(0.92–1.17) | <0.001 |
| 50–<100 | 3.17(2.84–3.54) | 1.18(1.04–1.33) | ||
| 100–<200 | 2.51(2.26–2.78) | 1.20(1.07–1.35) | ||
| 200–<350 | 1.15(1.04–1.27) | 0.81(0.72–0.91) | ||
| 350+ | Ref. | Ref. | ||
| WHO HIV disease stage (time-varying) | ||||
| I | Ref. | <0.001 | Ref. | <0.001 |
| II | 2.13(1.91–2.37) | 1.59(1.42–1.78) | ||
| III | 5.96(5.41–6.56) | 3.54(3.20–3.92) | ||
| IV | 7.62(6.85–8.48) | 3.44(3.06–3.87) | ||
| ALT (u/L, time-varying) | ||||
| ≤40 | Ref. | <0.001 | Ref. | 0.78 |
| >40 | 1.33(1.24–1.43) | 1.01(0.94–1.08) | ||
| Cotrimoxazole (time-varying) | ||||
| No | Ref. | <0.001 | Ref. | 0.11 |
| Yes | 1.28(1.20–1.36) | 1.05(0.99–1.12) | ||
| Isoniazid preventive therapy (time-varying) | ||||
| No | Ref. | <0.001 | Ref. | <0.001 |
| Yes | 2.29(1.58–3.32) | 2.25(1.55–3.27) | ||
| Nonadherence (time-varying) | ||||
| No | Ref. | <0.92 | ||
| Yes | 1.00(0.94–1.07) | |||
| Months on ART (time-varying) | ||||
| None | Ref. | <0.001 | Ref. | <0.001 |
| >0 and <3 | 3.34(3.15–3.54) | 1.57(1.47–1.68) | ||
| 3–<6 | 1.67(1.51–1.85) | 0.79(0.71–0.88) | ||
| 6–<12 | 1.48(1.34–1.65) | 0.73(0.65–0.81) | ||
| 12–<24 | 1.16(1.04–1.29) | 0.54(0.48–0.60) | ||
| 24–<36 | 0.91(0.79–1.04) | 0.41(0.36–0.48) | ||
| 36+ | 0.69(0.59–0.80) | 0.28(0.25–0.33) | ||
| ART agentsc | ||||
| NRTI | ||||
| d4T | Ref. | <0.001 | Ref. | <0.001 |
| AZT | 0.87(0.82–0.91) | 0.65(0.60–0.70) | ||
| TDF | 1.69(1.48–1.94) | 0.90(0.77–1.05) | ||
| NNRTI | ||||
| EFV | Ref. | <0.001 | Ref. | <0.001 |
| NVP | 0.49(0.47–0.52) | 0.36(0.34–0.39) | ||
Notes: HR, Hazard Ratio; CI, confidence interval; BMI, body mass index; MUAC, middle upper arm circumference; ALT, alanine aminotransferase; ART, antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; d4T, stavudine; AZT, zidovudine; TDF, tenofovir; EFV, efavirenz; NVP, nevirapine;
The comparison was conducted among women only, the reference group is non-pregnant women.
In multivariate analysis, BMI and MUAC were included into separate models. And the model with BMI was used get HR and 95%CI for other variables.
Only patients who started ART during the follow-up period were included into the analysis to examine the association of ART agents with TB risk.
Fig. 2.
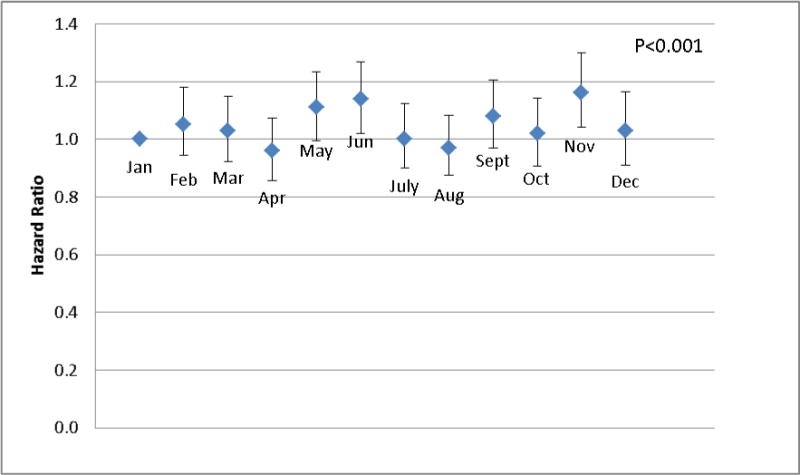
Hazard Ratio of incident tuberculosis by calendar months among HIV-infected adults in Dar Salaam, Tanzania. Note: The diamonds show the Hazard Ratios with January as reference (HR=1.0); The vertical Bars show the 95% confidence intervals. (The P value was calculated based likelihood ratio test by running the multivariate Cox proportional hazard model twice, with and without the 11 calendar-month indicators included)
Time-varying nutritional status was significantly associated with TB risk. Compared with patients with normal BMI (18.5–<25.0kg/m2), patients with BMI<17.0 had a 2-fold increased risk of TB, while overweight and obese patients had 36% and 45% percent reduced risks, respectively (P value for trend<0.001). Similar associations were found between TB risk and MUAC (HR, 3.15; 95%CI, 2.86–3.46, comparing the lowest vs. highest categories; P value for trend <0.001). Patients with severe, moderate, and mild anemia a 103%, 66%, and 22% higher risks of TB, respectively, compared to those without anemia (P value for trend<0.001, Table 2).
Lower CD4+ cell counts and advanced WHO HIV disease stage were significantly associated with a higher risk of developing TB. Compared with patients with CD4+ ≥350 cells/uL, those with CD4+<200 cells/uL had a significantly 5% to 20% higher risk of TB. Compared to patients who were WHO stage I, those who were WHO stage II, III, and IV had a 1.59, 3.54, and 3.44 times greater risk of TB, respectively (P for trend<0.001, Table 2)
After controlling for potential confounders, patients in the first 3 months of ART had a 57% higher risk of developing TB compared to patients who were not on ART. However, the risk of TB began to decrease with continued ART, patients who were on ART for 3–6, 6–12, 12–24, 24–36, ≥36 months had a 21%, 27%, 47%, 59%, and 72% reduced risks of developing TB compared to patients who were not on ART (Table 2). Among patients who have started ART in program, the relationship between risk of TB and duration on ART were further demonstrated in figure 3. From this graph we observed that the risk of TB increased over time before ART initiation, and reached highest level around ART initiation, after that the risk decreased over time with increasing months on ART (figure 3).
Fig. 3.
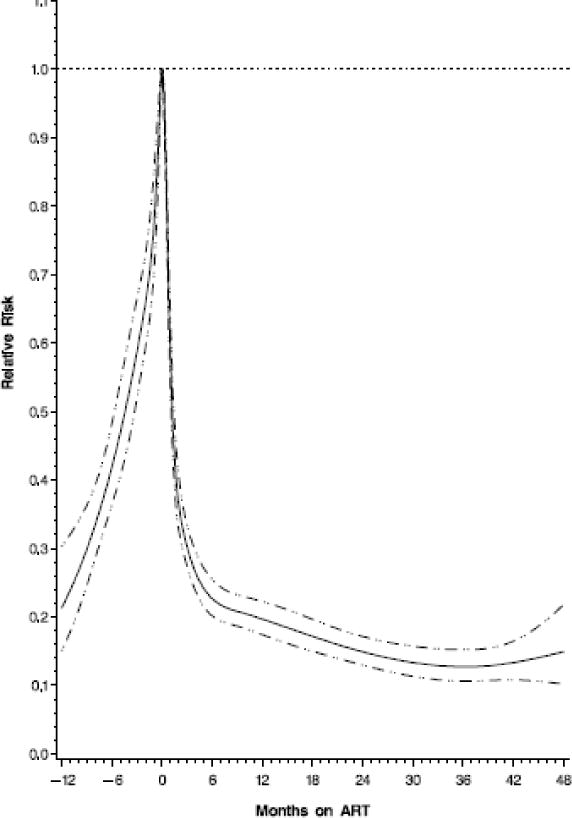
Hazard Ratio of incident tuberculosis by duration on ART (with zero as reference) among HIV-infected individuals in Dar es Salaam, Tanzania. Note: The horizontal dot line shows where the hazard ratio equal to 1; the solid line shows hazard ratio; the dot-dash lines show the 95% confidence intervals for hazard ratios; negative times represent time before ART initiation, only patients who have started ART during the follow-up were included to create the graph.
To compare the effect of different ART regimens on TB risk, a separate multivariate model was fit among patients who initiated ART during the follow-up period (n=53,056), Compared to patients receiving d4T-contaning regimens, those receiving AZT- or TDF-containing regimens had 35% (HR:0.65, 95%CI: 0.60–0.70) and 10% (HR:0.90,95%CI:0.77–1.05) reduced risks of incident TB, respectively; compared to patients receiving EFV-containing regimens, those receiving NVP-containing regimens had a 64% (HR:0.36,95%CI:0.34–0.39) reduced risk of incident TB (Table 2).
Discussion
In this large observational prospective cohort study of HIV-infected adults in Tanzania, we found that the incidence of TB was significantly lower for patients receiving ART than those who were not on ART. However, patients within the first three months following ART initiation had a temporary increased risk. Male gender, poor nutritional status, lower CD4 cell count, and advanced HIV disease stage were associated with a higher risk of developing active TB. In addition, we found a seasonal fluctuation of TB incidence with higher risk in the end of rainy seasons in Dar es Salaam, Tanzania (May, June and November).
It is well documented that ART effectively reduces TB incidence among population in HIV endemic areas[3, 4, 10–12]. ART suppresses viral activity and promotes the restoration of the immune system, hence leading to a reduced risk of re-activating latent TB or of emerging opportunistic infections. However, TB incidence may rise during the initial months on ART, which is largely due to ART-induced unmasking of subclinical active TB and delayed diagnosed of unspecific symptomatic patients in resource-limited settings[13–15]. The TB incidence pattern following ART initiation in the current study is consistent with findings from previous studies[16–20]. Data from South Africa[18, 20] and Nigeria[19] both demonstrated that TB incidence was higher in the first year of ART, then decreased over time thereafter. Similar to our previous finding among HIV-infected children, patients receiving NVP-containing ART regimens had a lower risk of developing of TB[21]. Due to the concerns over an advert drug interaction between NVP and rifampin (anti-TB therapy), EFV was recommend to replace NVP for TB-infected patients[5]. Therefore, the increased risk associated with EFV might be due to confounding by indication, meaning that patients who were suspected with TB infection but without enough evidence for diagnosis at ART initiation were more likely to be prescribed EFV-containing regimens.
Our study demonstrated that nutritional status is a strong predictor for TB risk among HIV-infected adults. The bidirectional association between malnutrition and TB has been well documented in general population[22, 23]. However, there has been little evidence among HIV-infected populations receiving ART[24]. Several studies have indicated that poor nutritional status is associated with higher risks of mortality and opportunistic infection, including TB, after ART initiation[25–27]. Furthermore, co-infection with HIV and TB exacerbates the malnutrition and wasting seen in TB or HIV infection alone, due to increased energy expenditure, decreased appetite, and nutrient malabsorption[28, 29]. To exclude the possibility of reverse causality, patients diagnosed with active TB within 30 days after enrollment were excluded from the current analysis. To clarify the temporal relationship between nutritional status and incident TB, the time-varying nutritional status indicators, measured by BMI, MUAC and hemoglobin level, were associated with outcomes at least one month later in our analyses. To control the high TB risk and sustain the ART treatment outcomes, randomized controlled trials are warranted to confirm findings from observational studies and identify effective strategies to improve and maintain nutritional status among HIV-infected individuals.
In this study, we also found the risk of TB was higher at the end of rainy seasons (May, June and November). This may be due to people spending more time indoors during rainy seasons, thereby increasing close contact and exposure time to TB-infected individuals. The seasonal variation across the year might also be related to the seasonal fluctuation in vitamin D status. Several studies have suggested that vitamin D deficiency impairs the immune response to mycobacterial infection[30]. Evidence from observational studies has shown that vitamin D deficiency was associated with higher risk of TB in various populations[31–34]. In a previous study from Tanzania, Sudfeld and colleagues [35] found that the period of June through August was associated with the highest risk of low vitamin D status (25(OH)D <30 ng/mL) in HIV-infected adults. This finding is consistent with our result that the higher risk of TB was observed in the similar period from May to November. Randomized controlled trials of vitamin D supplementation for prevention of TB appear to be warranted for HIV-infected populations.
Consistent with published data, we identified several other risk factors for incident TB among HIV-infected population, including male, lower CD4+ cell counts[18, 36, 37], advanced HIV disease stage[18, 37]. We found that patients enrolled in Kinondoni and Temeke districts had a higher risk of TB compared those enrolled in Ilala. In Dar es Salaam, people living in Ilala district tend to have a higher social economic status, which might be associated with lower prevalence and incidence of TB infection among the population.
Our study had several strengths and limitations. First, the majority of the TB diagnoses were based on clinical syndromes. Physicians ordered sputum smear test and chest X-ray for all diagnosed TB patients in this study, but only 12% of them had a positive result on sputum smear and 7% positive on chest X-ray. Sputum cultures were not available in the HIV care and treatment clinics in Dar es Salaam. Because of the high TB incidence among the target population, sputum microbiologic confirmation was not required for the diagnosis and treatment of TB for patients attending the HIV clinics. Second, since viral loads were not routinely measured in the study population, and socio-economic data and other lifestyle factors including smoking, and diet, were not collected, we were not able to take them into account and potential residual confounding from these factors cannot be excluded. The strengths of the study include the large sample size and relatively long follow-up period. More than 75% of the patients started ART during the follow-up and contributed data and person-years in both pre-ART and post-ART periods, this allows us to calculate the TB incidence by ART status, and examine the both short term and long term effects of ART on TB incidence.
In summary, our findings demonstrate that ART significantly reduced the incidence of active TB among HIV-infected individuals, despite a sharp increase in TB incidence during the initial months of ART due to unmasking of subclinical TB. Malnutrition, lower CD4 cell counts, and advanced WHO HIV disease state were significantly predictor of TB incidence, while seasonal variation played a role in the development of TB among HIV-infected population. A combined strategy targeting for improving and maintaining nutritional status, effective TB screening tools, timely initiation of ART, and controlling HIV disease progression is needed to reduce the high TB risk among HIV-infected population in resource-limited settings. In TB endemic regions, patients initiating ART, particularly males and those with poor nutritional status, should be closely monitored for active TB in the months following ART initiation. Further studies focused on nutritional interventions in the context of ART and HIV/TB co-infection are warranted.
Acknowledgments
We thank the Management and Development for Health (MDH), Dar es Salaam health delivery system, Muhimbili University of Health and Allied Sciences, Harvard School of Public Health, and the Ministry of Health and Social Welfare for guidance and collaboration in implementing this national HIV care and treatment program in Dar es Salaam, Tanzania; all the patients and staff of the MDH-supported care and treatment sites who have contributed to these findings.
Financial support
The HIV care and treatment program was supported by U.S. Presidents’ Emergency Plan for AIDS Relief (PEPFAR, grant number U51HA02522), and the Centers for Disease Control and Prevention (grant number 5U2GPS001966). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Role of authors:
E.L.: Study design, conduct data analysis, literature search, and manuscript writing;
A.M.: study design, editing;
D. Spiegelman: study design, data analysis plan, editing
D. Sando: data collection, editing;
G. C.: study design, data collection, editing;
E.H.: data analysis, editing;
P. D.: study design editing;
N. L.: data analysis, editing
C.R.S.: literature review, editing
W.W.F.: Study design, analysis plan, editing
All authors approved the final version of the manuscript.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization. Global tuberculosis report. Geneva, Swizerland: 2014. [Google Scholar]
- 2.USAIDS. THe GAP report. Geneva, Switzerland: 2014. 2014. [Google Scholar]
- 3.Lima VD, Granich R, Phillips P, Williams B, Montaner JS. Potential impact of the US President’s Emergency Plan for AIDS relief on the tuberculosis/HIV coepidemic in selected Sub-Saharan African countries. J Infect Dis. 2013;208:2075–2084. doi: 10.1093/infdis/jit406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suthar AB, Lawn SD, del Amo J, Getahun H, Dye C, Sculier D, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001270. doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Antiretroviral thearpy for HIV infection in adolescents and adults: recommendation for a public health approach. 2010 revision. [Google Scholar]
- 6.National Guideline for the Management of HIV and AIDS, National AIDS Control Programme (NACP), Third Edition. The United Republic of Tanzania, Ministry of Health and Social Welfare. 2008 [Google Scholar]
- 7.WHO. Vitamin and Mineral Nutrition Information System. Geneva, Switzerland: World Health Organization; 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. (WHO/NMH/NHD/MNM/11.1) [Google Scholar]
- 8.Andersen PK, Gill RD. Cox’s Regression Model for Counting Processes: A Large Sample Study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 9.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 10.Severe P, Juste MA, Ambroise A, Eliacin L, Marchand C, Apollon S, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–265. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samandari T, Agizew TB, Nyirenda S, Tedla Z, Sibanda T, Shang N, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 12.Golub JE, Pronyk P, Mohapi L, Thsabangu N, Moshabela M, Struthers H, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009;23:631–636. doi: 10.1097/QAD.0b013e328327964f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Worodria W, Massinga-Loembe M, Mayanja-Kizza H, Namaganda J, Kambugu A, Manabe YC, et al. Antiretroviral treatment-associated tuberculosis in a prospective cohort of HIV-infected patients starting ART. Clin Dev Immunol. 2011;2011:758350. doi: 10.1155/2011/758350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermans SM, Kiragga AN, Schaefer P, Kambugu A, Hoepelman AI, Manabe YC. Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in sub-Saharan Africa. PLoS One. 2010;5:e10527. doi: 10.1371/journal.pone.0010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinkhof MW, Egger M, Boulle A, May M, Hosseinipour M, Sprinz E, et al. Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin Infect Dis. 2007;45:1518–1521. doi: 10.1086/522986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girardi E, Sabin CA, d’Arminio Monforte A, Hogg B, Phillips AN, Gill MJ, et al. Incidence of Tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis. 2005;41:1772–1782. doi: 10.1086/498315. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7:e34156. doi: 10.1371/journal.pone.0034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akanbi MO, Achenbach CJ, Feinglass J, Taiwo B, Onu A, Pho MT, et al. Tuberculosis after one year of combination antiretroviral therapy in Nigeria: a retrospective cohort study. AIDS Res Hum Retroviruses. 2013;29:931–937. doi: 10.1089/aid.2012.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawn SD, Kranzer K, Edwards DJ, McNally M, Bekker LG, Wood R. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS. 2010;24:1323–1328. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li N, Manji KP, Spiegelman D, Muya A, Mwiru RS, Liu E, et al. Incident tuberculosis and risk factors among HIV-infected children in Tanzania. AIDS. 2013;27:1273–1281. doi: 10.1097/QAD.0b013e32835ecb24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8:286–298. [PubMed] [Google Scholar]
- 23.Macallan DC. Malnutrition in tuberculosis. Diagn Microbiol Infect Dis. 1999;34:153–157. doi: 10.1016/s0732-8893(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 24.Semba RD, Darnton-Hill I, de Pee S. Addressing tuberculosis in the context of malnutrition and HIV coinfection. Food Nutr Bull. 2010;31:S345–364. [PubMed] [Google Scholar]
- 25.Hanrahan CF, Golub JE, Mohapi L, Tshabangu N, Modisenyane T, Chaisson RE, et al. Body mass index and risk of tuberculosis and death. AIDS. 2010;24:1501–1508. doi: 10.1097/QAD.0b013e32833a2a4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu E, Spiegelman D, Semu H, Hawkins C, Chalamilla G, Aveika A, et al. Nutritional status and mortality among HIV-infected patients receiving antiretroviral therapy in Tanzania. J Infect Dis. 2011;204:282–290. doi: 10.1093/infdis/jir246. [DOI] [PubMed] [Google Scholar]
- 27.Melkamu H, Seyoum B, Dessie Y. Determinants of Tuberculosis Infection among Adult HIV Positives Attending Clinical Care in Western Ethiopia: A Case-Control Study. AIDS Res Treat. 2013;2013:279876. doi: 10.1155/2013/279876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Lettow M, Fawzi WW, Semba RD. Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutr Rev. 2003;61:81–90. doi: 10.1301/nr.2003.marr.81-90. [DOI] [PubMed] [Google Scholar]
- 29.Swaminathan S, Padmapriyadarsini C, Sukumar B, Iliayas S, Kumar SR, Triveni C, et al. Nutritional status of persons with HIV infection, persons with HIV infection and tuberculosis, and HIV-negative individuals from southern India. Clin Infect Dis. 2008;46:946–949. doi: 10.1086/528860. [DOI] [PubMed] [Google Scholar]
- 30.Martineau AR. Old wine in new bottles: vitamin D in the treatment and prevention of tuberculosis. Proc Nutr Soc. 2012;71:84–89. doi: 10.1017/S0029665111003326. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355:618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 32.Martineau AR, Leandro AC, Anderson ST, Newton SM, Wilkinson KA, Nicol MP, et al. Association between Gc genotype and susceptibility to TB is dependent on vitamin D status. Eur Respir J. 2010;35:1106–1112. doi: 10.1183/09031936.00087009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martineau AR, Nhamoyebonde S, Oni T, Rangaka MX, Marais S, Bangani N, et al. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci U S A. 2011;108:19013–19017. doi: 10.1073/pnas.1111825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sudfeld CR, Giovannucci EL, Isanaka S, Aboud S, Mugusi FM, Wang M, et al. Vitamin D status and incidence of pulmonary tuberculosis, opportunistic infections, and wasting among HIV-infected Tanzanian adults initiating antiretroviral therapy. J Infect Dis. 2013;207:378–385. doi: 10.1093/infdis/jis693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sudfeld CR, Wang M, Aboud S, Giovannucci EL, Mugusi FM, Fawzi WW. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PLoS One. 2012;7:e40036. doi: 10.1371/journal.pone.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin-Echevarria E, Serrano-Villar S, Sainz T, Moreno A, Casado JL, Dronda F, et al. Development of tuberculosis in human immunodeficiency virus infected patients receiving antiretroviral therapy. Int J Tuberc Lung Dis. 2014;18:1080–1084. doi: 10.5588/ijtld.13.0757. [DOI] [PubMed] [Google Scholar]
- 37.Venkatesh PA, Bosch RJ, McIntosh K, Mugusi F, Msamanga G, Fawzi WW. Predictors of incident tuberculosis among HIV-1-infected women in Tanzania. Int J Tuberc Lung Dis. 2005;9:1105–1111. [PubMed] [Google Scholar]


