Abstract
Background
Coffee consumption has been reported to be inversely associated with hepatocellular carcinoma (HCC), the most common type of liver cancer. Caffeine has chemopreventive properties, but whether caffeine is responsible for the coffee-HCC association is not well studied. In addition, few studies have examined the relationship by sex, and no studies have examined whether there is an association between coffee and intrahepatic cholangiocarcinoma (ICC), the second most common type of liver cancer.
Methods
In the Liver Cancer Pooling Project, a consortium of U.S.-based cohort studies, data from 1,212,893 individuals (HCC n=860, ICC n=260) in nine cohorts were pooled. Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CI) were estimated using proportional hazards regression.
Results
Higher coffee consumption was associated with lower risk of HCC (HR>3 cups/day vs. non-drinker, 0.73; 95% CI, 0.53-0.99; ptrend cups/day=<0.0001). More notable reduced risk was seen among women than men (pinteraction=0.07). Women who consumed more than three cups of coffee per day were at a 54% lower risk of HCC (HR, 0.46; 95% CI, 0.26-0.81), whereas men had more modest reduced risk of HCC (HR, 0.93; 95% CI, 0.63-1.37). The associations were stronger for caffeinated coffee (HR>3 cups/day vs. non-drinker, 0.71, 95% CI, 0.50-1.01) than decaffeinated coffee (HR, 0.92; 95% CI, 0.55-1.54). There was no relationship between coffee consumption and ICC.
Conclusions
These findings suggest that, in a U.S. population, coffee consumption is associated with reduced risk of HCC.
Impact
Further research into specific coffee compounds and mechanisms that may account for these associations is needed.
Keywords: coffee, diet, epidemiology, hepatocellular carcinoma, intrahepatic cholangiocarcinoma
INTRODUCTION
Primary liver cancer is the second leading cause of cancer death worldwide (1), and the seventh leading cause in the U.S. (2). Liver cancer incidence rates in the U.S. have been rising since 1980 (3), although the increase has not been significant in recent years (4). Hepatocellular carcinoma (HCC) is the dominant histologic type of liver cancer, accounting for approximately 65% of cases, while intrahepatic cholangiocarcinoma (ICC), the second most common histologic type, accounts for approximately 14% (5). HCC usually develops in the background of oxidative stress and inflammation, triggered by chronic infection with hepatitis B or C virus (HBV or HCV), excess alcohol consumption, aflatoxin exposure or obesity/diabetes (6). Based on a recent meta-analysis, potential common risk factors for HCC and ICC include chronic HBV and HCV, excessive alcohol use, and diabetes/obesity (7).
Most observational studies have shown a reduced risk of HCC associated with coffee consumption (8, 9). Caffeine, polyphenols (e.g., chlorogenic acid), and diterpenes (e.g., cafestol and kahweol) are thought to be, at least partially, responsible for this reduction in HCC risk (10). Experimentally, caffeine has been shown to inhibit hepatic carcinogenesis (11), potentially through an antioxidant, anti-inflammatory, or radical scavenging mechanisms (12).
Age-adjusted incidence of HCC in men is approximately three times higher than in women (13). This disparity has been hypothesized to be due to the greater prevalence of most known risk factors among men. However, these differences cannot fully explain the male predominance of these tumors (14). It is possible that coffee consumption differentially affects tumor risk in men and women by influencing hormone levels (15), obesity (16), diabetes (16), or other unknown factors.
While the association between coffee drinking and incidence of HCC has been studied in Asian and European populations (8), only one study has examined the association in a U.S. population (17). Additionally, no studies have examined the association between coffee consumption and ICC.
To examine the overall and sex-specific association of coffee with HCC and ICC, and determine whether the associations varied by caffeine content, we studied the hypothesis in a project that pooled data from nine U.S.-based cohort studies.
MATERIALS AND METHODS
Study Population
The Liver Cancer Pooling Project (LCPP) has been described previously (18). Briefly, all U.S.-based cohort studies that are members of the National Cancer Institute (NCI) Cohort Consortium were invited to participate in the LCPP. Of the 14 studies that agreed to participate, nine studies contributed data on both coffee consumption and liver cancer histology: NIH-AARP Diet and Health Study (AARP) (19), Agricultural Health Study (AHS) (20), United States Radiologic Technologists (USRT) Study (21), Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) (22), Women's Health Study (WHS) (23), Cancer Prevention Study–II Nutrition Cohort (CPS-II) (24), Iowa Women's Health Study (IWHS) (25), Black Women's Health Study (BWHS) (26), and Women's Health Initiative (WHI) (27) (Supplementary Table S1).
Outcomes
Incident primary liver cancer (defined as International Classification of Diseases, 10th edition [ICD-10] diagnostic code C22) among LCPP cohort members was ascertained by three methods: linkage to state cancer registries, medical record review, or a self-report to the parent cohort study. Cases missing histology information were excluded (n=832). Cases were then classified as HCC (International Classification of Diseases for Oncology, 3rd edition [ICD-O-3] histology codes of 8170-8175), ICC (ICD-O-3 histology codes of 8032-8033, 8041, 8050, 8070-8071, 8140-8141, 8160, 8260, 8480, 8481, 8490, and 8560), or other liver cancer (all other histology codes). Non-HCC or non-ICC cases were excluded from the analysis (n=171). The current analysis included 860 HCC cases, 260 ICC cases, and 1,211,773 non-cases.
Exposure
With the exception of WHI, all studies assessed coffee drinking over the past year (or 12 months) (Supplementary Table S2). WHI asked participants to report if they usually drink coffee every day. Additionally, WHI assessed only the number of caffeinated cups of coffee consumed and, thus, was not included in decaffeinated intensity or combination analyses. The remaining studies assessed caffeine content by asking participants to report caffeinated and decaffeinated coffee consumption separately or by asking participants the proportion of decaffeinated coffee consumed. Individuals were classified into mutually exclusive groups of caffeinated coffee drinkers, decaffeinated coffee drinkers, or a combination of caffeinated and decaffeinated coffee consumption. To examine trends in coffee consumption, both by caffeine content (using a stratified analysis) and overall, the number of cups of coffee, assumed to be approximately 8 ounces, per day was analyzed as: 0, >0-<1, 1-<2, 2-3, and >3 cups/day. Non-drinkers were defined as those individuals reporting little or no coffee consumption during the FFQ timeframe.
Statistical Analysis
Cox proportional hazard regression analysis was used to calculate adjusted hazard ratios (HRs) and 95% confidence intervals for the associations of coffee consumption with HCC and ICC. The proportional hazards assumption was tested using an interaction between coffee exposure (defined as continuous and categorical) and log(time) in models that included confounders and an interaction was observed (p<0.05). Thus, we present the overall HRs, averaging over varying baseline hazards ratios during follow-up, and the HRs for four time periods of follow-up, based on quartiles of follow-up time for HCC and ICC. Examination of coffee consumption by these differential follow-up times did not result in notable differences among the four lengths of follow-up.
Effect measure modification by sex, cigarette smoking (evaluated as never/ever and cigarettes/day [continuous]), body mass index (BMI, kg/m2; evaluated as continuous and dichotomous [<25 and ≥25]), and diabetes (yes/no) was assessed using likelihood ratio tests comparing regression models with and without a multiplicative term (28). We found no evidence of effect measure modification by smoking, BMI, or diabetes (p≥0.10). However, there was some evidence of effect measure modification by sex (p<0.10).
Potential confounders (29) included alcohol consumption (evaluated as ever/never [referent]; drinks/day [0, >0-<1, 1-3, and >3]), smoking (evaluated as never [referent]/former/current; cigarettes/day, pack-years, and smoking duration [all evaluated as continuous and categorized as quartiles of intake among smokers]), age at questionnaire administration (years, evaluated as continuous and categorical [<50, 50-59, 60-69, ≥70]), race (Caucasian [referent], African American, Asian/Pacific Islander, American Indian/Alaskan Native, Other), sex (male [referent]/female), education (some high school or less, high school/GED, some college/vocational training, college degree [referent], post-college), and BMI (evaluated as continuous and categorical [<18.5, 18.5-<25, 25-<30, and ≥30 kg/m2]). Variables remained in the adjusted model if they were associated with the exposure and outcome and the test of effect was significant (p<0.05) (30); age (continuous), sex, race, smoking (never/current/former and categorized cigarettes/day [0, ≤10, >10-≤15, >15-≤25, or >25]), alcohol consumption (categorized drinks/day [0, >0-<1, 1-3, >3]), and BMI (continuous) met this criterion and were included in all final models. We adjusted for study in all models. We also created a forest plot and used fixed-effects meta-analysis to estimate a summary HR and assess heterogeneity using I2. An I2 of 0% indicates no heterogeneity, whereas larger values indicate increasing heterogeneity between studies (31). Analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC) and STATA version 13 (StataCorp LP, College Station, TX). All p-values are two-sided.
Sensitivity Analyses
We examined caffeine content and intensity of coffee consumption using a model that jointly considered these terms. We also analyzed all confirmed or suspected HCC cases, which included HCC cases (ICD-O-3 histology codes of 8170-8175) and additional suspected HCC cases defined as ICD-O-3 histology codes of 8000, 8010, or missing. Finally, we conducted a meta-influence analysis for HCC, excluding one study at a time from the pooled analysis.
RESULTS
Demographic characteristics of HCC and ICC cases and non-cases are shown in Table 1 and Supplementary Table S3. Compared with non-cases, individuals who developed HCC or ICC were more likely to be older, male, Asian/Pacific Islander, overweight or obese, smoke, drink heavily, and have diabetes.
Table 1.
Characteristics of participants in the Liver Cancer Pooling Project by case status.
| Non-Cases (N=1,211,773) |
Hepatocellular Carcinoma (N=860) |
Intrahepatic Cholangiocarcinoma (N=260) |
||||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| Person-Years | 11,861,098 | 5,333 | 1,705 | |||
| Age at Entry (years) | ||||||
| <50 | 118,974 | (9.8) | 9 | (1.0) | 0 | (0.0) |
| 50-59 | 359,330 | (29.7) | 160 | (18.6) | 56 | (21.5) |
| 60-69 | 587,608 | (48.5) | 573 | (66.6) | 165 | (63.5) |
| ≥70 | 145,861 | (12.0) | 118 | (13.7) | 39 | (15.0) |
| Sex | ||||||
| Male | 491,326 | (40.5) | 618 | (71.9) | 134 | (51.5) |
| Female | 720,447 | (59.5) | 242 | (28.1) | 126 | (48.5) |
| Race | ||||||
| White | 1,050,854 | (87.4) | 702 | (83.1) | 229 | (88.4) |
| Black | 105,350 | (8.8) | 54 | (6.4) | 10 | (3.9) |
| Other | 45,878 | (3.8) | 89 | (10.5) | 20 | (7.7) |
| Missing | 9,691 | 15 | 1 | |||
| Body Mass Index (kg/m2) | ||||||
| <18.5 | 11,884 | (1.0) | 8 | (1.0) | 1 | (0.4) |
| 18.5-24.9 | 438,398 | (37.0) | 190 | (22.8) | 69 | (27.3) |
| 25-29.9 | 468,971 | (39.6) | 343 | (41.1) | 112 | (44.3) |
| ≥30 | 265,683 | (22.4) | 293 | (35.1) | 71 | (28.1) |
| Missing | 26,837 | 26 | 7 | |||
| Education | ||||||
| Some High School or less | 69,879 | (6.0) | 89 | (10.7) | 19 | (7.4) |
| High School | 235,132 | (20.0) | 183 | (22.0) | 49 | (19.1) |
| Some College/Vocational | 419,629 | (35.7) | 280 | (33.7) | 84 | (32.7) |
| College Degree | 222,206 | (18.9) | 143 | (17.2) | 63 | (24.5) |
| Graduate Degree | 227,373 | (19.4) | 136 | (16.4) | 42 | (16.3) |
| Missing | 37,554 | 29 | 3 | |||
| Diabetes | ||||||
| No | 1,121,080 | (92.8) | 620 | (72.4) | 228 | (87.7) |
| Yes | 86,506 | (7.2) | 236 | (27.6) | 32 | (12.3) |
| Missing | 4,187 | 4 | 0 | |||
| Alcohol (drinks/day) | ||||||
| Non-Drinker | 306,409 | (26.5) | 255 | (31.9) | 61 | (24.9) |
| >0-<1 drink | 638,748 | (55.1) | 347 | (43.4) | 129 | (52.7) |
| 1-3 drinks | 152,062 | (13.1) | 96 | (12.0) | 31 | (12.7) |
| >3 drinks | 61,187 | (5.3) | 101 | (12.6) | 24 | (9.8) |
| Missing | 53,367 | 61 | 15 | |||
| Cigarette Smoking Status | ||||||
| Non-Smoker | 530,557 | (44.8) | 248 | (29.7) | 92 | (35.9) |
| Former Smoker | 471,702 | (39.8) | 412 | (49.4) | 120 | (46.9) |
| Current Smoker | 182,506 | ( 15.4) | 174 | (20.9) | 44 | (17.2) |
| Missing | 27,008 | 26 | 4 | |||
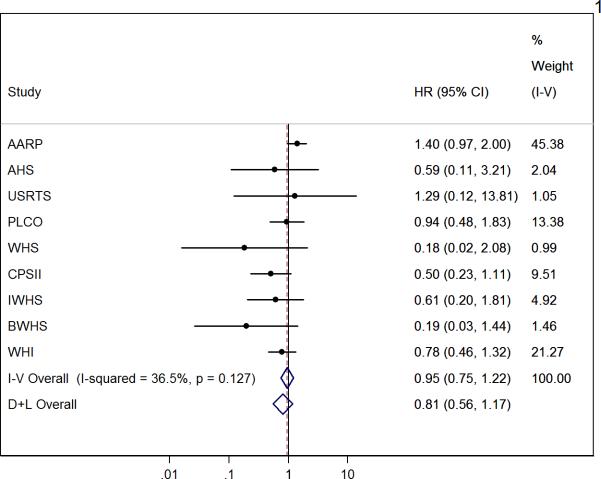
Figure 1 shows the study-specific and summary HRs for coffee consumption versus no consumption and HCC risk. The fixed-effects summary HR was 0.95 (95% CI, 0.75-1.22). While no significant heterogeneity was detected between studies, the inconsistency was moderate (I2=36.5%).
Figure 1.
Study-specific and Summary Hazard Ration (HR) and 95% Confidence Interval (CI) of Coffee Consumption versus No Consumption for Hepatocellular Carcinoma, Liver Cancer Pooling Project.
Table 2 presents the overall and stratified results by length of study follow-up for the pooled analysis. The group who consumed at least three cups of coffee per day were at 27% lower risk of HCC (HR, 0.73; 95% CI, 0.53-0.99), and there was evidence of an inverse dose-response relationship (HRcups/day=0.90; 95% CI, 0.85-0.94). There was no association between coffee consumption and ICC.
Table 2.
Adjusted* Hazard Ratios (HR) and 95% Confidence Intervals (CI) for Associations Between Coffee Consumption and Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma Incidence by Follow-Up Time, Liver Cancer Pooling Project.
| Hepatocellular Carcinoma |
|||||||
|---|---|---|---|---|---|---|---|
| Overall |
0-3.6 years |
3.7-6.7 years |
6.4-8.8 years |
≥8.9 years |
|||
| Coffee Consumption | Non-case N | Case N | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Non-Drinker | 172,865 | 85 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ever | 901,622 | 650 | 1.00 (0.79, 1.27) | 1.05 (0.65, 1.68) | 0.77 (0.50, 1.19) | 1.13 (0.68, 1.87) | 1.12 (0.69, 1.82) |
| Cups/day | |||||||
| >0-<1 | 164,839 | 138 | 1.24 (0.94, 1.64) | 1.43 (0.83, 2.48) | 0.95 (0.56, 1.60) | 1.33 (0.75, 2.37) | 1.31 (0.72, 2.40) |
| 1-<2 | 179,632 | 149 | 1.16 (0.88, 1.52) | 0.91 (0.51, 1.63) | 0.99 (0.59, 1.64) | 1.31 (0.74, 2.32) | 1.50 (0.87, 2.59) |
| 2-3 | 370,531 | 255 | 0.89 (0.68, 1.15) | 1.09 (0.65, 1.81) | 0.61 (0.38, 1.00) | 0.88 (0.51, 1.53) | 1.05 (0.62, 1.77) |
| >3 | 161,019 | 97 | 0.73 (0.53, 0.99) | 0.67 (0.35, 1.26) | 0.57 (0.32, 1.03) | 0.99 (0.54, 1.82) | 0.72 (0.39, 1.36) |
| Continuous, cups/day | 0.90 (0.85, 0.94) | 0.89 (0.81, 0.98) | 0.87 (0.78, 0.96) | 0.93 (0.85, 1.02) | 0.89 (0.81, 0.98) | ||
| p for trend† | <0.0001 | 0.02 | 0.006 | 0.1 | 0.02 | ||
| Intrahepatic Cholangiocarcinoma |
|||||||
|---|---|---|---|---|---|---|---|
| Overall |
0-3.6 years |
3.7-6.7 years |
6.8-9.2 years |
≥9.3 years |
|||
| Coffee Consumption | Non-case N | Case N | HR | HR | HR | HR | HR |
| Non-Drinker | 172,865 | 33 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ever | 901,622 | 199 | 0.93 (0.63, 1.37) | 0.83 (0.39, 1.75) | 1.19 (0.49, 2.85) | 0.75 (0.36, 1.58) | 1.05 (0.50, 2.21) |
| Cups/day | |||||||
| >0-<1 | 164,839 | 36 | 1.15 (0.70, 1.89) | 0.85 (0.30, 2.40) | 1.18 (0.40, 3.51) | 1.33 (0.54, 3.27) | 1.22 (0.44, 3.39) |
| 1-<2 | 179,632 | 33 | 0.79 (0.48, 1.30) | 0.69 (0.26, 1.83) | 1.01 (0.34, 2.97) | 0.87 (0.35, 2.16) | 0.60 (0.21, 1.74) |
| 2-3 | 370,531 | 85 | 0.93 (0.61, 1.42) | 0.86 (0.38, 1.94) | 1.22 (0.47, 3.12) | 0.63 (0.27, 1.47) | 1.18 (0.52, 2.66) |
| >3 | 161,019 | 40 | 1.00 (0.61, 1.63) | 1.12 (0.44, 2.86) | 1.67 (0.61, 4.59) | 0.59 (0.21, 1.67) | 0.81 (0.30, 2.23) |
| Continuous, cups/day | 1.00 (0.92, 1.08) | 1.04 (0.89, 1.22) | 1.12 (0.97, 1.30) | 0.83 (0.69, 0.99) | 0.98 (0.83, 1.15) | ||
| p for trend† | 0.9 | 0.6 | 0.1 | 0.04 | 0.8 | ||
Adjusted for: sex, age (continuous), race (white, black, other), cohort (AARP, AHS, USRT, PLCO, WHS, CPSII, IWHS, BWHS, WHI), BMI (continuous), smoking status (non-smoker, former smoker, current smoker), cigarette smoking intensity (0, ≤10, >10-≤15, >15-≤25, >25 cigarettes/day), alcohol (non-drinker, and >0-<1, 1-3, >3).
P-value for trend of continuous variable.
Table 3 shows the association of coffee consumption by caffeine content with HCC and ICC. For caffeinated coffee, individuals who consumed at least three cups of coffee per day had a modestly reduced risk of HCC (HR=0.71; 95% CI, 0.50-1.01). A trend of decreased HCC risk was also observed for caffeinated coffee consumption (HRcups/day, 0.91; 95% CI, 0.86-0.97; ptrend=0.002). However, little or no association was seen with decaffeinated coffee consumption (HR>3 cups vs. non-drinker=0.92; 95% CI, 0.55-1.54; HRcups/day=0.93; 95% CI, 0.86-1.02; ptrend=0.1) or with consumption of both caffeinated and decaffeinated coffee (HR>3 cups vs. non-drinker=0.61; 95% CI, 0.31-1.21; HRcups/day=0.89; 95% CI, 0.77-1.03; ptrend=0.1). The examination of ICC and consumption of caffeinated or decaffeinated coffee revealed null associations.
Table 3.
Adjusted* Hazard Ratios (HR) and 95% Confidence Intervals (CI) for Associations Between Coffee Consumption and Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma Incidence by Caffeine Content, Liver Cancer Pooling Project.
| Hepatocellular Carcinoma |
Intrahepatic Cholangiocarcinoma |
||||||
|---|---|---|---|---|---|---|---|
| Caffeinated Coffee | Non-case N | Case N | HR | 95% CI | Case N | HR | 95% CI |
| Non-Drinker | 172,865 | 85 | 1.00 | 33 | 1.00 | ||
| Ever | 521,438 | 379 | 1.00 | (0.77 - 1.28) | 119 | 0.91 | (0.60 - 1.37) |
| Cups/day | |||||||
| 0 | 172,865 | 85 | 1.00 | 33 | 1.00 | ||
| >0-<1 | 66,120 | 58 | 1.22 | (0.87 - 1.73) | 17 | 1.32 | (0.71 - 2.43) |
| 1-<2 | 102,435 | 85 | 1.19 | (0.87 - 1.62) | 15 | 0.59 | (0.32 - 1.10) |
| 2-3 | 246,378 | 174 | 0.95 | (0.72 - 1.26) | 57 | 0.91 | (0.58 - 1.43) |
| >3 | 106,505 | 62 | 0.71 | (0.50 - 1.01) | 30 | 1.08 | (0.63 - 1.83) |
| p for trend† | 0.002 | >0.99 | |||||
| Decaffeinated Coffee | |||||||
| Non-Drinker | 172,865 | 85 | 1.00 | 33 | 1.00 | ||
| Ever | 232,725 | 204 | 1.16 | (0.88 - 1.53) | 56 | 0.95 | (0.59 - 1.53) |
| Cups/day‡ | |||||||
| 0 | 130,187 | 63 | 1.00 | 18 | 1.00 | ||
| >0-<1 | 66,450 | 58 | 1.33 | (0.92 - 1.91) | 15 | 1.17 | (0.58 - 2.35) |
| 1-<2 | 47,791 | 51 | 1.38 | (0.95 - 2.02) | 10 | 0.94 | (0.43 - 2.07) |
| 2-3 | 75,840 | 64 | 0.97 | (0.67 - 1.40) | 20 | 1.11 | (0.56 - 2.17) |
| >3 | 22,745 | 21 | 0.92 | (0.55 - 1.54) | 6 | 1.03 | (0.39 - 2.70) |
| p for trend† | 0.1 | 0.6 | |||||
| Combination Coffee | |||||||
| Non-Drinker‡ | 130,187 | 63 | 1.00 | 18 | 1.00 | ||
| Ever | 130,379 | 44 | 0.48 | (0.29 - 0.79) | 20 | 1.72 | (0.71 - 4.16) |
| Cups/day‡ | |||||||
| 0 | 130,187 | 63 | 1.00 | 18 | 1.00 | ||
| >0-<1 | 27,696 | 10 | 0.58 | (0.28 - 1.21) | 3 | 1.45 | (0.37 - 5.68) |
| 1-<2 | 27,098 | 10 | 0.52 | (0.25 - 1.08) | 6 | 2.46 | (0.80 - 7.52) |
| 2-3 | 45,466 | 11 | 0.33 | (0.16 - 0.67) | 7 | 1.68 | (0.58 - 4.87) |
| >3 | 30,119 | 13 | 0.61 | (0.31 - 1.21) | 4 | 1.33 | (0.37 - 4.78) |
| p for trend† | 0.1 | 0.9 | |||||
Adjusted for: sex, age (continuous), race (white, black, other), cohort (AARP, AHS, USRT, PLCO, WHS, CPSII, IWHS, BWHS, WHI), BMI (continuous), cigarette smoking intensity (cigarettes/day), smoking status (non-smoker, former smoker, current smoker), cigarette smoking intensity (0, ≤10, >10-≤15, >15-≤25, >25 cigarettes/day), alcohol (non-drinker, and >0-<1, 1-3, >3 drinks/day).
P-value for trend of continuous variable.
WHI excluded from analysis, due to lack of information about decaffeinated coffee consumption.
As shown in Table 4 and Supplementary Table S4, there was evidence of a multiplicative interaction between coffee drinking and sex for HCC (pinteraction=0.07, χ2=3.37 [df=1]) but not for ICC (pinteraction=0.8, χ2=0.05 [df=1]). Comparing coffee drinkers to non-drinkers, women had a 22% decreased risk of HCC (HR=0.78; 95% CI, 0.56-1.10), while men did not (HR=1.21; 95% CI, 0.87-1.69). Among women who drank more than three cups of coffee per day, the risk of HCC was further reduced (HR=0.46; 95% CI, 0.26-0.81, ptrend=0.004). Men who drank more than three cups of coffee per day had little reduced risk of HCC (HR=0.93, 95% CI, 0.63-1.37) but the risk reduction associated with cups of coffee consumed per day was similar to women (ptrend=0.0004). The examination of coffee consumption and ICC stratified by sex found no notable associations among either men or women.
Table 4.
Adjusted* Hazard Ratios (HR) and 95% Confidence Intervals (CI) for Associations Between Coffee Consumption and Hepatocellular Carcinoma by Sex, Smoking Status, Body Mass Index, and Diabetes, Liver Cancer Pooling Project.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Coffee Consumption | Non-case N | Case N | HR | 95% CI | Non-case N | Case N | HR | 95% CI |
| Non-Drinker | 46,615 | 40 | 1.00 | 126,250 | 45 | 1.00 | ||
| Ever | 393,030 | 490 | 1.21 | (0.87 - 1.69) | 508,592 | 160 | 0.78 | (0.56 - 1.10) |
| Cups/day | ||||||||
| >0-<1 | 73,955 | 113 | 1.57 | (1.09 - 2.25) | 90,884 | 25 | 0.79 | (0.47 - 1.33) |
| 1-<2 | 71,352 | 103 | 1.35 | (0.93 - 1.95) | 108,280 | 46 | 1.01 | (0.66 - 1.53) |
| 2-3 | 170,339 | 195 | 1.06 | (0.75 - 1.51) | 200,192 | 60 | 0.71 | (0.48 - 1.06) |
| >3 | 76,461 | 79 | 0.93 | (0.63 - 1.37) | 84,558 | 18 | 0.46 | (0.26 - 0.81) |
| Continuous, cups/day | 0.90 | (0.86 - 0.96) | 0.87 | (0.79 - 0.96) | ||||
| p for trend† | 0.0004 | 0.004 | ||||||
| Non-Smokers |
Smokers |
|||||||
|---|---|---|---|---|---|---|---|---|
| Coffee Consumption | Non-case N | Case N | HR | 95% CI | Non-case N | Case N | HR | 95% CI |
| Non-Drinker | 115,235 | 48 | 1.00 | 57,789 | 37 | 1.00 | ||
| Ever | 374,559 | 172 | 0.94 | (0.68 - 1.30) | 527,629 | 478 | 1.10 | (0.79 - 1.55) |
| Cups/day | ||||||||
| >0-<1 | 87,498 | 39 | 0.91 | (0.59 - 1.40) | 77,356 | 99 | 1.52 | (1.04 - 2.23) |
| 1-<2 | 86,431 | 48 | 1.08 | (0.72 - 1.62) | 93,328 | 101 | 1.28 | (0.87 - 1.87) |
| 2-3 | 140,728 | 64 | 0.91 | (0.62 - 1.33) | 230,037 | 191 | 0.98 | (0.68 - 1.40) |
| >3 | 47,124 | 12 | 0.52 | (0.28 - 0.99) | 113,988 | 85 | 0.93 | (0.63 - 1.37) |
| Continuous, cups/day | 0.93 | (0.84 - 1.02) | 0.91 | (0.86 - 0.96) | ||||
| p for trend† | 0.1 | 0.0005 | ||||||
| BMI <25 kg/m2 |
BMI ≥25 kg/m2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Coffee Consumption | Non-case N | Case N | HR | 95% CI | Non-case N | Case N | HR | 95% CI |
| Non-Drinker | 67,651 | 25 | 1.00 | 105,214 | 60 | 1.00 | ||
| Ever | 339,706 | 147 | 0.82 | (0.53 - 1.25) | 561,916 | 503 | 1.07 | (0.81 - 1.42) |
| Cups/day | ||||||||
| >0-<1 | 63,633 | 33 | 1.02 | (0.60 - 1.72) | 101,206 | 105 | 1.33 | (0.96 - 1.85) |
| 1-<2 | 69,237 | 30 | 0.81 | (0.47 - 1.38) | 110,395 | 119 | 1.30 | (0.95 - 1.79) |
| 2-3 | 136,149 | 56 | 0.73 | (0.45 - 1.17) | 234,382 | 199 | 0.95 | (0.70 - 1.28) |
| >3 | 60,960 | 24 | 0.65 | (0.37 - 1.14) | 100,059 | 73 | 0.76 | (0.53 - 1.08) |
| Continuous, cups/day | 0.90 | (0.82 - 0.99) | 0.89 | (0.85 - 0.94) | ||||
| p for trend† | 0.03 | <0.0001 | ||||||
| No Diabetes |
Diabetes |
|||||||
|---|---|---|---|---|---|---|---|---|
| Coffee Consumption | Non-case N | Case N | HR | 95% CI | Non-case N | Case N | HR | 95% CI |
| Non-Drinker | 161,059 | 67 | 1.00 | 11,391 | 17 | 1.00 | ||
| Ever | 835,624 | 462 | 0.89 | (0.68 - 1.16) | 63,979 | 188 | 1.41 | (0.85 - 2.32) |
| Cups/day | ||||||||
| >0-<1 | 151,345 | 102 | 1.19 | (0.87 - 1.64) | 13,102 | 36 | 1.42 | (0.80 - 2.54) |
| 1-<2 | 165,484 | 104 | 1.02 | (0.75 - 1.40) | 13,769 | 45 | 1.60 | (0.91 - 2.81) |
| 2-3 | 344,302 | 174 | 0.75 | (0.56 - 1.01) | 25,558 | 81 | 1.40 | (0.83 - 2.38) |
| >3 | 150,340 | 73 | 0.68 | (0.48 - 0.95) | 10,231 | 24 | 0.98 | (0.52 - 1.83) |
| Continuous, cups/day | 0.88 | (0.83 - 0.93) | 0.94 | (0.87 - 1.03) | ||||
| p for trend† | <0.0001 | 0.2 | ||||||
Adjusted for: age (continuous), race (white, black, other), cohort (AARP, AHS, USRT, PLCO, WHS, CPSII, IWHS, BWHS, WHI), BMI (continuous), smoking status (non-smoker, former smoker, current smoker), cigarette smoking intensity (0, ≤10, >10-≤15, >15-≤25, >25 cigarettes/day), alcohol (non-drinker, and >0-<1, 1-3, >3 drinks/day).
P-value for trend of continuous variable.
Results from a model that jointly considered caffeine content and intensity of coffee consumption were not substantially different than our main stratified model (Supplementary Table S5). When we examined suspected HCC cases, results did not differ from our main analysis of confirmed HCC (Supplementary Tables S6). Finally, the meta-influence analysis for HCC (Supplementary Figure S1), revealed that the AARP cohort exerted a substantial influence on the overall results. Thus, we also present our results for only the AARP cohort (Supplementary Tables S7-S8) and excluding the AARP cohort (Supplementary Tables S9-S10).
DISCUSSION
This study examined the association between coffee and risk of incident HCC and ICC, stratified by caffeine content and sex. In our analyses, stronger associations between coffee consumption and HCC were observed for women. Among women who drank more than three cups of coffee per day, we found risk reductions for HCC of 54%.
Previous studies have reported that coffee consumption is associated with a decreased risk of HCC. In a recent meta-analysis, high levels of coffee consumption (versus none) was associated with a 56% reduction in risk of liver cancer; with each one cup of coffee consumed per day, a 20% reduction in risk of HCC was observed (8). Three additional studies published since this meta-analysis also noted an inverse association between coffee consumption and HCC (17, 32, 33). In the current study, drinking more than three cups of coffee per day (versus non-drinkers) was associated with a 27% decreased risk of HCC and increasing coffee consumption by one cup per day was associated with a 10% reduction in HCC risk.
While not statistically significant, there was evidence of modest heterogeneity among studies. As a sensitivity analysis, we conducted a meta-influence analysis. In this analysis, the HR for coffee drinkers versus non-drinkers was notably different when the AARP study was dropped compared to the overall result (Supplementary Figure S1). The results were quite different examining the AARP cohort versus the other pooled cohorts. Individuals in the AARP cohort consuming more than three cups of coffee per day had little to no reduced risk of HCC. When the AARP cohort was excluded, individuals drinking more than three cups of coffee per day had a 59% decreased risk of HCC (Supplementary Tables S7 and S9). It is unclear why estimates from the AARP cohort appear different, as cohort recruitment was very similar between the cohorts included in the LCPP (Supplementary Table S1). There are two possibilities. First, at the time of the AARP baseline questionnaire (1995), AARP was primarily composed of retirees. Thus, coffee consumption among retirees could be different than among workers or the general population. Alternatively, this could be due to chance. However, as AARP comprises over 45% of the LCPP study population, results stratified by cohort should be interpreted with caution.
The recent meta-analysis also reported that coffee consumption was associated a reduce risk of HCC in both men and women (8). This meta-analysis included two previous studies that reported coffee consumption among men was associated with a greater risk reduction of HCC (34, 35) and three studies that did not report a difference (36-38). In the current study, we saw coffee consumption was associated with a decreased risk of HCC, which was stronger among women. However, in the analysis stratified by cohort, the association of coffee-HCC by sex was quite different by cohort. In the AARP cohort, women with higher coffee consumption had a possible 23% decreased risk of HCC, but men did not. Conversely, in the other cohorts, men and women had a 52-67% reduced risk of HCC (Supplementary Table S8 and S10). Differences between previous studies and our study could partially be due to geographic differences, variability in coffee brew or preparation methods, bioavailability of coffee compounds, or chance.
Studies have shown that coffee brew and preparation methods vary widely by geographic location, even within the U.S. (39). The amounts of various compounds retained in coffee are highly dependent on the method of brewing (40). For example, boiled, Turkish, and French press methods retain the highest amounts of cafestol and kahweol, whereas negligible amounts are found in instant, drip filtered, or percolated coffee (41). Brewing strength (i.e., the concentration of coffee grounds per liter of water used for brewing) also affects the levels of cafestol and kahweol found in various coffee brewing methods (41). Preparation of coffee with added milk or creamer could also potentially affect the bioavailability of coffee compounds. A recent study, using 10% milk or non-dairy creamer, found no differences in bioavailability in phenolic coffee compounds (42), but another study, using instant coffee dissolved in only milk, found a 28% reduction in urinary excretion of cholorogenic acid (43). This suggests that consuming milk and coffee together could affect the bioavailability of compounds found in coffee.
Coffee is a mixture of many different compounds, such as carbohydrates, lipids, vitamins, alkaloids, nitrogenous molecules, and polyphenolic compounds (44). Animal studies have also provided support for the potential chemopreventive effect of coffee. For example, a murine study found that coffee decreased the incidence of tumors, including hepatocellular adenomas, and increased energy expenditure (45). The primary compounds in coffee that have been identified as possibly having chemopreventive effects include diterpenes (i.e., cafestol and kahweol), chlorogenic acid, and caffeine (40). Diterpenes are lipids that have been shown to inhibit phase I activating enzyme expression and enzymatic activity, induce phase II detoxifying enzymes, and regulate the Nrf2/ARE signaling pathway (10, 12, 40). Chlorogenic acid is a polyphenol that has been shown to increase activity of phase II detoxifying enzymes via the Nrf2/ARE signaling pathway (46). Caffeine intake increases metabolic rate and energy expenditure, and thus caffeine may regulate weight and reduce the risk of developing metabolic syndrome (12, 47). Additionally, caffeine may contribute to the antioxidant capacity of coffee (10, 12). A recent study from the European Prospective Investigation into Cancer and Nutrition (EPIC) found a 72% lower risk of HCC associated with high levels of coffee consumption (33). Consistent with the current report, the EPIC study noted inverse, monotonic associations between coffee and HCC among caffeinated coffee consumption (ptrend=0.009) but not decaffeinated coffee (ptrend=0.5) (33). While previous studies have reported an association between tea (33, 48), but not other types of caffeinated beverages (e.g., soda), and reductions in HCC risk (11), we were unable to evaluate tea or soda consumption in the present study.
Potential risk reduction of HCC due to coffee consumption is likely due to long-term consumption rather than transient exposure (12). Studies to date, including the current report, may not adequately captured long-term coffee consumption. All of the parent studies in the LCPP assessed coffee consumption for the 12 months prior to study baseline or less, which is the exposure included in the current report. This was assumed to be indicative of adult coffee consumption. However, there are a number of reasons, particularly due to health concerns, that may lead individuals to alter their coffee consumption, specifically caffeinated coffee (49). Thus, misclassification of long term exposure status could result from having only a single, self-reported measurement at study baseline, which does not account for the within person variability over time. However, we believe that this form of misclassification would likely be nondifferential with respect to liver cancer, and we would expect any potential bias to attenuate the estimates of risk, biasing them towards the null hypothesis (29). Therefore, the null results found for the overall association between coffee drinkers, versus non-drinkers, and HCC could potentially be due to misclassification. For instance, individuals classified as non-drinkers could have formerly been heavy coffee drinkers, which could be a potential explanation as to why among individuals consuming lower levels of coffee (<2 cups/day) we observed an increased risk of HCC, compared to non-drinkers. This is primarily being driven by the NIH-AARP study, where individuals may have altered their coffee consumption after retiring from the workforce.
While the pooled analysis included information on the major confounders (e.g., smoking and alcohol consumption), it did not include information on other potential confounders, such as HBV and HCV status, for all individuals. However, hepatitis B surface antigen (HBsAg) and antibody to hepatitis C virus (anti-HCV) were assessed in a case-control study nested within this study. Among the HCC cases tested (n=151), 39 (25.8%) were positive for anti-HCV and 5 (3.3%) were positive for HBsAg. Among the matched controls (n=375), 10 (2.7%) were positive for anti-HCV and 3 (0.8%) were positive for HBsAg. We examined the association between HCV and HBV status and coffee drinking, and found no association (data not shown). Additionally, the LCPP did not obtain and harmonize information on other dietary factors (e.g., total energy intake) that may be potential confounders as well. These results may also not be generalizable to non-white or Hispanic populations, as the cohorts included in this analysis were primarily composed of white, non-Hispanic participants.
Although not statistically significant, the analyses stratified by smoking, BMI, and diabetes are intriguing. In each of these models, higher coffee consumption was associated with a lower risk of HCC in the absence of smoking, overweight/obesity, or diabetes. This suggests that if coffee is working through an antioxidant, anti-inflammatory, or radical scavenging mechanism (12), it functions primarily at lower levels of oxidative stress and inflammation. However, in the presence of smoking, diabetes, or overweight/obesity, the potential risk reduction benefits from coffee could be overwhelmed by these highly pro-oxidative and inflammatory mechanisms (50).
This study had a large sample size to evaluate the association between coffee consumption and liver cancer incidence by subtype: HCC and ICC, although the number of ICC cases in this pooled analysis remained limited. The large sample size of the LCPP, compared to previous studies, also allowed us to stratify by caffeine content of coffee and sex; however, the number of cases for the stratified analyses is still relatively small. Additionally, previous studies have primarily been conducted in Europe and Asia (8), where coffee brew and preparation methods are different than in the U.S.
In conclusion, our finding of an inverse association between coffee consumption and HCC suggests that coffee consumption may modestly reduce the risk of HCC in the U.S. Further research is needed to elucidate the role of coffee consumption, including method of brew and preparation, in relation to HCC in the U.S.
Supplementary Material
Acknowledgments
Grant Support: NIH Intramural Research Program, National Cancer Institute (JL Petrick, ND Freedman, BI Graubard, MC Alavanja, L Beane-Freeman, J Koshiol, MS Linet, M Purdue, C Schairer, AJ Sigurdson, VV Sahasrabuddhe, KA McGlynn). National Cancer Institute Grants CA39742 (JN Poynter, K Robien), CA047988 (I Lee, JE Buring), HL043851 (I Lee, JE Buring), HL080467 (I Lee, JE Buring), HL099355 (I Lee, JE Buring), DK098311 (AT Chan), CA186107 (AT Chan), CA87969 (AT Chan), and CA167552 (AT Chan).
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
REFERENCES
- 1.Ferlay JSI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] International Agency for Research on Cancer; Lyon, France: 2013. [2 June 2014]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012 doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 4.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. The American journal of gastroenterology. 2014;109:542–53. doi: 10.1038/ajg.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:1198–203. doi: 10.1158/1055-9965.EPI-05-0811. [DOI] [PubMed] [Google Scholar]
- 6.Ambade A, Mandrekar P. Oxidative stress and inflammation: essential partners in alcoholic liver disease. International journal of hepatology. 2012;2012:853175. doi: 10.1155/2012/853175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. Journal of hepatology. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1413–21. e1. doi: 10.1016/j.cgh.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 9.Saab S, Mallam D, Cox GA, 2nd, Tong MJ. Impact of coffee on liver diseases: a systematic review. Liver international : official journal of the International Association for the Study of the Liver. 2014;34:495–504. doi: 10.1111/liv.12304. [DOI] [PubMed] [Google Scholar]
- 10.Muriel P, Arauz J. Coffee and liver diseases. Fitoterapia. 2010;81:297–305. doi: 10.1016/j.fitote.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Cadden IS, Partovi N, Yoshida EM. Review article: possible beneficial effects of coffee on liver disease and function. Aliment Pharmacol Ther. 2007;26:1–8. doi: 10.1111/j.1365-2036.2007.03319.x. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig IA, Clifford MN, Lean ME, Ashihara H, Crozier A. Coffee: biochemistry and potential impact on health. Food & function. 2014 doi: 10.1039/c4fo00042k. [DOI] [PubMed] [Google Scholar]
- 13.El-Serag HB, Kanwal F. Epidemiology of Hepatocellular Carcinoma in the United States: Where Are We? Where Do We Go? Hepatology. 2014 doi: 10.1002/hep.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73. e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schliep KC, Schisterman EF, Mumford SL, Pollack AZ, Zhang C, Ye A, et al. Caffeinated beverage intake and reproductive hormones among premenopausal women in the BioCycle Study. Am J Clin Nutr. 2012;95:488–97. doi: 10.3945/ajcn.111.021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi S, Saito K, Jia H, Kato H. An integrated multi-omics study revealed metabolic alterations underlying the effects of coffee consumption. PloS one. 2014;9:e91134. doi: 10.1371/journal.pone.0091134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Setiawan VW, Wilkens LR, Lu SC, Hernandez BY, Le Marchand L, Henderson BE. Association of Coffee Intake With Reduced Incidence of Liver Cancer and Death From Chronic Liver Disease in the US Multiethnic Cohort. Gastroenterology. 2015;148:118–25. doi: 10.1053/j.gastro.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGlynn KA, Sahasrabuddhe VV, Campbell PT, Graubard BI, Chen J, Schwartz LM, et al. Reproductive factors, exogenous hormone use and risk of liver cancer among U.S. women: Results from the Liver Cancer Pooling Project. British journal of cancer. 2015 doi: 10.1038/bjc.2015.58. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. American journal of epidemiology. 2001;154:1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 20.Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, et al. The Agricultural Health Study. Environmental health perspectives. 1996;104:362–9. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boice JD, Jr., Mandel JS, Doody MM, Yoder RC, McGowan R. A health survey of radiologic technologists. Cancer. 1992;69:586–98. doi: 10.1002/1097-0142(19920115)69:2<586::aid-cncr2820690251>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Kramer BS, Gohagan J, Prorok PC, Smart C. A National Cancer Institute sponsored screening trial for prostatic, lung, colorectal, and ovarian cancers. Cancer. 1993;71:589–93. doi: 10.1002/cncr.2820710215. [DOI] [PubMed] [Google Scholar]
- 23.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women's Health Study. Journal of women's health & gender-based medicine. 2000;9:19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 24.Calle EE, Rodriguez C, Jacobs EJ, Almon ML, Chao A, McCullough ML, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94:2490–501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 25.Munger RG, Folsom AR, Kushi LH, Kaye SA, Sellers TA. Dietary assessment of older Iowa women with a food frequency questionnaire: nutrient intake, reproducibility, and comparison with 24-hour dietary recall interviews. American journal of epidemiology. 1992;136:192–200. doi: 10.1093/oxfordjournals.aje.a116485. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women's Health Study: a follow-up study for causes and preventions of illness. Journal of the American Medical Women's Association. 1995;50:56–8. [PubMed] [Google Scholar]
- 27.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the Women's Health Initiative study design. Annals of epidemiology. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 28.Kleinbaum D. Logistic regression : a self-learning text second ed. Springer; New York: 2002. [Google Scholar]
- 29.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2008. [Google Scholar]
- 30.Kleinbaum DG, Klein M. Survival analysis : a self-learning text. 3rd ed. Springer; New York: 2012. [Google Scholar]
- 31.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai GY, Weinstein SJ, Albanes D, Taylor PR, McGlynn KA, Virtamo J, et al. The association of coffee intake with liver cancer incidence and chronic liver disease mortality in male smokers. British journal of cancer. 2013;109:1344–51. doi: 10.1038/bjc.2013.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bamia C, Lagiou P, Jenab M, Trichopoulou A, Fedirko V, Aleksandrova K, et al. Coffee, tea and decaffeinated coffee in relation to hepatocellular carcinoma in a European population: Multicentre, prospective cohort study. International journal of cancer Journal international du cancer. 2014 doi: 10.1002/ijc.29214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue M, Kurahashi N, Iwasaki M, Shimazu T, Tanaka Y, Mizokami M, et al. Effect of coffee and green tea consumption on the risk of liver cancer: cohort analysis by hepatitis virus infection status. Cancer Epidemiol Biomarkers Prev. 2009;18:1746–53. doi: 10.1158/1055-9965.EPI-08-0923. [DOI] [PubMed] [Google Scholar]
- 35.Gallus S, Bertuzzi M, Tavani A, Bosetti C, Negri E, La Vecchia C, et al. Does coffee protect against hepatocellular carcinoma? British journal of cancer. 2002;87:956–9. doi: 10.1038/sj.bjc.6600582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue M, Yoshimi I, Sobue T, Tsugane S, Group JS. Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: a prospective study in Japan. Journal of the National Cancer Institute. 2005;97:293–300. doi: 10.1093/jnci/dji040. [DOI] [PubMed] [Google Scholar]
- 37.Kurozawa Y, Ogimoto I, Shibata A, Nose T, Yoshimura T, Suzuki H, et al. Coffee and risk of death from hepatocellular carcinoma in a large cohort study in Japan. British journal of cancer. 2005;93:607–10. doi: 10.1038/sj.bjc.6602737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimazu T, Tsubono Y, Kuriyama S, Ohmori K, Koizumi Y, Nishino Y, et al. Coffee consumption and the risk of primary liver cancer: pooled analysis of two prospective studies in Japan. International journal of cancer Journal international du cancer. 2005;116:150–4. doi: 10.1002/ijc.20989. [DOI] [PubMed] [Google Scholar]
- 39.Hawkins DI, Roupe D, Coney KA. The Influence of Geographic Subcultures in the United States. NA - Advances in Consumer Research. 1981;8:713–7. [Google Scholar]
- 40.Cavin C, Holzhaeuser D, Scharf G, Constable A, Huber WW, Schilter B. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2002;40:1155–63. doi: 10.1016/s0278-6915(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 41.Urgert R, Vanderweg G, Kosmeijerschuil TG, Vandebovenkamp P, Hovenier R, Katan MB. Levels of the Cholesterol-Elevating Diterpenes Cafestol and Kahweol in Various Coffee Brews. J Agric Food Chem. 1995;43:2167–72. [Google Scholar]
- 42.Renouf M, Marmet C, Guy P, Fraering AL, Moulin J, Enslen M, et al. Effect of milk and non dairy creamer on human bioavailability of coffee phenolic acids. Faseb J. 2009:23. [Google Scholar]
- 43.Duarte GS, Farah A. Effect of Simultaneous Consumption of Milk and Coffee on Chlorogenic Acids' Bioavailability in Humans (vol 59, 7925, 2011). J Agric Food Chem. 2011;59:10772. doi: 10.1021/jf201906p. [DOI] [PubMed] [Google Scholar]
- 44.Spiller MA. The chemical components of coffee. In: Spiller GA, editor. Caffeine. CRC Press; Boca Raton: 1998. [Google Scholar]
- 45.Stalder R, Bexter A, Wurzner HP, Luginbuhl H. A carcinogenicity study of instant coffee in Swiss mice. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 1990;28:829–37. doi: 10.1016/0278-6915(90)90056-s. [DOI] [PubMed] [Google Scholar]
- 46.Boettler U, Volz N, Pahlke G, Teller N, Kotyczka C, Somoza V, et al. Coffees rich in chlorogenic acid or N-methylpyridinium induce chemopreventive phase II-enzymes via the Nrf2/ARE pathway in vitro and in vivo. Mol Nutr Food Res. 2011;55:798–802. doi: 10.1002/mnfr.201100115. [DOI] [PubMed] [Google Scholar]
- 47.Heckman MA, Weil J, Gonzalez de Mejia E. Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. Journal of food science. 2010;75:R77–87. doi: 10.1111/j.1750-3841.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- 48.Fon Sing M, Yang WS, Gao S, Gao J, Xiang YB. Epidemiological studies of the association between tea drinking and primary liver cancer: a meta-analysis. Eur J Cancer Prev. 2011;20:157–65. doi: 10.1097/CEJ.0b013e3283447497. [DOI] [PubMed] [Google Scholar]
- 49.Soroko S, Chang J, Barrett-Connor E. Reasons for changing caffeinated coffee consumption: the Rancho Bernardo Study. Journal of the American College of Nutrition. 1996;15:97–101. doi: 10.1080/07315724.1996.10718571. [DOI] [PubMed] [Google Scholar]
- 50.Kamath S, Lip GY. Fibrinogen: biochemistry, epidemiology and determinants. QJM : monthly journal of the Association of Physicians. 2003;96:711–29. doi: 10.1093/qjmed/hcg129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.