Abstract
The purpose of the study was to assess the impact of an educational intervention on prostate cancer screening behavior and knowledge. Participants were 104 African American men, 45 years and older, who had not been screened for prostate cancer with a prostate-specific antigen and/or digital rectal exam within the past year. All participants received an intervention delivered by trained lay community educators using a prostate cancer educational brochure developed in collaboration with the community, with structured interviews preintervention and 3 months postintervention. The main study outcomes included prostate-specific antigen screening rates during the 3-month interval and knowledge, barriers to screenings, and decisional conflict around screening. Compared with the 46 men who did not get screened, the 58 participants who got screened were more likely to have greater than a high school education, annual household incomes ≥$25,000, and a family history of non-prostate cancer (p < .05). Average knowledge scores increased, and barriers to screening scores decreased, from preintervention to postintervention only for participants who had been screened (p < .05). The results of this study demonstrate the feasibility and efficacy of an academic institution collaborating with the African American community to develop a successful prostate cancer educational intervention, an approach that can be expanded to other cancers and other chronic diseases.
Keywords: African American, cancer prevention and screening, community-based participatory research, community health promotion, health disparities
Prostate cancer (PCa) is the second leading cause of cancer-related death for men in the United States (American Cancer Society, 2007; Jemal et al., 2008). African American men bear a disproportionate burden for PCa incidence and mortality, having the highest incidence, poorest survival, and a twofold higher mortality when compared with other racial and ethnic groups in the United States (American Cancer Society, 2007; Austin et al., 1990; Merrill & Lyon, 2000; Ries et al., 2008).
The causes for these disparities in African Americans are complex and may include an increased genetic vulnerability for PCa, unequal access to adequate treatments, delay in diagnosis, and increased susceptibility to more aggressive forms of PCa (Berger, Satagopan, Lee, Taneja, & Osman, 2006; Merrill & Brawley, 1997). Addressing these disparities in African American men is complicated by the fact that the benefit of PCa screening has been a controversial issue. For instance, there is inconclusive evidence that early detection of PCa through screening actually improves disease-specific mortality (Coley, Barry, Fleming, Fahs, & Mulley, 1997; Coley, Barry, Fleming, & Mulley, 1997; Gerald et al., 2009; Schroder et al., 2009). In addition, there is an ongoing debate whether the potential benefits of screening outweigh the potential risks associated with treatment. These risks include impotence, incontinence, and anxiety of a cancer diagnosis. Also, there is the question of whether or not life expectancy is improved as a result of screening and subsequent treatment. Some prostate cancers are very slow growing and would not threaten the life of the patient if not treated.
In light of this information, most medical professionals and cancer-related organizations have promoted informed decision making such that patients are informed about the risks and benefits of PCa screening. A 2007 review of current cancer guidelines, practices, and prospects emphasized informed decision making and shared decision making so that the potential benefits, limitations, and harm associated with testing for and treating PCa are fully discussed (Krist, Woolf, Johnson, & Kerns, 2007; Volk, Spann, Cass, & Hawley, 2003; Watson et al., 2006). Informed decision making is also the cornerstone of new 2010 recommendations regarding PCa screening from the American Cancer Society (Wolf et al., 2010).
Several factors have been associated with an increase in PCa screening, including having a positive family history of prostate and other cancers, being older, being employed, having a higher income, having an intention to getting screened, perceiving one’s health as good or excellent, and having a usual source of health care (Ford, Vernon, Havstad, Thomas, & Davis, 2006; Niven, Herman, Pweinrich, & Weinrich, 2001). In addition, family and friends, having a trusting relationship with a health care provider, and recruitment strategies that are based in the community or where African Americans work and live have been shown to play an important role in the PCa screening decision-making process (Jones, Steeves, & Williams, 2009, 2010).
Research in the area of PCa educational interventions offers promise to improve knowledge about the disease and reducing decisional conflicts with regard to the risks and benefits associated with screening. However, existing studies have revealed mixed results regarding PCa screening outcomes (Davison, Kirk, Degner, & Hassard, 1999; Frosch, Kaplan, & Felitti, 2003; Partin et al., 2004; Schapira & VanRuiswyk, 2000; Volk et al., 2003). Interventions in these areas have used a variety of formats including videotapes, Internet interventions, pamphlets, and face-to-face education. Despite this increase in educational materials for PCa, there has been little effort to develop materials specifically for African American men. Given the state of disparities in incidence, mortality, and survival rates, it is critically important to develop culturally tailored educational interventions for African American men.
The present study examines the efficacy of an educational intervention in improving informed decision making with regard to PCa screening, screening rates, and PCa knowledge in primarily low-income African American men. The development of this intervention was driven by a community-academic linkage, using community-based participatory research (CBPR) methodology. A community advisory board (CAB) consisting of community residents and representatives was used in developing the content and language of the intervention brochure.
Method
Participants were recruited face-to-face from the lobby area of Matthew Walker Comprehensive Health Center (MWCHC) and at community events such as health fairs. MWCHC is a federally funded (Health Resources and Services Administration) community health center that serves primarily low-income communities. MWCHC serves more than 18,000 patients a year and the majority of their patient population is African American, uninsured, and has an annual household income of ≤$15,000. In addition, participants were recruited through flyers posted at community businesses and community centers. These flyers provided a brief description of the study, eligibility criteria for participating, and a number to call for those interested in participating. The recruitment was conducted by lay community educators who were trained to screen for study eligibility. These eligibility criteria included being self-identified African American, being male, not having been screened for PCa in the past year with a prostate specific antigen (PSA) test or digital rectal exam (DRE), being 45 years or older, and being a resident of Davidson County/Nashville, Tennessee, for at least 6 months. The rationale for selecting men over 45 was that organizations such as the American Cancer Society recommend that men begin at age 50 to be tested annually by PSA and DRE, whereas those at increased risk, such as African Americans and men with a family history of the disease, can start at 45 (U.S. Preventive Services Task Force, 2002). The study protocol was approved by the Meharry Medical College Institutional Review Board.
A series of workshops were conducted by the study investigators to train the lay community educators in recruiting eligible participants, obtaining written informed consent, administering the assessments, and delivering the intervention. Lay community educators were recruited from the focus groups that were conducted as part of the intervention development (see section below). Three were recruited and all were African American men. The trained lay community educators obtained informed consent from participants and conducted the preintervention assessments. The assessment information collected from participants included demographic characteristics (age, race, income, education, marital status, employment status), health insurance coverage, height and weight, information about barriers to PCa screening, and PCa screening history. The intervention involved educating participants about PCa using an educational brochure, which was developed in collaboration with the target community. The study lay community educators discussed the information in the brochure with participants and used active learning techniques such as having participants paraphrase what was discussed.
Intervention Development
CBPR principles provided the guidelines for intervention development. CBPR is a research approach that mandates a partnership between traditionally trained experts and members of a community, with all parties interested in addressing a common research problem. This approach requires for the community to be a full research partner, participating in the planning, development, implementation, evaluation, and dissemination of the research. CBPR is characterized by substantial community input in the development and implementation of a research proposal. The nine principles of CBPR encompass the following: (1) acknowledge the community, (2) foster colearning and capacity building for all, (3) build on strengths and resources within the community, (4) integrate and achieve a balance of all partners, (5) facilitate collaborative, equitable partnership in all phases of the research, (6) focus on the local relevance and determinants of health, (7) involve a cyclical process, (8) disseminate findings and knowledge gained to all partners and to involve all partners in the dissemination process, and (9) involve a long-term process and commitment (Israel et al., 2003).
Three focus groups were conducted to develop a catalog of barriers to PCa screening. The first focus group consisted of self-identified African American men above the age of 40 years who had been screened for PCa in the past year. The second focus group consisted of African American men 40 years and older and who had not been screened for PCa in the past year. The rationale for these groups was to gain understanding of barriers to PCa screening from the perspective of those who had been screened versus those who had not. The final focus group consisted of family members and significant others of participants in the first two focus groups. The rationale for this focus group was to gain insight and understanding of the barriers that family members identify for PCa screening. All the focus groups consisted of 8 to 10 participants.
The focus groups were conducted at MWCHC and were moderated by a pastor. This moderator was selected because he was a member of the target community, and he had experience moderating focus groups in past community-based projects. The moderator was provided with a list of “probing questions” to facilitate the discussions. Each participant provided informed consent prior to his participation, including an agreement (or refusal) to be video- and audio-taped during the sessions. Participants were also asked to provide permission to be contacted if they were selected to be on the CAB.
The CAB, consisting of five members each from the three focus groups, developed an educational brochure that would serve as the intervention to be administered by the lay community educators. CAB members provided informed consent prior to their participation and received a light meal and a $45 cash incentive for each CAB session. All CAB sessions were moderated by an African American pastor.
Three CAB sessions were used to develop the educational brochure. In Session 1, CAB members were provided a list of barriers generated in the focus groups, and the moderator engaged in a discussion to generate solutions to these barriers. “Prostate Cancer Screening: A Decision Guide for African Americans” from the CDC website (http://www.cdc.gov) was provided as homework reading for the next session. In Session 2, CAB members generated the content of the brochure. The content included a description of the prostate, screening options, reliability of these screening tests, impact of PCa on the African American population, and solutions to various barriers to screening. In Session 3, CAB members were presented with a draft of a brochure. They focused on modifying the brochure language to make it easy to read and understand. Focus group participants and members of the CAB were excluded from the study.
Intervention: Data Collection, Coding, and Analysis
Structured interviews with the study participants were conducted by lay educators at entry into the study and 3 months postintervention. Knowledge scores for PCa were measured by the Actual Knowledge of Prostate Cancer subscale of the Prostate Knowledge Questionnaire developed by Agho and Lewis (2001). This subscale has 21 items and was designed to measure the extent to which a person can recognize general factual information about PCa. The scale contains items such as “African American men are less likely to develop this type of cancer, True or False.” The advantage of this subscale is that it was designed to be used with African American men and has a Cronbach α of .87, which indicates good reliability. Responses on this subscale were summed to provide an overall prostate cancer knowledge score. The maximum knowledge score was 21, with the higher scores representing greater PCa knowledge.
Decisional conflict regarding PCa screening was measured by an 8-item version of the Decisional Conflict Scale adapted by Taylor et al. (2006). This adapted scale has a binary response format (“No = 1,” “Yes = 0”) with lower summed scores indicating greater level of informed decision making with respect to engaging in screening for PCa. The maximum score on this scale is 8 and the minimum is 0. This scale was designed for use with African Americans and has a Cronbach α of .76, which indicates adequate reliability.
Barriers to PCa screening were summed to create a total barrier score. Participants were presented with a list of 11 barriers to screening and were instructed to indicate all the barriers that applied to them. A score of “1” was given to any barrier that was reported. Scores on these 11 barriers were summed. The maximum total barrier score was 11, with the higher scores indicating that a participant perceived a greater number of barriers to screening for PCa.
The primary outcome of interest was screening for prostate cancer with the PSA test. The rationale for selecting this test is that MWCHC, which is the primary screening provider for this study, uses only PSA as part of their PCa screening protocol. Chi-square tests and the analysis of variance were used to examine the difference between demographic and lifestyle characteristics by screening status. A multivariate analysis of covariance (MANCOVA) was used to evaluate the associations between pre- and postintervention scores in PCa knowledge, barriers to screening, and screening status. To adjust for potential confounding, key demographic variables were controlled. A multiple linear regression model was conducted to examine the predictive function of demographic and lifestyle variables for the decisional conflict scores at postintervention. All data analyses were conducted using Version 9.2 of the SAS System for Windows (SAS Institute Inc., Cary, NC) and based on two-sided probability.
Results
Demographic and Lifestyle Characteristics
The intervention, as well as pre- and postintervention interviews, were delivered to 104 African American men, 58 (56%) of whom underwent PSA screening in the interval and 46 of whom did not. Demographic and lifestyle characteristics of the study participants are presented in Table 1. Differences between the screened and unscreened groups were not large, except that participants who reported getting screened were more likely to report having had greater than a high school education compared with those that did not get screened (48% vs. 13%), have an annual household income equal to or greater than $15,000 (61% vs. 38%), and were more likely to report having a family history of any other cancer except PCa (67% vs. 41%; all p < .05).
Table 1.
Demographic and Lifestyle Characteristics of the Participants at Entry Into the Study
| Variables | Eligible Participants, N = 104 (%) |
Screened, N = 58 (%) |
Not Screened, N = 46 (%) |
p Value |
|---|---|---|---|---|
| Age at interview (years) | 51.7 ± 6.6 | 51.4 ± 6.2 | 52.2 ± 7.1 | .557 |
| Education | ||||
| Less than or equal to high school | 69 (68%) | 29 (52%) | 40 (87%) | <.001 |
| More than high school | 33 (32%) | 27 (48%) | 6 (13%) | |
| Marital status | ||||
| Married/living with a partner | 29 (29%) | 17 (31%) | 12 (27%) | .644 |
| Divorced/widowed/separated | 41 (41%) | 24 (44%) | 17 (39%) | |
| Never married | 29 (29%) | 14 (25%) | 15 (34%) | |
| Employment status | ||||
| Employed | 49 (48%) | 30 (54%) | 19 (41%) | .217 |
| Unemployed | 53 (52%) | 26 (46%) | 27 (59%) | |
| Annual household income | ||||
| <$15,000 | 50 (50%) | 22 (39%) | 28 (62%) | .022 |
| ≥$15,000 | 51 (50%) | 34 (61%) | 17 (38%) | |
| Self-rated health | ||||
| Excellent/very good/good | 73 (72%) | 38 (68%) | 35 (76%) | .359 |
| Fair/poor | 29 (28%) | 18 (32%) | 11 (24%) | |
| Health insurance | ||||
| Yes | 42 (41%) | 26 (46%) | 16 (35%) | .266 |
| No | 61 (59%) | 31 (54%) | 30 (65%) | |
| Having personal doctor or health care provider | ||||
| Yes | 61 (60%) | 35 (60%) | 26 (59%) | .898 |
| No | 41 (40%) | 23 (40%) | 18 (41%) | |
| How long since last routine checkup? | ||||
| Within past year | 27 (28%) | 19 (34%) | 8 (19%) | .103 |
| More than 1 year ago | 71 (72%) | 37 (66%) | 34 (81%) | |
| Have any of your relatives had prostate cancer? | ||||
| Yes | 22 (21%) | 14 (25%) | 8 (17%) | .377 |
| No | 81 (79%) | 43 (75%) | 38 (83%) | |
| Have any of your relatives had any other cancer, except prostate cancer? | ||||
| Yes | 57 (55%) | 38 (67%) | 19 (41%) | .010 |
| No | 46 (45%) | 19 (33%) | 27 (59%) | |
| Have you ever had a PSA test or digital rectal exam? | ||||
| Yes | 27 (26%) | 11 (19%) | 16 (35%) | .076 |
| No | 76 (74%) | 46 (81%) | 30 (65%) | |
Note. PSA test = prostate-specific antigen test.
Relationship Between Screening Status and PCa Knowledge
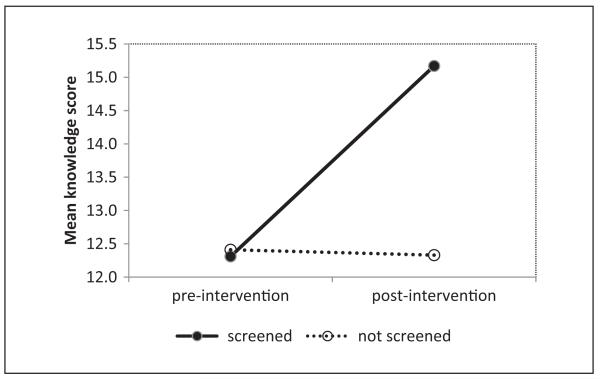
The MANCOVA conducted to investigate the relationships between prostate cancer pre- and postintervention knowledge scores and screening status revealed a significant interaction between screening status and knowledge scores (p = .04), as shown in Figure 1. The average knowledge scores increased from preintervention to postintervention for participants who had gotten screened, but not among participants who did not get screened.
Figure 1.
Interaction between screening status and time for knowledge scores
Barriers to Prostate Cancer Screening
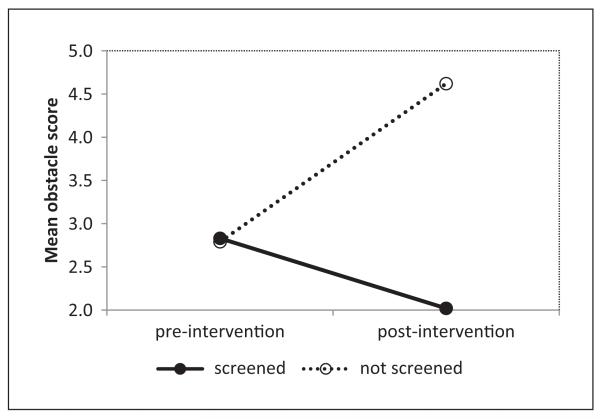
Barriers to PCa screening by screening status and time are illustrated in Table 2. Preintervention, there were no significant differences between those who subsequently got versus did not get screened in any of the nine barriers listed. Postintervention, however, the two groups differed in seven of the nine barriers. The percentages of the screened reporting barriers tended to decline from pre- to postintervention, whereas the percentages of the unscreened tended to rise. The largest barrier to PCa screening reported by participants in both groups was not having health insurance, but this percentage rose from 39% to 65% for the unscreened pre- to postintervention, while declining from 46% to 33% for the screened. In the MANCOVA conducted to investigate the relationships of total barrier scores and screening status between pre- and postintervention, there was a significant interaction between screening status and time (p = .003), which is visualized in Figure 2. The total barrier scores decreased at postintervention only for participants who had been screened, while increasing for participants who did not get screened.
Table 2.
Barriers to Prostate Cancer Screening by Screening Status at Preintervention and Postintervention
| Preintervention |
Postintervention |
|||||
|---|---|---|---|---|---|---|
| Barriers | Screened | Not Screened |
p Value | Screened | Not Screened |
p Value |
| Fear of finding out I have cancer | 25 (44%) | 12 (27%) | .073 | 10 (18%) | 8 (18%) | .967 |
| Not having health insurance | 26 (46%) | 18 (39%) | .508 | 19 (33%) | 30 (65%) | .001 |
| Cost of cancer screenings | 20 (35%) | 18 (39%) | .673 | 16 (28%) | 22 (49%) | .031 |
| Pain and discomfort of screenings | 19 (34%) | 14 (31%) | .764 | 16 (28%) | 19 (42%) | .120 |
| Difficulty getting time off work | 9 (16%) | 5 (11%) | .469 | 8 (14%) | 16 (36%) | .011 |
| Trouble remembering schedule screenings | 15 (26%) | 13 (28%) | .825 | 12 (21%) | 25 (54%) | <.001 |
| Not having enough information about screenings | 16 (28%) | 11 (24%) | .633 | 7 (12%) | 23 (50%) | <.001 |
| Not knowing where to get screened | 10 (18%) | 10 (22%) | .593 | 11 (19%) | 20 (43%) | .007 |
| Transportation issues | 11 (19%) | 13 (28%) | .285 | 9 (16%) | 19 (42%) | .003 |
Figure 2.
Interaction between screening status and time for barriers scores
Decisional Conflict for Prostate Cancer Screening
A multiple linear regression model was used to investigate the predictive function of demographic and lifestyle variables for decisional conflict for PCa screening at postintervention. There were no significant demographic or lifestyle predictors of decisional conflict. Furthermore, screening status was also a nonsignificant predictor of decisional conflict for PCa screening.
Discussion
In the 3 months following the community-derived intervention, 56% of the study participants reported getting a PSA test. This is higher than the 48% annual PCa screening rate in the State of Tennessee for 2008 (U.S. Department of Health and Human Services, n.d.). This result speaks to the effectiveness of the intervention in getting a majority of participants who had not gotten screened in the past 2 years to engage in screening. In fact, this intervention led to a higher screening rate than that found among men in the State of Tennessee.
The relationship between PCa knowledge and actual screening behavior is not well established. Studies have revealed mixed results, with some indicating that an increase in PCa knowledge is associated with an increase in PCa screening behavior, whereas others have found that screening behavior is either unaffected or actually decreases (Davison et al., 1999; Frosch et al., 2003; Partin et al., 2004; Schapira & VanRuiswyk, 2000; Volk et al., 2003). In this study, we found that only participants who had been screened by the end of the follow-up reported an increase in PCa knowledge. At present, it is unclear if these findings represent cultural differences, since a majority of the previous studies on PCa have been conducted with Caucasian populations. The intervention attempted to provide balanced information about the benefits, risks, and accuracy of current PCa screening tests. It may be that some participants recognized from this information that there is no viable alternative to current screening practices and decided to address their prostate health with the available screening options. Furthermore, the intervention contained information about barriers to screening and strategies to overcome these barriers. This information may have eased their perceptions about difficulties associated with screening. Indeed, among those who had the PSA testing done, the percentage reporting barriers declined in the postintervention period.
In this study, there were no significant demographic or lifestyle predictors of decisional conflict for PCa screening. Compared with men who did not get screened, those who screened did not report less decisional conflict about screening. Also, improving PCa knowledge apparently does not seem to influence decisional conflict. These results suggest that decisional conflict about PCa screening may be affected by other factors such as social influences (family and friends) or existing beliefs and perceptions about cancer screening such as fear of finding out about having cancer.
Level of education, income, and having been screened for PCa in the past were the only sociodemographic or lifestyle variables that we found had a significant relationship with screening status. Unlike prior research, this study did not find that being married, being older, having a family history of PCa, and having health insurance were significantly associated with being screened for PCa (Chiu, Anderson, & Corbin, 2005; Ford et al., 2006; Nivens et al., 2001; Spencer et al., 2006). However, our participants at baseline were a relatively similar with regard to not having been screened in the past year, tending to lessen differences often found between screening and nonscreening groups in cross-sectional studies.
An interesting finding of this study was the relationship between screening status and perceptions about barriers to screening. At preintervention, participants who were screened and those that did not get screened had very similar perceptions about barriers to getting screened. At postintervention, there were several social determinants of health that influenced screening behavior. For example, compared with participants who did not get screened, those who screened at postintervention were less likely to report not having health insurance, cost of cancer screenings, difficulty getting time off work, and transportation issues as barriers to screening. Perceptions about screening also played a role at postintervention. For example, compared with participants who did not get screened, those who screened at postintervention were less likely to report not having enough information about screenings and not knowing where to get screened.
The impact of social determinants of health and perceptions about screening at postintervention were reflected in the total barrier scores that decreased only for participants who had been screened. Barrier scores for participants who did not get screened actually increased at postintervention compared with their preintervention scores. These results suggest that engaging in screening activity may lead to a positive reassessment of the obstacles involved in getting screening. It is unclear why the total barrier scores increased among participants who did not get screened. One possible explanation is that this group discovered that the obstacles to screening were greater than they initially perceived. Another explanation is that participants in this group reported a greater number of barriers at postintervention as a way to justify to themselves and/or others their failure to get screened.
Strengths and Limitations
This study had some notable strengths including that the self-reported PCa screening was confirmed using the MWCHC clinic database. All the participants in this study who were screened reported using this medical facility for their screening. In addition, this study provided information about barriers to PCa screening for primarily low-income African American men, a group at high risk for PCa incidence and mortality. Limitations of this study include that the data were primarily cross-sectional in nature, hence causation cannot be inferred. In addition, a majority of the variables were based on self-report and respondents may be unwilling to reveal or may not have accurate knowledge about their health status. In addition, this study had a small and convenient sample, both of which may affect the generalizability of the results.
Conclusion
The results of this study demonstrate the feasibility and efficacy of collaborating with the target community to develop a PCa educational intervention that increased knowledge and screening rates. This methodology of using a community–academic collaboration to develop interventions can be expanded to other cancers and other chronic diseases.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:
This research was supported by Grants 110CMS030208-01, 5U01CA11461-05, and RO1 CA092447-08.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Agho AO, Lewis MA. Correlates of actual and perceived knowledge of prostate cancer among African Americans. Cancer Nursing. 2001;24:165–171. doi: 10.1097/00002820-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Austin JP, Aziz H, Potters L, Thelmo W, Chen P, Choi K, Rotman M. Diminished survival of young blacks with adenocarcinoma of the prostate. American Journal of Clinical Oncology. 1990;13:465–469. doi: 10.1097/00000421-199012000-00002. [DOI] [PubMed] [Google Scholar]
- Berger AD, Satagopan J, Lee P, Taneja SS, Osman I. Differences in clinicopathologic features of prostate cancer between black and white patients treated in the 1990s and 2000s. Urology. 2006;67:120–124. doi: 10.1016/j.urology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- American Cancer Society . Cancer facts and figures for African Americans 2007-2008. Author; Atlanta, GA: 2007. [Google Scholar]
- Chiu BC, Anderson JR, Corbin D. Predictors of prostate cancer screening among health fair participants. Public Health. 2005;119:686–693. doi: 10.1016/j.puhe.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Coley CM, Barry MJ, Fleming C, Fahs MC, Mulley AG. Early detection of prostate cancer. Part II: Estimating the risks, benefits, and costs. American College of Physicians. Annals of Internal Medicine. 1997;126:468–479. doi: 10.7326/0003-4819-126-6-199703150-00010. [DOI] [PubMed] [Google Scholar]
- Coley CM, Barry MJ, Fleming C, Mulley AG. Early detection of prostate cancer. Part I: Prior probability and effectiveness of tests. The American College of Physicians. Annals of Internal Medicine. 1997;126:394–406. doi: 10.7326/0003-4819-126-5-199703010-00010. [DOI] [PubMed] [Google Scholar]
- Davison BJ, Kirk P, Degner LF, Hassard TH. Information and patient participation in screening for prostate cancer. Patient Education and Counseling. 1999;37:255–263. doi: 10.1016/s0738-3991(98)00123-2. [DOI] [PubMed] [Google Scholar]
- Ford ME, Vernon SW, Havstad SL, Thomas SA, Davis SD. Factors influencing behavioral intention regarding prostate cancer screening among older African-American men. Journal of the National Medical Association. 2006;98:505–514. [PMC free article] [PubMed] [Google Scholar]
- Frosch DL, Kaplan RM, Felitti VJ. A randomized controlled trial comparing Internet and video to facilitate patient education for men considering the prostate specific antigen test. Journal of General Internal Medicine. 2003;18:781–787. doi: 10.1046/j.1525-1497.2003.20911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerald LCD, Grubb RL, III, Buys SS, Chia D, Church TR, Fouad MN, Berg CD. Mortality results form a randomized prostate-cancer screening trial. New England Journal of Medicine. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel BA, Schulz AJ, Parker EA, Becker AB, Allen AJ, Guzman JR. Critical issues in developing and following community based participatory research principles. In: Minkler M, Wallerstein N, editors. Community based participatory research for health. Jossey-Bass; San Francisco, CA: 2003. pp. 53–76. [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA: A Cancer Journal for Clinicians. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. 2008. [DOI] [PubMed] [Google Scholar]
- Jones RA, Steeves R, Williams I. How African American men decide whether or not to get prostate cancer screening. Cancer Nursing. 2009;32:166–172. doi: 10.1097/NCC.0b013e3181982c6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RA, Steeves R, Williams I. Family and friend interactions among African American men deciding whether to have a prostate cancer screening. Urolic Nursing. 2010;30:189–194. [PMC free article] [PubMed] [Google Scholar]
- Krist AH, Woolf SH, Johnson RE, Kerns JW. Patient education on prostate cancer screening and involvement in decision making. Annals of Family Medicine. 2007;5:112–119. doi: 10.1370/afm.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill RM, Brawley OW. Prostate cancer incidence and mortality rates among white and black men. Epidemiology. 1997;8:126–131. doi: 10.1097/00001648-199703000-00001. [DOI] [PubMed] [Google Scholar]
- Merrill RM, Lyon JL. Explaining the difference in prostate cancer mortality rates between white and black men in the United States. Urology. 2000;55:730–735. doi: 10.1016/s0090-4295(99)00564-6. [DOI] [PubMed] [Google Scholar]
- Nivens AS, Herman J, Pweinrich S, Weinrich MC. Cues to participation in prostate cancer screening: A theory for practice. Oncology Nursing Forum. 2001;28:1449–1456. [PubMed] [Google Scholar]
- Partin MR, Nelson D, Radosevich D, Nugent S, Flood AB, Dillon N, Wilt TJ. Randomized trial examining the effect of two prostate cancer screening educational interventions on patient knowledge, preferences, and behaviors. Journal of General Internal Medicine. 2004;19:835–842. doi: 10.1111/j.1525-1497.2004.30047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Edwards BK. National Cancer Institute. Bethesda, MD: 2008. SEER Cancer Statistics Review, 1975-2005. http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER website, 2007. [Google Scholar]
- Schapira MM, VanRuiswyk J. The effect of an illustrated pamphlet decision-aid on the use of prostate cancer screening tests. Journal of Family Practice. 2000;49:418–424. [PubMed] [Google Scholar]
- Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, ERSPC Investigators Screening and prostate-cancer mortality in a randomized European study. New England Journal of Medicine. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- Spencer BA, Babey SH, Etzioni DA, Ponce NA, Brown ER, Yu H, Litwin M. A population-based survey of prostate-specific antigen testing among California men at higher risk for prostate carcinoma. Cancer. 2006;106:765–774. doi: 10.1002/cncr.21673. [DOI] [PubMed] [Google Scholar]
- Taylor KL, Davis JL, 3rd, Turner RO, Johnson L, Schwartz MD, Kerner JF, Chikarlo Leak C. Educating African American men about the prostate cancer screening dilemma: A randomized intervention. Cancer Epidemiology, Biomarkers & Prevention. 2006;15:2179–2188. doi: 10.1158/1055-9965.EPI-05-0417. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Center for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Prevalence and trends data. (n.d.) http://apps.nccd.cdc.gov/brfss/display.asp?cat=PC&yr=2008&qkey=4423&state=TN.
- U.S. Preventive Services Task force Screening for prostate cancer: recommendations and rationale. Annals of Internal Medicine. 2002;137:915–916. doi: 10.7326/0003-4819-137-11-200212030-00013. [DOI] [PubMed] [Google Scholar]
- Volk RJ, Spann SJ, Cass AR, Hawley ST. Patient education for informed decision making about prostate cancer screening: a randomized controlled trial with 1-year follow-up. Annals of Family Medicine. 2003;1:22–28. doi: 10.1370/afm.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E, Hewitson P, Brett J, Bukach C, Evans R, Edwards A, Austoker J. Informed decision making and prostate specific antigen (PSA) testing for prostate cancer: A randomised controlled trial exploring the impact of a brief patient decision aid on men’s knowledge, attitudes and intention to be tested. Patient Education and Counseling. 2006;63:367–379. doi: 10.1016/j.pec.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Wolf AM, Wender RC, Etzioni RB, Thompson IM, D’Amico AV, Volk RJ, American Cancer Society Prostate Cancer Advisory Committee American Cancer Society guideline for the early detection of prostate cancer: Update 2010. CA: A Cancer Journal for Clinicians. 2010;60:70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]