Abstract
This study of vosaroxin evaluated dose-limiting toxicity (DLT), maximum-tolerated dose (MTD), pharmacokinetics (PK), clinical activity and pharmacodynamics in relapsed/refractory leukemia. Dosing was weekly (days 1, 8 and 15) or twice weekly (days 1, 4, 8 and 11). Seventy-three treated patients had a median age of 65 years, 85% had acute myeloid leukemia and 78% had refractory disease. Weekly schedule: 42 patients received 18–90 mg/m2; MTD was 72 mg/m2. Twice-weekly schedule: 31 patients received 9–50 mg/m2; MTD was 40 mg/m2. DLT was stomatitis; primary non-hematologic toxicity was reversible gastrointestinal symptoms and febrile neutropenia. Thirty-day all-cause mortality was 11%. Five patients had complete or incomplete remissions; median duration was 3.1 months. A morphologic leukemia-free state (bone marrow blast reduction to <5%) occurred in 11 additional patients. Antileukemic activity was associated with total dose or weekly time above 1 μmol/l plasma vosaroxin concentration (P<0.05). Vosaroxin exposure was dose proportional over 9–90 mg/m2. The average terminal half-life was ~25 h and clearance was non-renal. No induction or inhibition of vosaroxin metabolism was evident. Vosaroxin-induced DNA damage was detected as increased intracellular γH2AX. Vosaroxin had an acceptable safety profile, linear PK and encouraging clinical activity in relapsed/refractory leukemia.
Keywords: vosaroxin, quinolone derivative, relapsed/refractory, acute leukemia, phase 1, voreloxin
Introduction
Treatment outcomes for patients with relapsed or refractory acute leukemia, including acute myeloid leukemia (AML), are largely unsatisfactory despite the variety of available chemotherapeutic agents.1,2 Potential mechanisms of treatment resistance include overexpression of multidrug-resistance proteins,3,4 upregulation of antiapoptotic pathways such as bcl-25,6 and leukemic–stromal interactions within the bone microenvironment.7,8 Among the many new agents being developed with the intention to circumvent resistance mechanisms in AML are cell-cycle inhibitors, signal transduction inhibitors and other novel chemotherapeutic compounds.9–12
Vosaroxin (formerly voreloxin; SNS-595) is a novel, first-in-class anticancer quinolone derivative that induces site-selective DNA damage by intercalating DNA and inhibiting topoisomerase II, leading to apoptosis.13 In non-clinical studies, vosaroxin displayed potent activity in a broad array of mouse models, including human xenografts of diverse tissue origin, syngeneic and drug-resistant tumors,14 and a normal mouse model of marrow ablation.15 Vosaroxin's activity profile in these models was superior to conventional anticancer agents such as cisplatin, cytarabine, etoposide, doxorubicin and paclitaxel.14 At its maximum-tolerated dose (MTD) in a normal mouse model, vosaroxin ablated bone marrow more potently than cytarabine.15 In non-clinical toxicology studies, the primary adverse effects were myelosuppression and gastrointestinal toxicity. Vosaroxin is not a substrate for P-glycoprotein and its activity is independent of p53, thus it may have utility in bypassing important mechanisms of chemotherapy resistance.14,16
The cytotoxicity of cell-cycle-dependent agents such as vosaroxin is affected by the proliferation status of the malignant cell population. Based on vosaroxin's cell-cycle dependency, demonstrated in both in vitro and in vivo mouse models,13,14 we investigated two schedules of vosaroxin: traditional timed sequential therapy dosing (days 1, 8 and 15) and more frequent dosing (days 1, 4, 8 and 11) to provide more constant drug exposure and possibly less cell-cycle-based non-hematologic toxicity. This clinical phase Ib dose-escalation study in relapsed or refractory leukemia was designed to characterize the safety and pharmacokinetic (PK) profiles of vosaroxin, as well as its antileukemic and pharmacodynamic (PD) properties.
Materials and methods
Enrollment and eligibility
Patients were enrolled and treated at the H. Lee Moffitt Cancer Center & Research Institute (Tampa, FL, USA), MD Anderson Cancer Center (Houston, TX, USA), Indiana University Simon Cancer Center (Indianapolis, IN, USA) and Sidney Kimmel Cancer Center at Johns Hopkins University (Baltimore, MD, USA). The study protocol was approved by the institutional review board of each center, and patients provided informed consent in accordance with the principles of the Declaration of Helsinki. The clinical trial was registered at http://www.clinicaltrials.gov as NCT00246662.
Eligible patients were at least 18 years of age with a diagnosis of advanced relapsed or refractory leukemia not expected to respond to standard therapy. Leukemias could include WHO-classified AML, de novo or poor-risk myelodysplastic syndrome with an International Prognosis Scoring System score of at least 1.5, acute lymphoblastic leukemia or chronic myeloid leukemia in blast crisis. Entry criteria also included Eastern Cooperative Oncology Group performance status of ≤2, fewer than four previous induction/reinduction chemotherapy regimens, serum creatinine and total bilirubin ≤2 mg/dl, and alanine aminotransferase and aspartate aminotransferase ≤2.5 times the upper limit of normal.
Study design and treatment schema
This was a phase Ib, open-label, dose-escalation study. Patients were sequentially assigned to one of two vosaroxin dosing regimens (schedules) in groups of 3–6, beginning at 18 mg/m2 once weekly (days 1, 8 and 15) for 3 weeks (weekly schedule) or 9 mg/m2 twice weekly (days 1, 4, 8 and 11) for 2 weeks (twice-weekly schedule) in a 28-day cycle or until peripheral blood counts recovered. Dose escalation was independent for each schedule as shown in Table 1. The dose was increased for sequential groups until a protocol-defined dose-limiting toxicity (DLT) occurred in at least one patient, then the group was expanded to six patients. If a patient did not receive all cycle 1 doses or was not assessable for DLT, the patient was considered unevaluable and was replaced. Additional patients could be added at the discretion of the clinical team to better characterize possible toxicity. If no DLT occurred, dose escalation followed a modified Fibonacci sequence. The MTD was defined as the dose level below that at which two or more of six patients experienced a DLT. Toxicity was graded using the National Cancer Institute Common Terminology Criteria version 3.0.17
Table 1.
Treatment schema, dose escalation and dose-limiting toxicity
| Schedule dose level | No. of treated patients | No. of evaluable patients | Type of DLT | No. of patients who had DLT |
|---|---|---|---|---|
| Weekly (days 1, 8 and 15) for 3 weeks | ||||
| 18 mg/m2 | 3 | 3 | None | |
| 27 mg/m2 | 6 | 3 | None | |
| 38 mg/m2 | 3 | 3 | None | |
| 50 mg/m2 | 8 | 6 | Grade 4 thrombocytopenia ongoing >8 weeks in the absence of leukemia | 1 |
| 60 mg/m2 | 4 | 4 | None | |
| 72 mg/m2 a | 12 | 12 | Bowel obstruction | 1 |
| Grade 3 stomatitis | 1 | |||
| 90 mg/m2 | 6 | 5 | Grade 3 stomatitis | 4 |
| Twice weekly (days 1, 4, 8 and 11) for 2 weeks | ||||
| 9 mg/m2 | 4 | 3 | None | |
| 13.5 mg/m2 | 4 | 3 | None | |
| 19 mg/m2 | 3 | 3 | None | |
| 25 mg/m2 | 3 | 3 | None | |
| 30 mg/m2 | 3 | 3 | None | |
| 40 mg/m2 a | 11 | 10 | Grade 3 stomatitis | 3 |
| 50 mg/m2 | 3 | 3 | Grade 3 or 4 stomatitis | 3 |
Abbreviation: DLT, dose-limiting toxicity.
Maximum-tolerated dose.
DLT was assessed in cycle 1 from the time of first treatment through day 29. A DLT was defined as clinically significant, treatment-related, grade 4 neutropenia or thrombocytopenia that persisted >8 weeks in the absence of leukemia, or any grade 3 or greater non-hematologic adverse event, regardless of duration (excluding nausea, vomiting or diarrhea controlled with antiemetic/antidiarrheal therapy; alopecia; infection/febrile neutropenia controlled by antibiotics; and grade 3 aspartate aminotransferase or alanine aminotransferase lasting <7 days).
Patients could complete up to four cycles of treatment; two additional cycles could be completed if persistent or recurrent leukemia was absent and toxicity was acceptable. Any cycle could be delayed to allow recovery from toxicity and one vosaroxin dose reduction (25%) was permitted. Supportive care, including antiemetics, myeloid growth factors and transfusions of packed red blood cells and platelets, was permitted when appropriate.
Response evaluation
A bone marrow aspirate or biopsy and peripheral blood sample were obtained at the end of each cycle (~day 28) to assess blast reduction/clearance and myelosuppression. Response categories were based on International Working Group response criteria.18 Morphologic leukemia-free state required bone marrow blasts to be <5%. For complete remission (CR), bone marrow blasts had to be <5% with no leukemic blasts in peripheral blood and no evidence of extramedullary disease, neutrophils >1×109/l and platelets ≥100×109/l. If recovery of platelets was incomplete, the response was CRp (CR with incomplete platelet recovery).
PK and correlative PD studies
The schedule of patient blood and urine collection and the analytical method for the determination of plasma and urine vosaroxin concentrations are described in the Supplementary Materials and Methods.
For correlative PD studies, peripheral blood blasts were obtained from 10 patients treated in the weekly schedule on days 1 and 8 (predose and 2 h postdose) at Johns Hopkins Sidney Kimmel Cancer Center. Samples were stained with antibody to phosphorylated H2AX (γH2AX)19,20 and examined by flow cytometry. In brief, Ficoll-enriched blasts were fixed in 70% ethanol and stored at 4 °C until staining in TST buffer (TBS, 4% FBS, 0.1% Triton X-100). Cells were incubated in either diluted (1:500) antiphospho-Ser139-histone H2AX (Upstate Cell Signaling Solutions, Lake Placid, NY, USA) or diluted (1:500) mouse IgG1 isotype control (Southern Biotech, Birmingham, AL, USA). After 2 h incubation, cells were exposed for 1 h to diluted (1:200) Alexa Fluor 488 goat antimouse IgG (H+L) (Molecular Probes, Eugene, OR, USA) with 5 μg/ml RNase, fixed in 2% formaldehyde in TBS and stored at 4 °C until flow cytometric analysis. Cellular debris was gated out and overlays of the experimental and isotype-control histograms were created. To determine the percentage of blasts exhibiting DNA damage, areas of overlap were attributed to nonspecific staining and subtracted from the experimental histograms. In each overlay, the percentage of blasts positive for γH2AX (%γH2AX) was calculated as the number of cells stained specifically for γH2AX divided by the total blast cell number. Each analysis was performed on a standard sampling of 106 cells. The s.d. for this method (triplicate sampling) is ≤0.3 fold.21
Results
Patient demographics
Seventy-five patients enrolled in the study, including 44 in the weekly schedule (42 treated) and 31 in the twice-weekly schedule. Two enrolled patients were not treated because one did not meet entry criteria and one experienced pulmonary emboli before treatment. Characteristics of the 73 treated patients are shown in Table 2. Patients were predominantly white (73%), male (62%) and had a median age of 65 years. In all, 85% of patients had AML; most were extensively pretreated (median 4 previous chemotherapy regimens), and 78% had refractory disease.
Table 2.
Characteristics of treated patients
| Characteristic | Vosaroxin schedule |
Total (n = 73) |
||||
|---|---|---|---|---|---|---|
| Weekly (n = 42) |
Twice weekly (n = 31) |
No. | % | |||
| No. | % | No. | % | |||
| Age, years | ||||||
| Median | 65 | 65 | 65 | |||
| Range | 21–81 | 26–85 | 21–85 | |||
| Sex | ||||||
| Male | 27 | 64 | 18 | 58 | 45 | 62 |
| Female | 15 | 36 | 13 | 42 | 28 | 38 |
| Race or ethnicity | ||||||
| Asian | 1 | 2 | 1 | 3 | 2 | 3 |
| Black or African American | 6 | 14 | 3 | 10 | 9 | 12 |
| Hispanic or Latino | 4 | 10 | 5 | 16 | 9 | 12 |
| White | 31 | 74 | 22 | 71 | 53 | 73 |
| ECOG performance status | ||||||
| 0–1 | 38 | 90 | 30 | 97 | 68 | 93 |
| 2 | 4 | 10 | 1 | 3 | 5 | 7 |
| Diagnosis | ||||||
| AML | 35 | 83 | 27 | 87 | 62 | 85 |
| ALL | 4 | 10 | 4 | 13 | 8 | 11 |
| MDS transformed to AML | 3 | 7 | 0 | 0 | 3 | 4 |
| Disease status | ||||||
| Relapsed | 7 | 17 | 7 | 23 | 14 | 19 |
| Refractorya | 34 | 81 | 23 | 74 | 57 | 78 |
| Others | 1 | 2 | 1 | 3 | 2 | 3 |
| No. of prior chemotherapy regimens | ||||||
| 0–3 | 24 | 57 | 15 | 48 | 39 | 53 |
| 4–9 | 18 | 43 | 16 | 52 | 34 | 47 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ECOG, Eastern Cooperative Oncology Group; MDS, myelodysplastic syndrome.
Defined as not achieving complete remission after most recent prior leukemia therapy.
Safety profile
Seventy-three patients received at least one dose of vosaroxin. Dose escalation and DLT are shown in Table 1. The MTD was determined to be 72 mg/m2 for the weekly schedule and 40 mg/m2 for the twice-weekly schedule. Grade 3 stomatitis was the most common DLT, affecting five patients in the weekly schedule and six patients in the twice-weekly schedule. In cycle 1, 27% of patients overall experienced stomatitis of any grade (12% grade 1–2; 15% grade ≥3) with a median onset of 14 days (range, 3–20 days) and a median duration of 15 days (range, 5–39 days). The incidence and severity of stomatitis was associated with the total vosaroxin dose in cycle 1 and the weekly time above a plasma vosaroxin concentration of 1 μmol/l (P<0.05), independent of schedule. The stomatitis and other gastrointestinal symptoms were generally manageable and reversible with standard supportive care. Other frequent non-hematologic toxicity (>30% of patients) that was not dose-limiting included general infection, nausea, febrile neutropenia, diarrhea, fatigue, anorexia, cough, peripheral edema, vomiting and dyspnea. Non-hematologic toxicity occurring across all grades in at least 20% of patients overall is shown in Table 3.
Table 3.
Nonhematologic toxicity occurring across all grades in at least 20% of patients overall (N = 73)
| System adverse event | Vosaroxin weekly (n = 42) |
Vosaroxin twice weekly (n = 31) |
Total (n = 73) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 or 2 |
Grade ≥3 |
Grade 1 or 2 |
Grade ≥3 |
All |
||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Dermatologic | ||||||||||
| Petechiae | 8 | 19 | 0 | 0 | 6 | 19 | 1 | 3 | 15 | 21 |
| Gastrointestinal | ||||||||||
| Nausea | 24 | 57 | 0 | 0 | 21 | 68 | 1 | 3 | 46 | 63 |
| Diarrhea | 22 | 52 | 1 | 2 | 12 | 39 | 1 | 3 | 36 | 49 |
| Stomatitisa | 4 | 10 | 5 | 12 | 6 | 19 | 6 | 19 | 21 | 29 |
| Abdominal pain | 11 | 26 | 1 | 2 | 3 | 10 | 1 | 3 | 16 | 22 |
| General | ||||||||||
| Fatigue | 19 | 45 | 1 | 2 | 14 | 45 | 0 | 0 | 34 | 47 |
| Peripheral edema | 15 | 36 | 1 | 2 | 9 | 29 | 0 | 0 | 25 | 34 |
| Pyrexia | 10 | 24 | 1 | 2 | 6 | 19 | 4 | 13 | 21 | 29 |
| Infection b | 5 | 12 | 22 | 52 | 10 | 32 | 11 | 36 | 48 | 66 |
| Febrile neutropenia | 0 | 0 | 28 | 67 | 0 | 0 | 15 | 48 | 43 | 59 |
| Metabolic | ||||||||||
| Anorexia | 17 | 41 | 1 | 2 | 12 | 39 | 0 | 0 | 30 | 41 |
| Pulmonary | ||||||||||
| Dyspnea | 13 | 31 | 0 | 0 | 10 | 32 | 1 | 3 | 24 | 33 |
| Pleural effusion | 9 | 21 | 1 | 2 | 5 | 16 | 1 | 3 | 16 | 22 |
Note: Toxicity was graded using the National Cancer Institute Common Terminology Criteria version 3. Patients experiencing multiple episodes of the same event were counted once at the highest event grade.
Dose-limiting toxicity.
Comprises 20 individual adverse event terms representing various types of general infection, sepsis, bacteremia and pneumonia.
Myelosuppression and its associated complications such as infections were expected and observed in this study of patients with advanced leukemia. Hematologic DLT (prolonged platelet recovery) occurred in one patient at 50 mg/m2 in the weekly schedule. For the two patients in the weekly schedule who had a CR, the times to recovery of peripheral absolute neutrophil counts to >1×109/l were 37 and 50 days, and the times to recovery of platelets to ≥100×109/l were 28 and 49 days, respectively. Only one patient in the twice-weekly schedule had a CR (time to recovery was 37 days for both absolute neutrophil counts and platelets), and given the small number of patients, comparison between schedules is not possible. Myelosuppression was managed with growth factor support and blood transfusion according to institutional standard of care.
Non-hematologic toxicity based on laboratory data was minimal following vosaroxin treatment at doses up to 90 mg/m2 weekly for 3 weeks and 50 mg/m2 twice weekly for 2 weeks. No grade 3 or greater treatment-related hepatic or renal dysfunction was reported. No clinically significant trend in non-hematologic toxicity was noted over time. No clinical manifestation of QTc prolongation, such as torsades de pointes, was observed.
Eight deaths occurred within 30 days (range, 11–30 days) after the first dose of vosaroxin, including six in the weekly schedule and two in the twice-weekly schedule, for a 30-day all-cause mortality rate of 11%. The primary cause of death for five patients (7%) was infection (four patients) and intestinal obstruction (one patient).
Pharmacokinetics
Blood samples were obtained for PK evaluation from 72 patients who received at least one dose of vosaroxin. Following a short intravenous administration (~10 min), plasma vosaroxin concentrations declined in a biphasic manner (Supplementary Figure 1), consisting of an initial rapid decline followed by a prolonged terminal phase with a half-life of ~25 h (Table 4). The average total body clearance was 4 l/h (2 l/h/m2) and apparent volume of distribution at steady state (Vss) was 120 l, exceeding the volume of total body water.
Table 4.
Pharmacokinetic parameters by dose group in cycle 1 (N = 72)
| Schedule day | Dose (mg/m2) | N | AUCinf (h ng/ml) |
CL (l/h) |
T1/2 (h) |
MRT (h) |
Vss (l) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | |||
| Weekly | ||||||||||||
| Day 1 | 18–90 | 41 | 31 245 | 65 | 3.92 | 34 | 25.13 | 25 | 32.17 | 29 | 120 | 30 |
| Day 1 | 72a | 11 | 39 450 | 64 | 4.16 | 37 | 26.60 | 23 | 33.89 | 30 | 130 | 27 |
| Twice weekly | ||||||||||||
| Day 1 | 9–50 | 31 | 14 212 | 62 | 4.37 | 30 | 26.58 | 35 | 33.91 | 38 | 142 | 38 |
| Day 4 | 9–50 | 30 | 17 067 | 70 | 4.29 | 32 | 28.21 | 32 | 34.43 | 32 | 145 | 39 |
| Day 8 | 9–50 | 29 | 15 637 | 68 | 4.54 | 32 | 22.30 | 27 | 28.61 | 29 | 126 | 38 |
| Day 11 | 9–50 | 28 | 15 983 | 75 | 4.52 | 31 | 23.74 | 32 | 30.79 | 36 | 136 | 45 |
Abbreviations: AUC, area under the concentration versus time curve; AUCinf, AUC from time zero to infinity; AUC72h, AUC from time zero to 72 h postdose; CL, total body clearance estimated from dose/AUCinf; Cmax 5min, observed peak or maximum concentration at the end of infusion; MRTinf, mean residence time when the drug concentration versus time profile is extrapolated to infinity; t1/2, terminal half-life; Vss, apparent volume of distribution at steady state.
AUC72h = 32547h ng/ml (CV 51%); Cmax 5min = 1916ng/ml (CV 24.8%) (accumulation ratio, AUC72d4/AUC72d1 and AUC72d11/AUC72d1).
Plasma vosaroxin exposure (AUC) increased proportionally with an increase in dose over a dose range of 9–90 mg/m2. No significant differences in clearance were noted based on age (overall and <65 years versus ≥65 years), sex, body weight or body surface area. After repeated doses (twice-weekly schedule), drug accumulation was 1.2 for day 4/day 1 (P<0.05, n=29) and day 11/day 1 (P<0.05, n=28). Vosaroxin clearance was comparable in patients receiving vosaroxin weekly or on days 1, 4, 8 and 11, indicating no induction or inhibition of vosaroxin metabolism after repeated doses.
Renal excretion of vosaroxin and its metabolites, N-desmethyl-vosaroxin and O-desmethyl-vosaroxin, was determined following the first dose of vosaroxin in eight patients in the weekly schedule at 18 mg/m2 and 27 mg/m2. N-desmethyl-vosaroxin was the only metabolite detected in urine and the ratio of N-desmethyl-vosaroxin to vosaroxin excretion was 0.43 (coefficient of variation 40%). The average renal excretion of the total dose infused was 2.35% (coefficient of variation 79%) for vosaroxin and 0.99% (coefficient of variation 84%) for N-desmethyl-vosaroxin, suggesting non-renal clearance.
Plasma vosaroxin concentrations were sustained for a long period of time above 0.1 μmol/l (40.1 ng/ml), the half-maximal (50%) inhibitory concentration (IC50), and 1 μmol/l, the IC90 in vitro in the MV4-11 AML cell line,15 at vosaroxin doses of 72 mg/m2 weekly or 40 mg/m2 twice weekly. Mean plasma vosaroxin concentrations were maintained at or above the IC50 and IC90 for 117 and 34 h per week, respectively, in the weekly schedule, and for 158 and 22 h per week, respectively, in the twice-weekly schedule.
Response
A morphologic leukemia-free state (bone marrow blasts reduced to <5%) was achieved in 16 patients (22%) at a median of 32.5 days (range, 21–51 days) after the first dose of vosaroxin, including in 12 patients treated once weekly with ≥50mg/m2 and 4 treated twice weekly with ≥30mg/m2. Five of these patients had responses of CR or CRp; all but one response occurred with weekly treatment, and responses were maintained for 1.7–19.2 months (median 3.1 months). Two patients had an unfavorable karyotype (Southwest Oncology Group classification)22 and one (72 mg/m2) had previous autologous stem cell transplantation. Characteristics of the five responding patients are shown in Table 5.
Table 5.
Characteristics of patients with a disease response of CR or CRp
| Schedule dose group |
Age (years) |
Disease status |
Cytogenetic risk categorya |
Cytogenetics at baseline |
Cytogenetics at response | IWG response |
Duration of response (months) |
|---|---|---|---|---|---|---|---|
| Weekly | |||||||
| 50 mg/m2 | 66 | First relapse | Intermediate | 46,XY | 46,XY | CRp | 2.4 |
| 72 mg/m2 | 41 | Refractory | Unfavorable | 47,XY, 9qht, t(11;19)(q23;p13.1),+21 | Not done | CRp | 3.1 |
| 72 mg/m2 | 40 | Refractory | Not done | Not done | Not done | CR | 1.7 |
| 90 mg/m2 | 71 | Primary refractory | Unfavorable | 46,XX, der(7)t;(5;7) (p13,p11.2) | 46,XX | CR (CRc) | 9.1 |
| Twice weekly | |||||||
| 40 mg/m2 | 73 | Refractory | Intermediate | 46,XX | 46,XX | CR | 19.2 |
Abbreviations: CR, complete response; CRc, cytogenetic complete response; CRp, complete response with incomplete recovery of platelets (<100 × 109/l); IWG, International Working Group.
Southwest Oncology Group classification.
Bone marrow blast reduction to <5% after vosaroxin treatment was associated with the total dose (cycle 1) or weekly time above a plasma vosaroxin concentration of 1 μmol/l (P<0.05). Most patients with an antileukemic response (10 of 16) maintained these concentrations longer than 20 h, including two patients who had a response of CR and one who had a CRp. The other two patients who had responses of CR or CRp maintained vosaroxin plasma concentrations >1 μmol/l for 10 and 16 h.
Pharmacodynamics
Vosaroxin is a replication-dependent DNA damaging agent13 that induces DNA double-strand breaks. Among the cellular responses to double-strand breaks is phosphorylation of histone H2AX on Ser139, producing γH2AX.23 Therefore, the presence and magnitude of γH2AX is an indication of persistent, unrepaired DNA damage.19 γH2AX increased in 7 of 10 patients after treatment with the first dose of vosaroxin at 50 mg/m2 or higher (Figure 1). Before treatment on day 8, γH2AX levels remained higher than at baseline in 9 of 10 patients, suggesting persistent DNA damage long after the initial dose was administered. The increase in intracellular γH2AX at 2 h after vosaroxin administration appeared to be greater when antileukemic activity was evident. On day 1, the increase in γH2AX was 2.6-fold for patients who had bone marrow blasts <5% after treatment versus 1.8-fold for patients whose blasts were ≥5%. On day 8, the increase in γH2AX was 3.8-fold for patients who had bone marrow blasts <5% after treatment versus 1.7-fold for those with blasts ≥5%.
Figure 1.
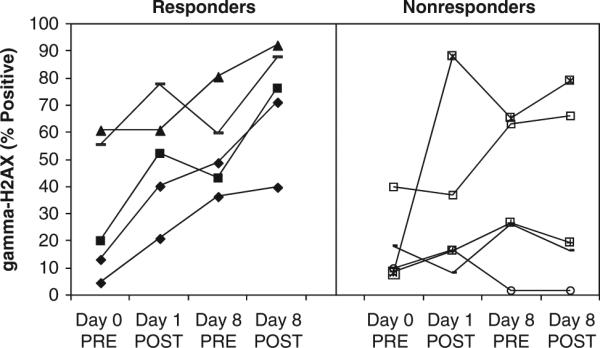
Change in γH2AX in patient peripheral blood blasts at 2 h following vosaroxin treatment in the weekly schedule on days 1 and 8 (50 mg/m2, n=1; 60 mg/m2, n=2; 72 mg/m2, n=5; 90 mg/m2, n=2) at one study center. Responders (filled symbols: 50 mg/m2, n=1; 60 mg/m2, n=1; 72 mg/m2, n=1; 90 mg/m2, n=2) had bone marrow blasts <5% after treatment and non-responders (open symbols: 60 mg/m2, n=1; 72 mg/m2, n=4) had bone marrow blasts ≥5% after treatment.
Discussion
We tested vosaroxin, a novel anticancer quinolone derivative, in patients with relapsed or refractory leukemia using two treatment schedules based on potential effects on drug-induced changes in leukemic cell growth kinetics. Dose-dependent activity was seen in both schedules with similar safety profiles, except the incidence of febrile neutropenia and infections appeared to be higher in the weekly schedule, possibly due to the longer dosing interval (15 days versus 11 days for the twice-weekly schedule) that could prolong myelosuppression. Given the heterogeneity of the patient population, the limitations of a small sample size, and the nonrandomized phase I study design, it is not possible to identify a preferred schedule between the two studied. Both weekly and twice-weekly schedules warrant further investigation to optimize dose regimens of vosaroxin for the treatment of leukemias.
In patients with solid tumors treated with vosaroxin at lower doses (up to 75 mg/m2/28-day cycle), the primary DLT was myelosuppression.24 In patients with advanced leukemia treated with vosaroxin at higher doses (up to 270 mg/m2/cycle in the current study), the DLT was stomatitis. The 30-day all-cause mortality rate of 11% was within the lower expected range for cytotoxic therapy in this patient population.25
Vosaroxin exhibited linear PK over a dose range of 9–90 mg/m2 after a single dose and after repeated doses. The lack of sex and age differences in PK characteristics is particularly relevant because AML primarily affects older individuals (predominantly males). Consistent with non-clinical in vitro studies26 and a previous clinical study in patients with advanced solid tumors,24 there was no evidence of induction or inhibition of vosaroxin metabolism in patients with leukemia. Given vosaroxin's minimal metabolism and non-renal clearance, the potential for drug–drug interaction with vosaroxin is low. Thus, vosaroxin PK is unlikely to be altered in patients with renal impairment or non-obstructive hepatic impairment.
Vosaroxin-induced DNA damage, as measured by an increase in intracellular γH2AX levels following vosaroxin treatment, was evident within hours of vosaroxin administration. Interestingly, persistent DNA damage was demonstrated at 1 week after drug administration compared with baseline. Vosaroxin-induced DNA damage at 2 h after drug administration was of greater magnitude in patients whose bone marrow blasts were reduced to <5% after treatment compared with non-responding patients. Although the limited number of samples tested precludes a definitive conclusion, this finding provides a rationale for evaluating intracellular γH2AX levels as a potential biomarker for clinical response in future vosaroxin studies.
Sixteen patients (22%) had bone marrow blast clearance following vosaroxin administration, beginning at 50 mg/m2 weekly and 30 mg/m2 twice weekly. Within this group, vosaroxin achieved remission in five patients, an encouraging finding for patients who received multiple previous chemotherapy regimens and were refractory to the most recent treatment course. Clinical activity was apparent below the MTD in the weekly schedule whereas remission was not achieved until the MTD was reached in the twice-weekly schedule. Antileukemic activity was associated with total dose and with the time above 1 μmol/l vosaroxin concentration in plasma, suggesting that the dose and the length of time above a certain concentration threshold may be important for attaining optimal antileukemic activity. Such clinical activity suggests a potentially unique and potent effect against leukemic cells with acquired resistance to other classes of chemotherapeutic agents.
In patients with AML, anthracyclines are traditionally administered on consecutive days for 3 days or for 2 days in combination with continuous intravenous infusion of cytarabine for 5–7 days. Idarubicin and daunorubicin are both rapidly metabolized to the less active idarubicinol and daunorubicinol, respectively, and are extensively distributed out of plasma to tissues and blood cells. Active concentrations above IC50 are maintained intracellularly for a prolonged period (more than 7 days), and intracellular exposure to daunorubicin was shown to be a factor in response.27–29 In contrast, vosaroxin has much lower clearance, smaller volume of distribution and exposure well above antileukemic levels in vitro during the long elimination phase. Vosaroxin concentrations in blood were approximated in peripheral blood cells and concentrated ~30-fold in bone marrow in rat tissue distribution studies, suggesting that high concentrations are maintained in the relevant compartments. The mechanistic differences resulting in part from vosaroxin's selective interaction with DNA and less reactive chemical core13 and its more limited distribution to normal tissues suggest a lower potential for the off-target organ toxicities common to the anthracyclines.
In summary, vosaroxin was well tolerated with intriguing evidence of clinical activity in patients with advanced leukemia. Vosaroxin is currently being studied in a phase 3 trial in combination with cytarabine in patients with first relapsed or refractory AML (VALOR).
Supplementary Material
Acknowledgements
The authors thank John Feaster for statistical analyses, Kristi Mahadocon and David Arnold for statistical programming and acknowledge the contributions of the Sunesis project team: Dan Adelman, Jeff Silverman, Ute Hoch, Michelle Arkin, Matt Suster and Peggy Kegley. This work was supported by research funding from Sunesis Pharmaceuticals, Inc.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Conflict of interest
TC, RSA, JAF and GCM are employees of Sunesis Pharmaceuticals, Inc., and own company stock. FR, HMK, FJG, AFL and JEK were advisors or paid consultants during study conduct. FR, HMK and AFL received honoraria and FR, FJG and JEK received research funding from Sunesis Pharmaceuticals, Inc. RMR and LDC declare no potential conflict of interest.
References
- 1.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–1163. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 2.Litzow MR, Othus M, Cripe LD, Gore SD, Lazarus HM, Lee SJ, et al. Failure of three novel regimens to improve outcome for patients with relapsed or refractory acute myeloid leukaemia: a report from the Eastern Cooperative Oncology Group. Brit J Haematol. 2010;148:217–225. doi: 10.1111/j.1365-2141.2009.07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy: a Southwest Oncology Group study. Blood. 1997;89:3323–3329. [PubMed] [Google Scholar]
- 4.Leith CP, Kopecky KJ, Chen IM, Eijdems L, Slovak ML, McConnell TS, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: a Southwest Oncology Group study. Blood. 1999;94:1086–1099. [PubMed] [Google Scholar]
- 5.Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M, et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108:2358–2365. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Min YH, Eom JI, Cheong JW, Maeng HO, Kim JY, Jeung HK, et al. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: its significance as a prognostic variable. Leukemia. 2003;17:995–997. doi: 10.1038/sj.leu.2402874. [DOI] [PubMed] [Google Scholar]
- 7.Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113:6215–6224. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9:1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 9.Fathi AT, Karp JE. New agents in acute myeloid leukemia: beyond cytarabine and anthracyclines. Curr Oncol Rep. 2009;11:346–352. doi: 10.1007/s11912-009-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giles F, Vey N, DeAngelo D, Seiter K, Stock W, Stuart R, et al. Phase III randomized, placebo-controlled, double-blind study of high dose continuous infusion cytarabine alone or with laromustine (VNP40101M) in patients with acute myeloid leukemia in first relapse. Blood. 2009;114:4027–4033. doi: 10.1182/blood-2009-06-229351. [DOI] [PubMed] [Google Scholar]
- 11.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–137. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 12.Shah BD, Lancet JE. Frontline treatment of acute myeloid leukemia: are we making progress? In: Govindan R, editor. American Society of Clinical Oncology Education Book. American Society of Clinical Oncology; Alexandria, VA: 2009. pp. 372–377. [Google Scholar]
- 13.Hawtin RE, Stockett DE, Byl JA, McDowell RS, Nguyen T, Arkin MR, et al. Voreloxin is an anticancer quinolone derivative that intercalates DNA and poisons topoisomerase II. PLoS ONE. 2010;5:e10186. doi: 10.1371/journal.pone.0010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoch U, Lynch J, Sato Y, Kashimoto S, Kajikawa F, Furutani Y, et al. Voreloxin, formerly SNS-595, has potent activity against a broad panel of cancer cell lines and in vivo tumor models. Cancer Chemother Pharmacol. 2009;64:53–65. doi: 10.1007/s00280-008-0850-3. [DOI] [PubMed] [Google Scholar]
- 15.Scatena CE, Kumer JL, Arbitrario JP, Howlett AR, Hawtin RE, Fox JA, et al. Voreloxin, a first-in-class anticancer quinolone derivative, acts synergistically with cytarabine in vitro and induces bone marrow aplasia in vivo. Cancer Chemother Pharmacol. 2010;66:881–888. doi: 10.1007/s00280-009-1234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Kolk DM, de Vries EG, Müller M, Vellenga E. The role of drug efflux pumps in acute myeloid leukemia. Leuk Lymphoma. 2002;43:685–701. doi: 10.1080/10428190290016773. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0. 8/06. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 18.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Banath JP, Olive PL. Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks. Cancer Res. 2003;63:4347–4350. [PubMed] [Google Scholar]
- 20.Olive PL, Banath JP. Phosphorylation of histone H2AX as a measure of radiosensitivity. Int J Radiat Oncol Biol Phys. 2004;58:331–335. doi: 10.1016/j.ijrobp.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 21.Karp JE, Ricklis RM, Balakrishnan K, Briel J, Greer J, Gore SD, et al. A phase 1 clinical-laboratory study of clofarabine followed by cyclophosphamide for adults with refractory acute leukemias. Blood. 2007;110:1762–1769. doi: 10.1182/blood-2007-03-081364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slovak ML, Kopecky KH, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 23.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 24.Advani RH, Hurwitz HI, Gordon MS, Ebbinghaus SW, Mendelson DS, Wakelee HA, et al. Voreloxin, a first-in-class anticancer quinolone derivative, in relapsed/refractory solid tumors: a report on two dosing schedules. Clin Cancer Res. 2010;16:2167–2175. doi: 10.1158/1078-0432.CCR-09-2236. [DOI] [PubMed] [Google Scholar]
- 25.Kell J. Treatment of relapsed acute myeloid leukemia. Rev Recent Clin Trials. 2006;1:103–111. doi: 10.2174/157488706776876445. [DOI] [PubMed] [Google Scholar]
- 26.Evanchik MJ, Allen D, Yoburn JC, Silverman JA, Hoch U. Metabolism of (+)-1,4-dihydro-7-(trans-3-methoxy-4-methylamino-1-pyrrolidinyl)-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid (voreloxin; formerly SNS-595), a novel replication-dependent DNA-damaging agent. Drug Metab Dispos. 2009;37:594–601. doi: 10.1124/dmd.108.023432. [DOI] [PubMed] [Google Scholar]
- 27.Speth PA, Linssen PC, Boezeman JB, Wessels HM, Haanen C. Leukemic cell and plasma daunomycin concentrations after bolus injection and 72 h infusion. Cancer Chemother Pharmacol. 1987;20:311–315. doi: 10.1007/BF00262582. [DOI] [PubMed] [Google Scholar]
- 28.Speth PA, Minderman H, Haanen C. Idarubicin v daunorubicin: preclinical and clinical pharmacokinetic studies. Semin Oncol. 1989;16(Suppl 2):2–9. [PubMed] [Google Scholar]
- 29.Galettis P, Boutagy J, Ma DD. Daunorubicin pharmacokinetics and the correlation with P-glycoprotein and response in patients with acute leukaemia. Br J Cancer. 1994;70:324–329. doi: 10.1038/bjc.1994.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


