Abstract
Despite efforts to discover the cellular pathways regulating breast cancer metastasis, little is known as to how prolactin (PRL) cooperates with extracellular environment and cytoskeletal proteins to regulate breast cancer cell motility and invasion. We implicated serine-threonine kinase p21-activated kinase 1 (PAK1) as a novel target for PRL-activated Janus-kinase 2 (JAK2). JAK2-dependent PAK1 tyrosyl phosphorylation plays a critical role in regulation of both PAK1 kinase activity and scaffolding properties of PAK1. Tyrosyl phosphorylated PAK1 facilitates PRL-dependent motility via at least two mechanisms: formation of paxillin/GIT1/βPIX/pTyr-PAK1 complexes resulting in increased adhesion turnover and phosphorylation of actin-binding protein filamin A. Increased adhesion turnover is the basis for cell migration and phosphorylated filamin A stimulates the kinase activity of PAK1 and increases actin-regulating activity to facilitate cell motility. Tyrosyl phosphorylated PAK1 also stimulates invasion of breast cancer cells in response to PRL and three-dimensional (3D) collagen IV via transcription and secretion of MMP-1 and MMP-3 in a MAPK-dependent manner. These data illustrate the complex interaction between PRL and the cell microenvironment in breast cancer cells and suggest a pivotal role for PRL/PAK1 signaling in breast cancer metastasis.
5.1 Role of Prolactin in Regulation of Breast Cancer Cell Motility
Prolactin (PRL) is a peptide hormone secreted from the anterior pituitary and was originally discovered in the early twentieth century as a hormone that regulates milk production in mammals [1, 2]. In addition to lactation, PRL was also implicated in mammary gland growth and development [3–6]. Significant progress was made in determining PRL-mediated signaling pathways upon the characterization of the prolactin receptor (PRLR) in the 1980s [7]. The PRLR is a transmembrane protein that belongs to the cytokine receptor superfamily and is expressed in variety of tissues, most notably the mammary epithelium [8]. The PRLR has no intrinsic kinase activity and relies on nonreceptor tyrosine kinases to facilitate PRL-mediated downstream signaling pathways. The most well characterized mediator of PRL signaling is the nonreceptor tyrosine kinase Janus-kinase 2 (JAK2) [9–11]. Upon PRL binding to its receptor, PRLRs dimerize, resulting in the activation of JAK2, as characterized by autophosphorylation of Tyr1007/1008, and promoting tyrosyl phosphorylation of the PRLR [12–14]. PRL signaling induces the activation of several signaling cascades, including the signal tranducers and activators of transcription (STATs), mitogen-activated protein kinases (MAPKs), protein kinase C, and phosphatidylinositol 3-kinase (PI3K) [15–21]. Since then, PRL signaling has been shown to regulate a variety of normal and pathological cell processes, one of which is cell motility.
Cell migration is critical for many vital biological functions, including embryonic development, the inflammatory immune response, wound repair, tumor formation and metastasis, and tissue remodeling and growth. The actin cytoskeleton provides both the protrusive and contractile forces required for cell migration via a combination of actin polymerization and depolymerization, actin filament cross-linking, and the interaction of myosin-based motors with actin filaments [22]. The complexity of cell motility and the fact that it is regulated by many hormones, cytokines, and growth factors suggest that multiple signaling mechanisms exist to regulate this process.
Little is known about the mechanisms that underlie the process of PRL-induced cell motility and its putative role in breast cancer metastasis. PRL was previously shown to act as a chemoattractant for human breast carcinoma [23]. Actin-based structures are most commonly controlled by small Rho-GTPases Rac1, Cdc42, and RhoA and these proteins are activated by guanine nucleotide exchange factors (GEFs) and repressed by GTPase-activating proteins (GAPs). PRL can activate Rac1 and several pathways have been implicated in this Rac-dependent regulation [24–26]. The first pathway has been shown to depend on PRL-induced activation of tyrosine kinase Tec which associates with and enhances activity of Vav1, the GEF factor for Rac1 [24]. According to the second proposed mechanism, PRL induces activity of serine-threonine kinase Nek3 (NIMA-related kinase 3) followed by activation of Vav1/Vav2 and subsequent activation of Rac1 [27, 28]. In addition, PRL stimulation also induces an interaction between Nek3 and focal adhesion protein paxillin and significantly increases paxillin serine phosphorylation [28]. In addition to Rac, PRL also activates another small GTPase Cdc42 that plays an important role in development and differentiation of mammary epithelia [25]. We have recently proposed two novel mechanisms to regulate PRL-dependent breast cancer cell motility: (1) through a serine-threonine kinase p21-activated kinase 1 (PAK1) and its substrate, the actin-binding protein filamin A and (2) through regulation of adhesion turnover ([29]; see below).
Cell migration depends on optimal levels of cell adhesion. The mechanisms that regulate focal adhesion assembly, maturation, and turnover are not well understood and have become a critical area of emerging interest. Over 180 proteins are found in adhesions, many of which exhibit multiple protein–protein interactions [30]. Cell adhesion regulated by both PRL- and extracellular matrix (ECM)/integrin-dependent pathways is essential for all aspects of normal mammary gland development and function (reviewed in [31–33]). PRL also regulates activation of numerous proteins participating in breast cancer cell adhesion. Thus, in early studies it has been noticed that PRL dramatically changes adhesiveness of breast cancer cells [34]. PRL activates focal adhesion kinase (FAK) and eventually induces phosphorylation of paxillin, an event that is essential to the rapid turnover of adhesions during cell motility [35]. FAK is a nonreceptor tyrosine kinase that mediates integrin signaling and regulates focal adhesion assembly and maturation during cell spreading and migration through phosphorylation of various adhesion proteins (reviewed in [36]). PRL causes tyrosyl phosphorylation of paxillin in an Src/FAK-dependent manner and serine phosphorylation of paxillin by serine-threonine kinase Nek3 ([28, 37]). In addition, transmembrane glycoprotein signal regulatory protein-α (SIRPα) has been implicated in the PRL- and integrin-activated cross talk in breast cancer cells [38]. We will discuss the role of PRL-activated serine-threonine kinase PAK1 in the regulation of breast cancer cell adhesion (see below). Thus, PRL has evidently been shown to increase cell motility in breast cancer cells.
Epidemiologic studies also linked elevated level of circulating PRL to breast cancer metastases [39–41]. In addition, PRLR expression has been found in patients with colorectal cancer, with high concordance between primary tumors and corresponding metastases [42]. These data, combined with animal studies reporting increased metastases with PRL administration [43], suggest that PRL is involved in the development of metastasis and tumor progression.
We have previously found that the serine-threonine kinase PAK1, a downstream effector for both Cdc42 and Rac1, participates in PRL-dependent signaling. We have shown that PAK1 is a novel substrate of the JAK2 tyrosine kinase and that PRL-activated JAK2 phosphorylates PAK1 in vivo and in vitro. PAK1 tyrosines 153, 201, and 285 were identified as sites of JAK2 tyrosyl phosphorylation by mass spectrometry and two-dimensional (2D) peptide mapping [44].
The aim of this review is to introduce tyrosyl phosphorylated PAK1 as a novel player in the field of PRL signaling and to discuss several mechanisms of pTyr-PAK1-dependent regulation of breast cancer cell motility, adhesion, and invasion.
5.2 p21-Activated Kinase 1 (PAK1)
5.2.1 PAK1 Structure and Activation
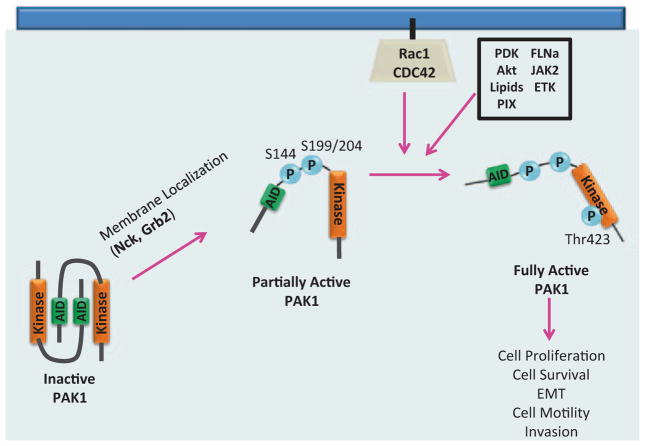
The PAKs are an evolutionarily conserved six member family of serine/threonine kinases and can be categorized into two groups based on structure and function: Group I (PAKs 1–3), which are activated in a GTPase-dependent or independent manner in response to extracellular signals, and Group II (PAKs 4–6), which are generally not regulated by Rho-GTPases but most likely through intramolecular mechanisms (reviewed in [45]). PAK1, a Group I member, is the most well-studied representative of the six PAK family members and is widely expressed in a variety of tissues. PAK1 plays a pivotal role in a range of cellular processes including cell proliferation, survival, motility, and invasion. PAK1 consists of an N-terminal regulatory domain containing a GTPase binding domain (GBD) that is partially overlapped with an autoinhibitory domain (AID). PAK1 enzymatic activity derives from its C-terminal serine/threonine kinase domain. The N-terminal regulatory domain of PAK1 has additional sites of protein–protein interaction that can mediate PAK1 activation and localization, including five classical proline-rich regions (PXXP), two of which facilitate binding to adaptor proteins Nck and Grb2. PAK1 also contains a nonclassical proline-rich region (PXP) that mediates interaction with the p21-interacting exchange factor PIX. In addition, there are three nuclear localization signals (NLS) and multiple phosphorylation sites, seven of which (serines 21, 57, 144, 149, 199, 204, and threonine 423) are sites of PAK1 autophosphorylation. PAK1 activation and localization are dependent on protein–protein interactions and both autophosphorylation and direct phosphorylation of PAK1 by other kinases (Fig. 5.1).
Fig. 5.1.
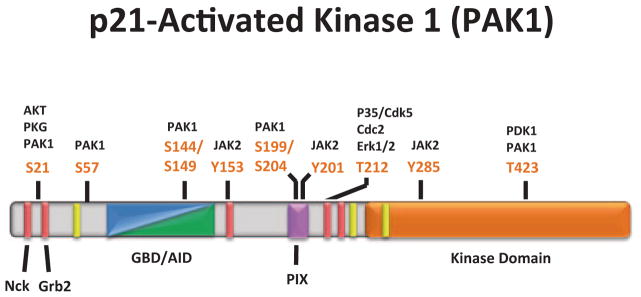
PAK1 domain structure and phosphorylation sites. The N-terminal regulatory region of PAK1 is composed of overlapping GBD/AID domains (aa 70-149, blue/green), five proline-rich regions (bright red), one nonclassical proline-rich region (aa 182–203, pink), and three nuclear localization signals (yellow). The C-terminal kinase domain (aa 249–545) is represented by the bright orange region. The two most N-terminal proline-rich regions (aa 12–18 and aa 40–47) mediate Nck/Grb2 binding, respectively, and subsequent PAK1 membrane localization. The non-classical proline-rich region regulates PIX/PAK1 binding and subsequent localization of PAK1 to adhesion complexes as well as facilitates PAK1 kinase activity. There are seven PAK1 autophosphorylation sites (S21, S57, S144, S149, S199, S204, and T423) that modulate PAK1 kinase activity, in addition to other sites phosphorylated by protein kinases that mediate PAK1 activity and localization. (Modified from Bokoch 2003)
PAK1 was initially discovered as an effector protein for two members of the Rho-family of small GTPases, Cdc42, and Rac [46]. These Rho-family GTPases serve as activators of PAK1 kinase activity. Inactive PAK1 resides in the cytoplasm as a homodimer, where the AID of one PAK1 molecule is obstructing the kinase domain of the other and vice versa (Fig. 5.2; [47]). Binding of Cdc42 or Rac1 to PAK1’s GBD induces a PAK1 conformational change that allows autoinhibitory relief and autophosphorylation of several sites on PAK1, keeping it in an open and active conformation. Recent studies suggest that membrane localization of inactive PAK1 via the adaptor proteins Nck and Grb2 promotes a semi-open conformation of PAK1 which facilitates autophosphorylation of several serines, including Ser199 and Ser204, and promotes initial kinase activation of PAK1. This semi-open/semi-active PAK1 is more susceptible to interaction with Rac1 or Cdc42. Rho-GTPase/PAK1 binding facilitates a fully open PAK1 conformation and allows autophosphorylation of Ser144 in the GBD and Thr423, the major PAK1 autophosphorylation site mediating PAK1 kinase activity, in the kinase domain [48].
Fig. 5.2.
Model for PAK1 activation. Inactive PAK1 is localized to the cytoplasm as a homodimer. Upon binding to Nck or Grb2, PAK1 is localized to the plasma membrane and undergoes a slight conformational change that facilitates partial autophosphorylation. PAK1 is now more susceptible to Rac1/Cdc42 binding, leading to a further conformational change, autophosphorylation at T423, and fully active PAK1 kinase activity. PAK1 can also be activated by interaction with Akt, lipids, PIX, FLNa, ETK, JAK2 or by direct T423 phosphorylation by PDK1. (Modified from Parrini et al. 2009)
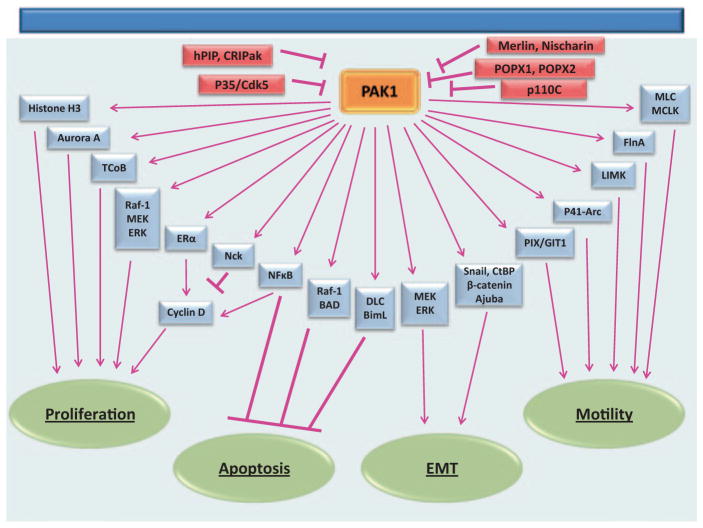
PAK1 activation is not solely dependent on GTPases, since the interaction of PAK1 with a variety of different proteins can regulate PAK1 kinase activity. Membrane localized PAK1 can be activated by direct phosphorylation on the critical Thr423 by phosphoinositide-dependent kinase 1 (PDK1) [49]. Similarly, certain lipids at the plasma membrane, like phosphatidic acid and sphingosine, can bind to the regulatory domain of PAK1 and induce kinase activation and subsequent autophosphorylation to the same extent as GTPase-activation of PAK1 [50]. Also at the plasma membrane, the actin cross-linking protein filamin A (FlnA) can mediate PAK1 kinase activity in two ways: by binding directly to the PAK1 GBD, therefore stimulating PAK1 activation, and facilitating the interaction of PAK1 with lipids [51, 52]. Akt (protein kinase B) can directly activate PAK1 and phosphorylate Ser21 on PAK1 which negatively mediates Nck/Grb binding and membrane localization [53, 54]. Some proteins, such as the guanine exchange factor (GEF) PIX, can induce PAK1 activation in both a GTPase-dependent and independent manner. The binding of PAK1 to PIX localizes PAK1 to cell–matrix adhesions and can directly mediate PAK1 activation, and since PIX is a Rac1-specific GEF, PIX can indirectly activate PAK1 through activation of Rac1 [55, 56]. Interestingly, PAK1 can also be activated upon tyrosyl phosphorylation. The nonreceptor tyrosine kinase Etk/BMX can tyrosyl phosphorylate PAK1 and induce PAK1 kinase activation; however, the sites for Etk-induced phosphorylation have not been mapped [57]. The nonreceptor tyrosine kinase JAK2 can also tyrosyl phosphorylate and activate PAK1, and we will review this activation and downstream effects on PAK1 signaling in this chapter [44]. Downregulation of PAK1 kinase activity is also important, since hyperactivation of PAK1 can induce mammary gland tumor growth [58]. PAK1 enzymatic activation and localization to focal adhesions can be inhibited when the tumor suppressor protein Merlin is bound to the PAK1 GBD [59]. Likewise, the integrin-binding protein Nischarin can bind to the kinase domain of activated PAK1, greatly reducing PAK1 kinase activity [60]. Cystein-rich protein CRIPak has also been identified as an inhibitor of PAK1 [61]. Human PAK1-interacting-protein 1 (hPIP) binds to first 70 amino acids of PAK1 and blocks kinase activity [62]. P35/Cdk5 phosphorylates PAK1 and inhibits kinase activity while phosphatases POPX1 and POPX2 dephosphorylate threonine 423 of PAK1 and also inhibit it [63, 64]. Protein kinase p110C binds to amino acids 210–332 of PAK1 and inhibits it [65].
The diverse means in which PAK1 is regulated lends PAK1 to participate in a variety of fundamentally different cellular processes (Fig. 5.3).
Fig. 5.3.
PAK1 regulates different cellular functions, including cell proliferation, survival, cell motility, and EMT. PAK1 kinase activity and/or interaction with various proteins mediate PAK1s variable functions within the cell
5.2.2 PAK1 Acts as a Scaffold
While PAK1 kinase activity plays a major role in PAK1 downstream signaling events, PAK1 can also act independent of its kinase activity as a molecular scaffold to facilitate the interaction between different proteins. Thus, PAK1 can regulate the actin cytoskeleton in both kinase-dependent and independent ways. PAK1 mutants with a modified N-terminus have dramatic effects on the actin cytoskeleton regardless of the presence of an active kinase domain [46, 66]. PAK1 overexpression has been shown to increase random cell movement irrespective of its kinase activity [67]. Also, an SH3 domain of PIX protein binds to a noncanonical proline-rich region on PAK1 independently of PAK1 kinase activity, and through association with GIT1, localizes PAK1 to the adhesion protein paxillin to regulate cell adhesion [55, 68]. These data, in combination with the fact that overexpression of kinase-dead PAK1 facilitates the formation of focal adhesions and stabilizes stress-fibers [69], suggest a role for the scaffolding abilities of PAK1 in regulation of cytoskeletal and adhesion dynamics.
PAK1 has also been shown to act as a scaffold in coordinating signaling between Raf-1, MEK, and ERK proteins upon cell adhesion to fibronectin or treatment with PDGF [70]. Overexpression of a kinase-dead PAK1 increased phosphorylation of MEK and ERK in kinase-independent way [71]. In addition to the scaffolding function, PAK1 phosphorylates both MEK and Raf-1 to amplify the ERK signaling [72]. The MAPK pathway is not the only pathway that benefits from the nonenzymatic activity of PAK1. Akt, a major regulator of several cell survival pathways, is activated upon phosphorylation by PDK1 and the plasma membrane localization of Akt is important for the activation [73, 74]. Higuchi et al. demonstrated that upon growth factor stimulation Akt binds to the C-terminal domain of PAK1 and is consequently targeted to the plasma membrane. At the plasma membrane, PAK1 can also bind to PDK1, bringing together both Akt and PDK1 thereby facilitating Akt stimulation by PDK1. These findings confirm scaffolding, kinase-independent functions of PAK1 [75]. There is also evidence of kinase-independent roles for PAK1 in regulation of the cell cycle. Overexpression of the kinase inhibitory domain of PAK1 (AID) induces cell cycle arrest and decreases cyclin D1 and D2 expression independently of PAK1 kinase activity [76]. We have previously demonstrated that three tyrosines on PAK1 molecules and PAK1-Nck interaction play a critical role in PAK1-dependent regulation of cyclin D1 promoter activity in response to PRL and proposed that Nck-PAK1 complex (formation of which does not depend on PAK1 kinase activity) can sequester PAK1 in cytoplasm to prevent PAK1 nuclear shuttling thereby inhibiting PAK1-dependent activation of cyclin D1 promoter [77].
The multifunctionality of PAK1 as both a kinase and a scaffolding protein allow PAK1 to modulate a diverse array of cell processes, such as cell proliferation, survival, motility, and invasion.
5.2.3 PAK1 Regulates Cell Proliferation
PAK1 has been shown to stimulate cell proliferation (Fig. 5.3). Thus, highly proliferating human breast cancer cell lines and tumor tissues have been shown to contain hyperactive PAK1 and its upstream regulator Rac3 [78]. Tyrosyl phosphorylation of PAK1 by nonreceptor tyrosine kinase Etk/Bmx leads to increased proliferation of human breast cancer MCF-7 cells [57]. In addition, expression of kinase-active T423E PAK1 mutant in mammary glands induces hyperplasia in the mammary epithelium [79]. One of the first conclusive evidence that PAK1 has a role in cell cycle regulation was the finding that overexpression of activated PAK1 in human breast cancer cells leads to the abnormal accumulation of centrosomes and aberrant mitoses [80]. Furthermore, PAK1 is present at histone complexes, centrosomes, and at mitotic spindles during mitosis [81]. During the early stages of mitosis, DNA must be tightly packed into chromosomes to allow for proper gene segregation through a process called chromosome condensation. This process is highly regulated by various posttranslational modifications to the DNA-bound histone protein complexes. One such event is the phosphorylation of Ser10 on histone H3 that is necessary for the initiation of chromosome condensation [82, 83]. Li et al. reported that active PAK1 can translocate into the nucleus (via the PAK1 NLSs, Fig. 5.1) where it can directly bind to and phosphorylate histone H3 on Ser10, promoting chromosome condensation and aiding in the progression of metaphase to anaphase during mitosis [81]. PAK1 can also mediate proper formation of the mitotic spindle and microtubule dynamics during mitosis. Tight regulation of microtubule dynamics is absolutely required for proper spindle formation and chromosomal segregation. Tubulin cofactor B (TCoB) assists in the assembly of α and β-tubulin and is localized at the centrosomes. PAK1 has been implicated in TCoB activity during mitosis. PAK1 colocalizes with TCoB at the centrosome and phosphorylates two serine residues on TCoB, encouraging the microtubule polymerization activity of TCoB [84]. PAK1 can also regulate the formation of the mitotic spindle at the centrosome through Aurora A. Aurora A is a serine/threonine kinase that is present at the centrosome throughout mitosis and is responsible for the recruitment of several microtubule-associated proteins required for proper spindle formation. Regulation of Aurora A is important, since knockdown of Aurora A leads to abnormal maturation of the centrosome [85]. Through interaction with PIX/GIT1 complex, PAK1 is localized to the centrosome where it directly induces Aurora A activation by phosphorylating Thr288 and Ser342 [86]. These data combined with the fact that hyperactive PAK1 in cells leads to aneuploidy [80] suggest an important role for PAK1 in chromosome segregation and microtubule regulation during mitosis. PAK1 can also induce expression of cyclin D1, one of the key mediators of cell cycle progression. Overexpression of active PAK1 increases cyclin D1 expression in breast cancer cells while knockdown of PAK1 significantly reduces cyclin D1 expression [87]. Our lab demonstrated that PRL-mediated activation of PAK1 increased nuclear localization of PAK1 and activation of cyclin D1 promoter and that PAK1/Nck binding inhibited PAK1 nuclear localization and cyclin D1 promoter activity [77]. Balasenthil et al. proposed that PAK1 can increase cyclin D1 transcription through two independent pathways—the NFκB pathway and phosphorylation of S305 of estrogen receptor alpha (ERα) [87, 88]. PAK1 has been previously shown to directly phosphorylate ERα at Ser305 and promote its transactivation functions [79]. Interestingly, PAK1 itself is activated by estrogen suggesting a positive feedback loop [89]. Lastly, PAK1 can regulate cell proliferation through activation of the Ras/ERK pathway [90–92]. The regulation of the ERK pathway by PAK1 requires both kinase-dependent and independent functions of PAK1 as stated earlier. Thus, these data describe a multifunctional role for PAK1 in the regulation of cell proliferation and cell cycle progression.
5.2.4 PAK1 Regulates Cell Survival
PAK1 also plays a role in cell survival (Fig. 5.3). PAK1 inhibits the release of pro-apoptotic factors from the mitochondria. BAD is a proapoptotic protein that binds and inhibits the prosurvival proteins Bcl-2 and Bcl-X. Phosphorylation of BAD at Ser112 and Ser136 blocks BAD binding to Bcl-XL and promotes cell survival [93]. PAK1 promotes cell survival by directly phosphorylating these serines on BAD [94]. Also, we had previously mentioned that PAK1 can act as a scaffold for Raf-1, MEK, and ERK, promoting the activation of the MAPK pathway in the regulation of cell proliferation; however, PAK-mediated phosphorylation of Raf-1 can also mediate cell survival. Raf-1 phosphorylation at Ser338 by PAK1 can induce the translocation of Raf-1 to the mitochondria where it binds to Bcl-2 and phosphorylates BAD at Ser112, thus providing an additional mechanism in which PAK1 can regulate BAD activity and prevent apoptosis [95]. PAK1-mediated BAD phosphorylation was described as a critical event in survival signaling induced by the HIV viral Nef protein [96]. In addition to directly regulating BAD activity, PAK1 induces the degradation of proapoptotic proteins such as BimL [97]. Typically, dynein light chain 1 (DLC1) is bound to BimL, preventing BimL from inactivating Bcl-2, thus promoting cell survival, that is until BimL is released upon proapoptotic signals [98, 99]. PAK1 can phosphorylate both DLC1 and BimL leading to DLC1/BimL degradation and therefore promoting cell survival [97]. Another mechanism in which PAK1 can regulate cell survival is through activation of the NFκB pathway [100–102]. PAK1 mediates NFκB activation by Ras, Raf-1, and Rac1 and expression of active PAK1 can stimulate NFκB on its own without activation of the inhibitor of κB kinases [100]. Friedland et al. discovered that PAK1-induced NFκB activation prevented apoptosis in three-dimensional (3D) cultures of mammary epithelial cells [102]. During Helicobacter pylori infection of human epithelial cells, PAK1 activates NFκB via activation of upstream regulatory kinase NIK (NFκB-inducing kinase) [103]. Furthermore, PAK1 also inhibits apoptosis by phosphorylating and inactivating cell survival forkhead transcription factor, FKHR [89]. Hence, PAK1 has both direct and indirect roles to play in the regulation of cell survival.
5.2.5 PAK1 Regulates the Actin Cytoskeleton
The first described and most well-understood function of PAK1 is the role for PAK1 in the regulation of the actin cytoskeleton and cell motility (Fig. 5.3). Active PAK1 is localized to areas of actin remodeling, such as filopodia and lamellipodia of motile cells, membrane ruffles, and pinocytosis vesicles [46, 104, 105]. Overexpression of kinase-active PAK1 induces the formation of lamellipodia and membrane ruffles [46, 104]. PAK1 phosphorylates a variety of different actin cytoskeleton proteins such as Lim Kinase 1 (LIMK1), p41-Arc, filamin A, myosin light chain (MLC) and myosin light chain kinase (MLCK). LIMK1 is a kinase that upon activation can phosphorylate and inactivate cofilin, an actin-binding protein. Cofilin depolymerizes actin fibers in ruffles and lamellipodia, promoting actin recycling and retrograde flow [106, 107]. PAK1 can directly phosphorylate and activate LIMK1, resulting in downstream inactivation of cofilin and subsequent stabilization of actin filaments [108, 109]. Actin filaments stabilization allows for the efficient formation of protrusive structures, such as lamellipodia and filopodia during cell motility [109]. Proper protrusion formation also relies on the creation of a branched actin network. The Arp2/3 complex is a complex of proteins that facilitates branching actin filaments by binding to existing actin fibers and providing nucleation sites for new actin filaments at a 70° angle from the original filament. The nucleation property of the Arp2/3 complex requires p41-Arc protein, which can be regulated by PAK1. Phosphorylation of p41-Arc on Thr21 by PAK1 induces the localization of p41-Arc to the Arp2/3 complex, facilitating actin nucleation and branching during cell motility, while blocking PAK1-mediated phosphorylation of p41-Arc inhibits cell motility [110]. Similarly, PAK1 can bind to the actin-cross-linking protein filamin A. Serine phosphorylation of FlnA by PAK1 at Ser2152 results in PAK1-dependent membrane ruffling [51]. FlnA in turn activates PAK1, furthering PAK1 downstream actin-modulating signals. Actin stress-fibers are anchored by focal adhesions to provide support and bind to nonmuscle myosins that regulate tension and contraction. PAK1 can modify actin–myosin binding and focal adhesion assembly by interacting and phosphorylating MLC and MLCK. MLCK typically phosphorylates MLC at Ser19, promoting actin–myosin binding and increased contractility [111]. PAK1, however, can phosphorylate Ser439 and Ser991 of MLCK and inhibit MLCK [112]. Inhibition of MLCK by PAK1 reduces stress fiber formation and leads to the disassembly of focal adhesions, both processes that are necessary to promote cell motility. PAK1 can directly phosphorylate MLC at Ser19, promoting myosin–actin binding which may regulate the contraction of the trailing edge of motile cells [67, 113]. Furthermore, PAK1 facilitates integrin-mediated cell adhesion [114–116], as activation of PAK1 promoted disassembly of actin stress, abolishment of focal adhesion, and reduction of cell attachment, while PAK1 silencing enhanced cell adhesion and/or spreading and led to increased size and number of mature focal adhesion [117–122].
5.2.6 Role of PAK1 in EMT
Epithelial-mesenchymal transition (EMT) is a process where tightly adhered, non-motile epithelial cells lose their epithelial characteristics and display the loose adherence and motile phenotypes of mesenchymal cells. EMT was first described in the context of embryogenesis, where it leads to the generation of mesenchymal cells. Epithelial cells undergoing EMT acquire a morphology that is appropriate for migration through the extracellular environment, and for settlement in areas of new organ formation. In recent years, EMT-like processes have been the focus of active research on their potential role as determinants of cancer cell invasion and metastasis. Certain proteins can be used as markers for pathogenic EMT, including E-cadherin, N-cadherin, and vimentin. EMT is characterized by the reduction of E-cadherin expression and an increase in both N-cadherin and vimentin expression. E-cadherin transcription is controlled by various transcription factors (TFs), one of which is Snail [123, 124]. The Snail superfamily of TFs is composed of two families; the Snail family (Snail, SNAILP, and SLUG), and the Scratch family (SCRATCH1 and SCRATCH2) (reviewed in [125]). Members of the Snail family have been shown to regulate EMT [124, 126].
PAK1 has been implicated in regulation of EMT by findings that E-cadherin expression in MCF-7 cells was downregulated upon transfection of PAK1, and conversely, E-cadherin expression in MDA-MB-435 cells was upregulated through inhibition of PAK1 expression [127]. A similar effect of PAK1 has been also demonstrated in keratinocytes [128]. On the other side, PAK1 was also shown to be required for the stabilization of adherent junctions through a recently discovered target of PAK1 Ajuba, an actin-binding protein that colocalizes with cadherins [129]. The role of PAK1 in the stabilization of E-cadherin cell–cell junction has also been shown during zebrafish epiboly [130].
PAK1 has been shown to regulate E-cadherin expression through Snail [127]. Yang et al. demonstrated that PAK1 phosphorylates Ser246 on Snail. Serine phosphorylation of Snail facilitates the accumulation of Snail in the nucleus and promotes transcriptional repression of E-cadherin. Knockdown of PAK1, or mutation of serine 246 on Snail to an alanine, leads to increased cytoplasmic Snail and a reduction of Snail repressor activity [127]. In contrast, the same lab had previously demonstrated that PAK1 phosphorylates corepressor CtBP (C-terminal binding protein 1) that leads to translocation of CtBP from the nucleus into the cytoplasm and relieves its corepressor activity toward the E-cadherin promoter. They demonstrated that CtBP-mediated repression of the E-cadherin promoter was relieved by transfection of PAK1 [131]. If so, the effect of PAK1 on E-cadherin expression may be either stimulating through inhibition of CtBP or repressive through activation of Snail.
PAK1 also interacts with β-catenin and promotes β-catenin activation in gastric epithelial cells [132]. Phosphorylation of β-catenin at Ser675 by PAK1 increases the stability and transcriptional activity of β-catenin in colorectal cells [133]. PAK1 knockdown in human colorectal cell lines inhibits β-catenin expression, β-catenin transcriptional activity, and the expression of c-Myc and suppresses the tumor growth and metastasis in mouse model [134].
Recently, PAK1 has been shown to activate β-catenin transcriptional activities and promote EMT in podocytes [135].
In addition, PAK1 can mediate peroxisome proliferator-activated receptor gamma (PPARgamma)-induced EMT of intestinal epithelial cells through activation of the ERK1/2 pathway [136].
5.2.7 Role of PAK1 in Breast Cancer
PAK1 plays an important role in such vital processes like cell proliferation, survival, cell motility and EMT, therefore it is no surprise that misregulation of PAK1 activity is present in many cancers. Altered expression and/or activation of PAK1 is evident in various cancers, including brain, pancreas, colon, bladder, ovarian, hepatocellular, urinary tract, renal cell carcinoma, thyroid, and breast cancers ([137–146], reviewed in [147]). Of these cancers, the role for PAK1 in breast cancer has been studied to the most extent ([58, 80, 87, 148–152], reviewed in [147, 153]). PAK1 is overexpressed or upregulated in some breast cancers. The PAK1 gene is localized within the 11q13 region, and 11q13.5 → q14 amplifications involving the PAK1 locus are found in 17 % of breast cancer [154, 155]. Overexpression of PAK1 was observed in 34 of 60 breast tumor specimens [87] and expression of PAK1 in human breast tumors correlates with tumor histologic grade [80, 150]. PAK1 expression and activity were higher in human breast tumors as compared to their adjacent controls [156]. Furthermore, expression of PAK1 in human breast tumors correlates with tamoxifen resistance [150]. PAK1 kinase activity can also be increased in human breast tissue by the upregulation of Rac3 activity or Rac1 expression [78, 157]. In a transgenic mouse model, PAK1 hyperactivation (PAK1 T423E mutant) leads to the formation of mammary gland tumors [58]. Of particular interest, PAK1 plays a critical role in premalignant progression of MCF10 series of human breast epithelial cell lines grown in 3D reconstituted basal membrane overlay cultures [158]. It has been demonstrated that expression of a kinase-dead PAK1 mutant in highly invasive breast cancer cell lines led to reduced invasiveness [69]. Conversely, hyperactivation of the PAK1 pathway in the noninvasive breast cancer cell line MCF-7 promotes cell migration and anchorage-independent growth [80]. As we described above, PAK1 phosphorylates several transcription factors, among them CtBP1 and Snail both of which are important for EMT [127, 131]. Another possible mechanism of PAK1-mediated malignant transformation is the enhancement of PAK1-regulated cell motility because PAK1 kinase activity participates in directional motility and PAK1 directly phosphorylates cytoskeletal proteins as we discussed above. For example, depletion of PAK1 has been shown to contribute to breast cancer cell invasion through cofilin-dependent mechanism [159]. Thus, PAK1 has become one of the focal points in the investigation into the mechanism and onset of human breast cancer. Recently, our lab has demonstrated a role for PRL-mediated tyrosyl phosphorylation of PAK1 in breast cancer cell motility, adhesion and invasion.
5.3 PRL Regulates Breast Cancer Cell Motility Through Tyrosyl Phosphorylated PAK1
5.3.1 JAK2 Tyrosyl Phosphorylates and Activates PAK1 in Response to PRL
In 2007, we demonstrated that PAK1 is a novel substrate of the JAK2 tyrosine kinase and that PRL-activated JAK2 phosphorylates PAK1 in vivo. PAK1 tyrosines 153, 201, and 285 were identified as sites of JAK2 tyrosyl phosphorylation by mass spectrometry and 2D peptide mapping. Our findings indicated that this phosphorylation plays an important role in cell survival and in the regulation of cyclin D1 promoter activity [44, 77].
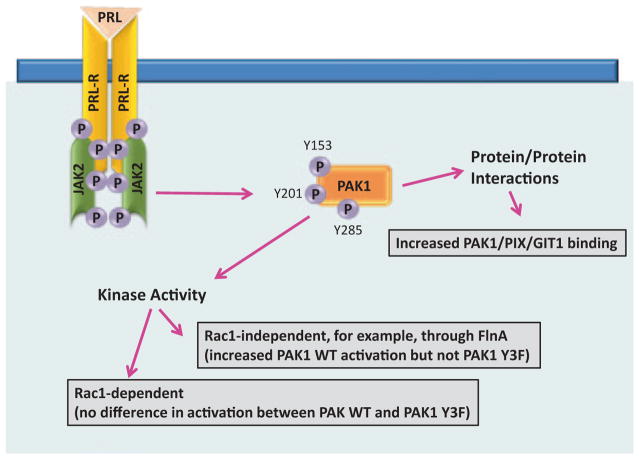
In an attempt to understand the mechanism of JAK2-dependent activation of PAK1, we first focused on testing PAK1 kinase activity in an in vitro kinase assay with P32-ATP and exogenous H4 histone as a substrate. Indeed, PAK1 kinase activity was increased in the presence of overexpressed activated JAK2 but not kinase dead JAK K882E. Active JAK2 had no effect on the kinase activity of the PAK1 Y3F mutant in which the three JAK2 phosphorylation sites (Tyr(s) 153, 201 and 285) were mutated to phenylalanine [44]. PRL treatment activated both PAK1 WT and PAK1 Y3F (which is catalytically active). However, in the presence of PRL, the kinase activity of PAK1 WT was significantly stronger than PAK1 Y3F in MCF-7, T47D and TMX2–28 breast cancer cell lines (Fig. 5.4; [29, 160]). Heregulin (HRG), a ligand for HER3 (human epidermal growth factor receptor-3) and HER4 (human epidermal growth factor receptor-4), activates both PAK1 WT and PAK1 Y3F to the similar extent confirming that PAK1 Y3F retains its kinase activity. How does PRL activate JAK2-phosphorylation-deficient mutant PAK1 Y3F? Presumably, it works through Rac1. Indeed, both PAK1 WT and PAK1 Y3F were similarly activated by either activated Rac1 V12 or by activated Cdc42 L61 [160]. This Rac1/Cdc42-dependent activation is pTyr-PAK1-independent, therefore PAK1 Y3F mutant exhibits some kinase activity in response to PRL (black bars for PAK1 Y3F in Fig. 5.4).
Fig. 5.4.
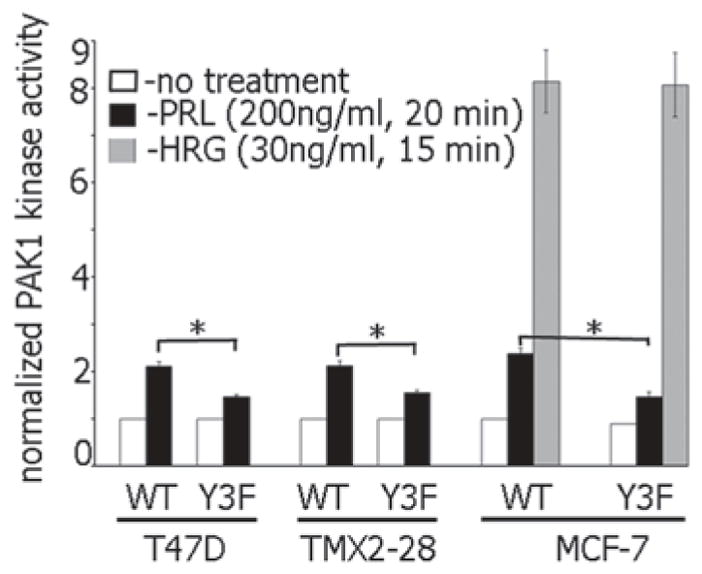
Tyrosyl phosphorylation of Tyr 153, 201, and 285 is required for maximal PAK1 kinase activity in response to PRL but not heregulin. Indicated cell lines stably overexpressing PAK1 WT or PAK1 Y3F were deprived of serum and treated with or without prolactin (PRL) or heregulin (HRG). Myc-PAK1 was IP’ed and subjected to an in vitro kinase assay with H4 histone as a substrate. Relative PAK1 kinase activity was then normalized by the amount of IP’d PAK1 for each lane and plotted. Bar represent mean ± S.E., *, p < 0.05 compared with the same cells without treatment (n = 3)
However, PAK1 is activated by GTPase-independent mechanisms as well. As we discussed above, membrane recruitment of PAK1 by Nck and Grb2 adapter proteins results in the stimulation of PAK1 kinase activity through interaction with lipids such as sphingosine or phosphatidic acid [50]. Membrane-localized PAK1 can also be activated by PDK1 [49]. The nonreceptor tyrosine kinase Etk/BMX can tyrosyl phosphorylate PAK1 and induce PAK1 kinase activation [57]. In addition, PAK1 can be directly activated by Akt [54] and FLNa [51]. Which mechanism acts in response to PRL? It has been demonstrated that PAK1 phosphorylates FLNa on Ser 2152 and FLNa activates PAK1 in a positive feedback loop [51]. We have shown than Ser-phosphorylation of FLNa was increased when FLNa was coexpressed with PAK1 and constitutively active JAK2 V617F as compared to coexpression of FLNa with kinase inactive JAK2 mutant K882E. Furthermore, we have demonstrated that PAK1 phosphorylates Ser 2152 of FLNa to a greater extent when PAK1 is tyrosyl-phosphorylated by JAK2 in response to PRL [29]. Thus, PRL can induce PAK1 kinase activity in two ways: first in a GTPase-dependent manner, activating both PAK1 WT and PAK1 Y3F, but also through pathway(s) that are pTyr-PAK1-dependent, further activating PAK1 WT but not PAK1 Y3F, for example, through filamin A (Fig. 5.5).
Fig. 5.5.
PRL stimulates kinase activity of PAK1 and PAK1 ability to form protein–protein interaction. PRL binding induces dimerization of the PRLR and subsequent activation of JAK2. JAK2 phosphorylates PAK1 on three tyrosines, Tyr 153, 201 and 285. Tyrosyl phosphorylation of PAK1 enhances both a Rac-dependent (Rider et al. 2013) and Rac-independent PAK1 kinase activity (Hammer et al. 2013), and also the ability for PAK1 to act as a molecular scaffold (Hammer et al., unpublished)
Moreover, PAK1 has a dual activity. First, PAK1 is a serine-threonine kinase and this activity depends on activation of the PAK1 kinase domain. Second, as we discussed above, PAK1 acts as a scaffold for many proteins, for example, for Raf-1, MEK and Erk [70] and this PAK1 activity depends on its ability to initiate protein–protein interactions. One might speculate that tyrosyl phosphorylated PAK1 may create additional docking sites to recruit SH2-domain containing proteins to facilitate local activation of recruited proteins and amplify PRL-dependent signaling. In such case, pTyr-PAK1 will be able to recruit additional proteins to function as a scaffold to locally amplify PRL signaling.
Thus, PRL may stimulate both PAK1 activities: kinase activity and scaffolding ability of PAK1 through JAK2-dependent tyrosyl phosphorylation of Tyr(s) 153, 201, and 285.
In our search for scaffolding activities of pTyr-PAK1, we focused on βPIX/GIT1 proteins. A proline-rich motif of PAK1 (residues 182–203) binds directly to the SH3 domain of GEF βPIX [55].The PIX proteins associate with G protein-coupled receptor kinase-interacting target 1 (GIT1), a GTPase activating protein (GAP) for Arf, that targets adhesion complexes by binding to paxillin [68]. βPIX and GIT1 can homodimerize and form large aggregates in the cell [161, 162]. This oligomerization is essential for localization to sites of adhesion since mutations that disrupt either GIT-βPIX association or βPIX homodimerization result in diffuse cytoplasmic localization of both proteins [163, 164]. PAK1 is an important component of this complex and formation of the four-molecule PAK1/βPIX/GIT1/paxillin signaling module transiently targets PAK1 to the sites of adhesion [165–168]. To provide insight into whether tyrosyl phosphorylation of PAK1 increases the ability of PAK1 to bind βPIX and GIT1, we immunoprecipitated PAK1 from the lysates of PAK1 WT and PAK1 Y3F cells treated with PRL over a time course and assessed these immunoprecipitates for endogenous βPIX. The quantification of PAK1 and βPIX bands in the immunoprecipitates showed that PRL increased association of βPIX with PAK1 WT about 8.5-fold. The amount of endogenous βPIX bound to PAK1 Y3F was left unchanged during PRL treatment. Next, we also assessed GIT1 associated with PAK1 WT and PAK1 Y3F upon PRL treatment. We demonstrated that threefold more GIT1 was associated with tyrosyl phosphorylated PAK1 WT than with PAK1 Y3F. These data demonstrate that tyrosyl phosphorylation of PAK1 regulates its binding activity toward βPIX and GIT1. We hypothesize that phosphorylation at position Y285 may affect the interaction with βPIX by inducing a conformational change that makes the proline-rich motif of PAK1 more accessible to βPIX (Hammer et.al. unpublished).
Overall, PAK1 WT and PAK1 Y3F have similar kinase activity in response to active Rac1/Cdc42. PRL stimulates both PAK1 WT and Y3F through Rac1 and additionally activates PAK1 WT (but not PAK1 Y3F) by Rac1-independnet mechanism(s) which depends on tyrosyl phosphorylation (for example, by local activation by filamin A). Furthermore, pTyr-PAK1 is able to recruit additional proteins to function as a scaffold to locally amplify PRL signaling further (for example, via βPIX/GIT1 recruitment). These two activities are interconnected because high local concentration of PAK1 in PAK1/βPIX/GIT1/paxillin complexes permits autophosphorylation, stimulating the kinase activity of PAK1 (Fig. 5.5).
5.3.2 Tyrosyl Phosphorylated PAK1 Regulates the Actin Cytoskeleton and Cell Motility in Response to PRL Through Filamin A
PAK1 is major regulator of actin cytoskeleton dynamics and can bind a variety of different actin-modulating proteins. We have implicated tyrosyl phosphorylation of PAK1 in the regulation of unstimulated phagokinesis, which is a combination of two processes that are dependent upon changes in the actin cytoskeleton: cellular movement and phagocytosis [44]. Later, we have demonstrated that overexpression of WT PAK1 enhanced the ability of PRL to induce cell ruffling. In contrast, overexpression of PAK1 Y3F failed to increase ruffling [169]. Membrane ruffling has been observed in many cell types in response to certain extracellular factors, and on motile cells where they are believed to be required for directed cell migration. Thus, the formation of membrane ruffles may be considered as a sign of increased response to external stimuli and of elevated cell migration (for review, [170, 171]). We extended our findings and demonstrated that overexpression of PAK1 WT strongly enhances cell migration in response to PRL in both cell wounding and Boyden chamber assays [29]. In an attempt to understand the mechanism of the amplifying effect of tyrosyl phopshorylated PAK1 on cell motility, we focused on filamin A for several reasons. First, the actin-binding protein FLNa is a binding partner of PAK1 [51]. Second, we have previously implicated FLNa in PRL-dependent signaling through adapter protein SH2B1β [169]. Filamin A is a 280 kDa actin cross-linking protein containing an N-terminal actin-binding domain and a rod region containing 24 immunoglobulin-like repeats (Fig. 5.6; reviewed in [172]). The last repeat of the rod region enables the FLNa molecules to dimerize, allowing for a flexible structure mediating the actin gelation activity of filamins. Filamins have > 90 interacting partners, including adapter proteins, small GTPases, transmembrane receptors, and membrane channels [173]. FLNa participates in the activation of various kinases as well as being regulated by kinases itself. FLNa binding to PAK1 enhances the kinase activity of PAK1, which subsequently phosphorylates FLNa at Ser 2152, resulting in PAK1-dependent membrane ruffling [51]. FLNa also stimulates PAK1 by interacting with sphingosine kinase 1, which phosphorylates sphingosine, leading to the direct activation of PAK1 [52]. As a potent actin cross-linking protein, FLNa regulates cell migration, although the role of FLNa in this process is controversial. Thus, multiple studies have demonstrated a positive impact of FLNa on the migration of different cell types (for example, [174–177]). One of the first noted defects of FLNa-deficient melanoma cells (M2 cells) was the inability to migrate due to inefficient polarization and continuous blebbing, which was rescued once FLNa was stably reexpressed (A7 cells) [174]. In contrast, FLNa overexpression inhibits neuronal migration [178] and downregulation of FLNa stimulates cancer cell migration, invasion, and metastatic formation [179]. In support of the latter finding, we demonstrated that the depletion of FLNa increased basal nonstimulated migration of T47D cells. However, PRL-induced cell migration was suppressed by FLNa knock-down. These previously published and our current results suggest that at normal expression levels, FLNa activity should be strongly regulated to coordinate cell migration. In addition, we show that PAK1 phosphorylates Ser 2152 of the actin-binding protein filamin A to a greater extent when PAK1 is tyrosyl-phosphorylated by JAK2 in response to PRL. Downregulation of PAK1 or filamin A abolishes the effect of PRL on cell migration. Thus, these data bring some insight into the mechanism of PRL-stimulated motility of breast cancer cells [29].
Fig. 5.6.
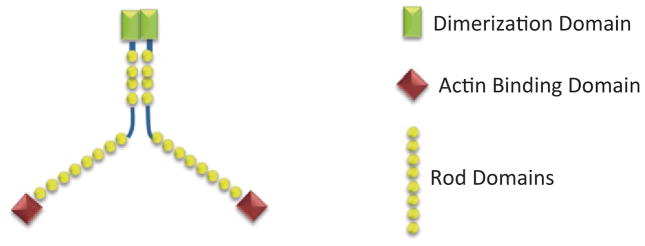
Schematic diagram of FLNa dimer. FLNa consists of an N-terminal actin-binding domain, a rod domain containing 24 IgG-like repeats and C-terminal dimerization domain. (modified from Cukier et al. 2007)
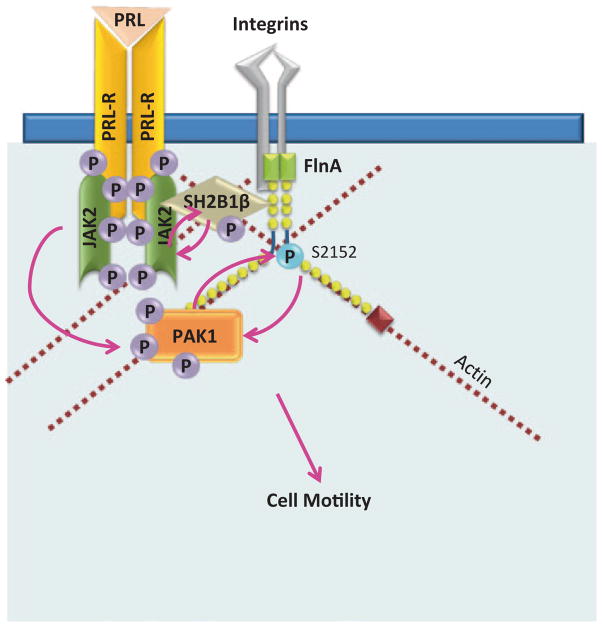
We have proposed a model for PRL-dependent regulation of the actin cytoskeleton (Fig. 5.7; [29, 169]). According to this model, upon ligation of PRLR and activation of JAK2, adapter protein SH2B1β translocates to activated PRLR-JAK2 complexes, where it cross-links actin filaments via its two actin-binding domains and binds to FLNa [169, 180]. PRL-activation of JAK2 also leads to tyrosyl phosphorylation of PAK1, thereby increasing PAK1’s activities (both the kinase and scaffolding activities) and stimulating phosphorylation of FLNa. FLNa, in turn, activates PAK1, binds to SH2B1β and relocates more SH2B1β to the JAK2/PAK1/FLNa complex. Because SH2B1β enhances the tyrosine kinase activity of JAK2 [181], the formation of this multiprotein complex results in enhancement of JAK2 activation and further activation of the JAK2/PAK1/FLNa-actin complex, leading to actin cytoskeleton reorganization.
Fig. 5.7.
Tyrosyl phosphorylated PAK1 regulates cell motility in response to PRL through filamin A. PRL-activated JAK2 tyrosyl phosphorylates PAK1 increasing PAK1 kinase activity and scaffolding ability of PAK1. pTyr-PAK1 has increased FlnA interaction and phosphorylates FlnA on Ser2152. FlnA activates PAK1 in a positive feedback loop. JAK2 also phosphorylates SH2B1β, which binds to FlnA and cross-links actin filaments. Positive feedback from FlnA to PAK1 and SH2B1β to JAK2 facilitates the formation of more JAK2/SH2B1β/FlnA/pTyr-PAK1 complexes that regulate actin remodeling during enhanced cell motility in response to PRL
5.3.3 PRL-Mediated pTyr-PAK1 Regulation of Adhesion Turnover
Cell adhesion is the basis for cell migration. Dynamic changes in cell–matrix adhesions are necessary for both cell spreading and cell motility. The rapid assembly and disassembly of adhesions during cell migration is called adhesion turnover. Upon contact with the ECM, or in response to external stimuli, there is clustering and activation of the cell-surface proteins integrins, the chief proteins regulating cell–matrix adhesion. Integrin clustering induces autophosphorylation of FAK on Tyr397, enhancing FAK kinase activity and recruits the adhesion scaffolding protein paxillin ([182, 183], reviewed in [184]). Localized FAK and paxillin, along with the actin-binding protein talin, at integrin clusters form small adhesion complexes, called nascent adhesions, at the distal edge of the lamellipodium [185]. Nascent adhesions are unstable and can either immediately disassemble, or mature into larger focal complexes. Nascent adhesion maturation requires FAK-mediated phosphorylation of paxillin on two tyrosines, Tyr31 and Tyr118 [182]. This tyrosyl phosphorylation of paxillin increases the affinity of paxillin for FAK, recruits a variety of kinases, scaffolding proteins, and regulators of GTPase activity, and induces the maturation of the nascent adhesion into a focal complex [185–187]. Focal complexes can then either further mature into larger focal adhesions, or disassemble (turnover), a process that occurs rapidly during cell motility. Modulation of adhesion dynamics is both tightly regulated and highly complex.
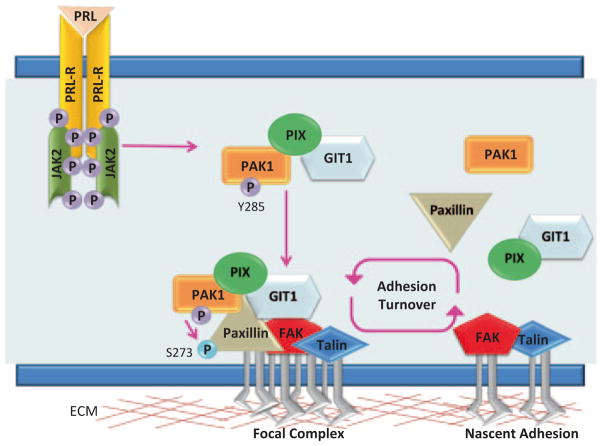
PAK1 activity has been implicated in regulating cell–matrix adhesion dynamics. PAK1 is localized at adhesions, where it regulates both adhesion assembly and disassembly [117, 119, 188, 189]. One of the first observations was that overexpressed PAK1 WT and kinase-dead PAK1 localized to the focal adhesions and caused the accumulation of focal points [117]. These data were conformed later by demonstration that overexpression of kinase-dead PAK1 facilitates the formation of focal adhesions [66, 69]. Several years later, PAK1 was shown to be directly involved in mediating adhesion turnover, a process that could be reversed upon expression of the AID domain, suggesting that PAK1 kinase activity was necessary for proper adhesion turnover [119, 189]. It turns out that both PAK1 kinase activity and scaffolding properties are required to modulate adhesion turnover in motile cells. In order for PAK1 to be localized to adhesion complexes, PAK1 must bind to the βPIX protein as well as the GIT1 protein, however this interaction is completely independent of βPIX’s GEF activity and GIT1’s GAP activity [55, 167]. βPIX binds to noncanonical proline-rich motif on PAK1 (Fig. 5.8) and this interaction is negatively regulated by autophosphorylation of PAK1 at Ser199/Ser204 [55, 165]. As mentioned previously, PAK1/βPIX binding does increase PAK1 activation and PAK1 can subsequently phosphorylate βPIX at Ser340; however, this phosphorylation does not regulate PAK1/βPIX interaction and the physiological relevance of this event has yet to be uncovered [190, 191]. The complex formation of PAK1 and βPIX is not sufficient to locate PAK1 at adhesion complexes. However, βPIX binds to GIT1 and GIT1 binds to paxillin thereby targeting this trimolecular complex to adhesions (Fig. 5.8; [168]). Once the GIT1/βPIX/PAK1 complex arrives at the adhesion complex, PAK1 can phosphorylate Ser273 on paxillin, increasing the affinity of GIT1 for paxillin and recruiting more GIT1/βPIX/PAK1 complexes to the adhesion [189]. At the same time, serine phosphorylation of paxillin reduces the affinity of FAK to paxillin, setting the stage for adhesion disassembly yet freeing FAK to facilitate the formation of new nascent adhesions [189]. Thus, PAK1 can facilitate adhesion turnover by means of its scaffolding and enzymatic activity.
Fig. 5.8.
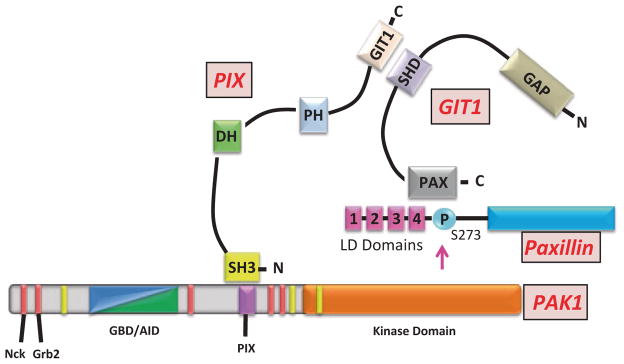
GIT1/βPIX/PAK1/paxillin complex. PAK1 binds to the N-terminal SH3-domain of βPIX. βPIX has a DBL-homology (DH) domain and a plextrin homology (PH) domain, and binds to GIT1 through a C-terminal GIT1-binding domain. GIT1 has a C-terminal paxillin-binding domain (PAX) that binds to the LD4 domain of paxillin and SHD domain that binds to βPIX. PAK1 can phosphorylate Ser273 of paxillin at adhesion complexes
We have recently shown that PRL-mediated tyrosyl phosphorylation of PAK1 regulates adhesion turnover. When breast cancer cells stably overexpressing either PAK1 WT or PAK1 Y3F were plated on collagen IV in the presence of PRL, PAK1 WT cells displayed a motile phenotype, while PAK1 Y3F cells were more round and well-spread (Hammer et.al., unpublished). Amount of cells adherent to collagen in the presence of PRL was also dependent on tyrosyl phosphorylated PAK1. We have demonstrated that PRL-induced tyrosyl phosphorylation of PAK1 facilitates PAK1/βPIX/GIT1 binding and the localization of PAK1 to small adhesion complexes. These data confirm that tyrosyl phosphorylation of PAK1 increases the ability for PAK1 to create protein–protein interactions. Furthermore, PRL/JAK2 induces kinase activity of pTyr-PAK1, therefore pTyr-PAK1 phosphorylates Ser273 on paxillin in response PRL to a greater extent than PAK1 Y3F mutant. Using phospho-specific antibodies directed to single phosphorylated tyrosines on PAK1, we identified Tyr285 as a site of PRL-dependent phosphorylation of PAK1 by JAK2. Our immunofluorescence analysis revealed that pTyr285-PAK1 localized to small adhesion complexes in the cells treated with PRL. Finally, we have performed time-lapse confocal fluorescence microscopy video recording of the cells treated with PRL. We have shown that PRL-mediated tyrosyl phosphorylation of PAK1 has a direct effect on the rate of adhesion turnover. Tyrosyl phosphorylated PAK1 increased the rates of both adhesion assembly and disassembly in breast cancer cells plated on collagen in response to PRL, while mutation of the single Tyr285 completely abolished the effect of PRL on adhesion turnover (Hammer et. al., unpublished).
We have proposed a model for PRL-dependent regulation of adhesion turnover that integrates our finding with previous studies (Fig. 5.9). PRL-activated JAK2 phosphorylates PAK1 on Tyr285, facilitating the formation of the GIT1/βPIX/pTyr-PAK1 complex and subsequent formation of new paxillin-containing focal complexes. At these complexes, pTyr-PAK1 phosphorylates paxillin at Ser273, recruiting more GIT1/βPIX/pTyr-PAK1 and releasing FAK from the focal complex. The accumulation of GIT1/βPIX/pTyr-PAK1 at the focal complexes facilitates both adhesion assembly and disassembly, thereby regulating cell motility.
Fig. 5.9.
PRL-dependent tyrosyl phosphorylation of PAK1 regulates adhesion turnover. PRL-activated JAK2 phosphorylates PAK1 at Tyr285 and stimulates both PAK1 activities: kinase activity and ability of PAK1 to form the GIT1/βPIX/pTyr285 PAK1 complex. This complex localizes to small adhesion complexes, the amount of which is increased by PRL treatment. Increased GIT1/βPIX/pTyr-285PAK1 association leads to enhanced phosphorylation of paxillin on Ser 273 that results in enhanced adhesion turnover and finally to increased cell motility
5.3.4 Role of PRL-Activated PAK1 in Breast Cancer Cell Invasion
Cells adhere to the ECM throughout most of their lifetime. The molecular composition of the ECM, ECM stiffness, specific association of multiple growth factors/cytokines with the matrix and “dimensionality” play major roles in the response of cells to their local matrix microenvironment [192].
The 3D matrix is a critical component of mammary tissue development not only under physiological but also in pathophysiological conditions. In vivo, women with dense mammary tissue, which is associated with increasing amount of collagen in the stroma of breast tissue are at 4–6 times greater risk of breast cancer as compared to those with no densities, and have a poor prognosis ([193–196]; reviewed in [197]). In vitro, mammary epithelial cells grown in 3D reconstituted basal membrane overlay cultures form spheroids with lumens (acini) that resemble secretory alveoli of normal mammary tissue. Increasing 3D matrix tension affects mammary cell morphogenesis and physiological functions [198–200]. Furthermore, reciprocal interactions between mammary epithelial cells, ECM and ECM remodeling enzymes are critical for development and differentiation during mammary gland development. Loss of this interaction leads to tumor progression (reviewed in [201]). It is now well documented that the interaction of cells with 2D substrates is significantly different than the more natural 3D environments that cells are embedded within in vivo [202–204]. Furthermore, the molecular composition of the ECM, specific association of multiple growth factors and cytokines with the matrix, along with the aforementioned “dimensionality” play roles in the responses of cells to their local matrix microenvironment [192]. Cells embedded in 3D matrix have higher amounts of ligated matrix-receptors as compared to the cells grown on the top of a thin film of matrix. Collagen receptors, such as integrins and discoidin domain receptors (DDRs), are signal transducting receptors. Integrin clustering initiates an array of signaling cascades, including activation of the Rho family of small GTPases, MAPKs, and PI3-kinases [205], and any of them can lead to regulation of matrix metalloproteinases (MMP) expression.
MMPs are a family of Zn2+-dependent enzymes composing 23 members. MMP-1 (collagenase 1) is a major proteinase of the MMP family that specifically degrades type I collagen, a major component of the ECM. It also degrades other fibrillar collagens of types II, III, VII, VIII, X, XI as well as gelatins, aggrecan, entactin, tenascin, and perlican [206–208]. As these collagen types are the most abundant proteins in the body, MMP-1 is critical for the modeling and remodeling of the ECM [209]. In clinical studies, increased MMP-1 expression is associated with the incidence or invasiveness of several cancers: colorectal, esophageal, pancreatic, gastric, breast, and malignant melanoma [210–215]. Increased MMP-1 expression is also associated with advanced stages of breast cancer and may be a predictive marker for the development of invasive disease [216]. MMP-3, or stromelysin 1, can degrade a variety of ECM substrates, such as type III, IV, V, VII, and IX–XI collagens, laminins, fibronectin, osteopontin, and proteoglycans. MMP-3 is expressed by stromal cells during normal mammary gland development and is strongly upregulated during postlactational mammary involution when considerable ECM remodeling and alveolar apoptosis occur. MMP-3 is upregulated in many breast tumors and contributes to cancer development. Indeed, mice overexpressing MMP-3 show excessive side branching and eventual tumor formation in the mammary gland ([217–219]; reviewed in [220–222]). MMP-3 induces EMT in mammary cells through cleavage of E-cadherin, expression of Rac1b and transcriptional factor Snail [223]. MMP-2 and MMP-9 are both type IV collagenases that contribute to tumor invasion in vitro because of their ability to break down basement membrane, degrading collagen IV in particular [224, 225]. Elevated circulating MMP-9 levels have been demonstrated in patients with breast cancer and MMP-2 and/or MMP-9 release has been associated with tumor invasion and metastasis ([226, 227]; reviewed in [228–231]). The expression of MMPs is regulated at the transcriptional and posttranscriptional levels (including the stability of mRNA and protein as well as the release and activation of protein) by a number of hormones, growth factors, and cytokines [232]. Despite efforts to discover the cellular pathways regulating MMPs, little is known as to how different cytokines cooperate with cytoskeletal proteins to regulate MMP expression.
PRL regulation of MMP expression and breast cancer cell invasiveness is complex. It has been shown that PRL-induced activation of MAPK/AP-1 pathway is inversely related to PRL-induced STAT5 activation [233–235]. Thus, PRL together with IGF-I promotes MMP-2 expression and cell invasion (AP-1 targets MMP-2 gene) [236]. In contrast, WT STAT5a overexpression inhibits MMP-2 transcription/activity while reduction of STAT5 by siRNA or inhibition of STAT5 activity increases the PRL-dependent transcription/activity of MMP-2 and invasiveness [235, 237]. T47 cells overexpressing degradation-resistant PRLR demonstrated increased proliferation and invasiveness while silencing of PRLR dramatically reduced the cell invasion and MMP-9 secretion [238]. In vivo, murine PRL-induced mammary carcinomas with lower level of pSTAT5 demonstrate higher level of MMP-9 expression [234]. Recently, these findings have been linked to the ECM stiffness [233]. Thus, in compliant 3D collagen I, PRL signaling has been shown to be mediated predominantly through the STAT5 pathway. This pathway results in prodifferentiation outcome with no MMP2 expression and absences of invasion. In contrast, in stiff collagen I, PRL signaling is mediated by Src/FAK and pERK 1/2 pathways resulting in MMP2 expression and enhanced invasion. Thus, increased stiffness of the ECM switches the signal in breast cancer cells from differentiation toward enhanced tumorigenic processes [233] PAK1 also plays a pivotal role in the regulation of cell transformation, invasion, and MMP secretion. Role of PAK1 in the EMT and breast and other cancers has been discussed above. Of particular interest, PAK1 plays a critical role in premalignant progression of MCF10 series of human breast epithelial cell lines grown in 3D reconstituted basal membrane overlay cultures [158]. Thus, PAK1 expression and activity increased with premalignant progression from normal mammary cells through hyperplasia, atypical hyperplasia, to ductal carcinoma while dominant-negative PAK1 or knock-down PAK1 reduced cell proliferation, migration, invasion, and pericellular proteolysis of collagen IV in these 3D cultures [158]. PAK1 has been also implicated in MMP regulation. TNFα-induced MMP-9 is mediated through PAK1 and JNK activation [239]. PAK1 regulated IL-1β-induced production and activity of MMP-13 and MMP-14 (MT1-MMP) in synoviocytes during inflammatory joint disease [240] as well as MMP-2 activity in ovarian cancer cells [241]. In addition, inhibition of Rac1 reduces MMP-1 expression [242].
We have recently implicated both PRL and PAK1 together with 3D collagen IV in the regulation of breast cancer invasion. PAK1 stimulates the PRL-dependent invasion of TMX2–28 cells (highly invasive ER-negative clone of MCF-7 cells) through Matrigel. We have shown that TMX2–28 cells stably overexpressing PAK1 WT have upregulated expression and secretion of MMP-1, -2, and -3 when they grow in 3D collagen IV, which makes up 31 % of Matrigel protein composition. PRL-induced PAK1 tyrosyl phosphorylation leads to a further increase in MMP-1 and MMP-3 expression and cell invasion in MAPK-dependent manner.
Why have we seen these effects only in 3D collagen IV? Different collagens regulate expression of different MMPs. Collagen I induces expression/secretion of MMP-1 and MMP-9 ([239, 243–247], while collagen IV upregulates expression of MMPs 2 and 9 [248]. MMP-2 is often constitutively expressed and controlled through a unique mechanism of enzyme activation. MT1-MMP (MMP-14)-mediated activation of pro-MMP-2 is upregulated by 3D collagen I in endothelial cells [249–251], fibroblasts [252–254], and cancer cell lines [255–259].
Embedding cells in a 3D matrix can amplify signals from ligated integrins/DDRs and lead to MMP expression. Furthermore, there are numerous reports of “cross talk” and “synergy” between signaling by ECM receptors and by various growth factors and cytokines. Such cross talk involves cooperation in the downstream signal transduction pathways.
Another possible explanation of how 3D collagen results in elevated expression of MMPs as compared to 2D collagen relates to the physical properties of 3D matrixes. ECM physical properties often refer to rigidity, porosity, insolubility, topography, and other characteristics that are essential for its scaffolding role in supporting tissue structure and integrity, and for its role in migration and anchorage of the cell (reviewed in [205, 208]). The aforementioned recent paper from Dr. Schuller’s lab has evidently demonstrated the role of 3D collagen I stiffness in the production of MMP-2 [233].
The actin cytoskeleton appears to be the major cellular system for transduction of force generated by the external network. Cytoskeletal stretching correlates with the recruitment of adhesion complex proteins and triggers signals resulting in the induction of a matrix-degrading protease (reviewed in [260, 261]). This may explain our data demonstrated that 3D collagen I induces expression of only MMP-9 while 3D collagen IV upregulates expression of MMP-1, 2, and 3 but not MMP-9 [160]. Collagen I is a fibril-forming collagen while collagen IV is a network forming collagen. We can speculate that cells embedded in the network formed by 3D collagen IV, but not collagen I, can sense geometry and the external force generated by this network. We speculate that, in addition to the ligation of different receptors, physical properties of the 3D collagen IV network activate cytoskeletal-triggered signaling pathways that are distinct from those activated by 3D fibrillar collagen I that results in induction of distinct MMPs.
Another possible explanation of how 3D collagens can induce MMPs expression is the observation that the ECM acts as a “sink” or “reservoir” for growth factors/cytokines. Indeed, the ECM is essential for shaping the concentration gradient for many growth factors, including bone morphogenetic protein, fibroblasts growth factor, Hedgehog, and Wnts [208, 262, 263]. We can speculate that the 3D collagen IV network retains PRL to a better extent than 3D fibrillar collagen I or 2D collagens, therefore leading to amplified PRL signal which leads to MMP-1 and -3 productions.
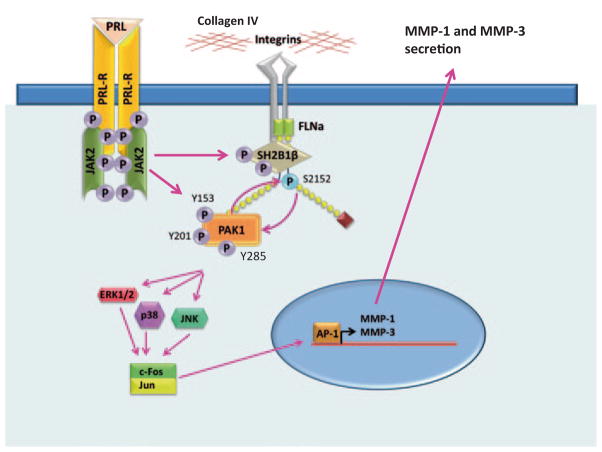
We have hypothesized that contact with 3D collagen IV may be an important invasive stimulus for breast cancer cells (Fig. 5.10). Mammary cells are normally surrounded by basement membrane, comprised mostly of type IV collagen. In normal cells, signals from collagen IV do not induce MMP expression. In contrast, in breast cancer cells PRL initiates the JAK2-dependent tyrosyl phosphorylation of PAK1, increasing PAK1 signaling. Importantly, PAK1 expression is also elevated in breast cancer [87]. Filamin A can serve as a bridge between activated integrins and pTyr-PAK1 to integrate signals from cytokines (PRL) and the ECM (collagen IV). PAK1 activates Erk 1/2, p38 MAPK, and JNK 1/2, each of which can activate AP-1. Genes encoding MMP-1 and -3 have an AP-1 binding site supporting the transcription of these MMPs after induction by PAK1. MMP-1 degrades type I collagen, which is a major component of the ECM and MMP-3 degrades collagen IV which is a main component of basement membrane. We have also shown that secretion of MMP-1 and -3 is required for PRL-dependent invasion [160]. Given the complexity of these signaling cascades it is likely that additional signaling molecules are also involved in the modulation of MMP expression.
Fig. 5.10.
PRL-dependent tyrosyl phopshorylated PAK1 and three-dimensional (3D) collagen IV regulate MMP-1 and MMP-3 production and invasion via MAPK pathways. PRL-activation of JAK2 leads to tyrosyl phosphorylation of PAK1 on tyrosines 153, 201, and 285, thereby increasing PAK1 activities and stimulating phosphorylation of FLNa. Phosphorylated FLNa stimulates the kinase activity of PAK1 in a positive feedback loop. In turn, FLNa binds to β-integrin and transduces signals from surrounding matrix to inside of a cell. 3D collagen IV-induced signals, in combination with pTyr-PAK1, produce intense synergistic increases in MMP-1 and MMP-3 production via MAPK pathways. MMP-1 degrades type I collagen, which is a major component of the ECM and MMP-3 degrades collagen IV which is a main component of basement membrane resulting in increased invasion of breast cancer cells in response to PRL
5.4 Conclusion and Future Directions
PRL binding to the PRLR induces receptor dimerization and activation of the non-receptor tyrosine kinase JAK2. Activated JAK2 phosphorylates the serine/threonine kinase PAK1 on three tyrosines 153, 201, and 285. This tyrosyl phosphorylation of PAK1 enhances such important PAK1 functions as kinase activity and the ability to form protein–protein interactions. Both of these PAK1 activities are important for adhesion, motility, and invasion of breast cancer cells in response to PRL. During cell adhesion, PRL promotes formation of the GIT1/βPIX/pTyr285-PAK1 complex. This complex localizes to small adhesion sites (adhesion complexes), the amount of which is increased by PRL treatment. In these small adhesion complexes at cell periphery PAK1 phosphorylates serine 273 on paxillin that results in enhanced adhesion turnover. Enhanced adhesion turnover facilitates cell motility and, indeed, PRL stimulates breast cancer cell motility.
In addition to GIT1/βPIX/pTyr285 PAK1-dependent mechanism, we implicated actin-binding protein filamin A in the regulation of cell motility. Tyrosyl phosphorylation of PAK1 in response to PRL increases PAK1/FLNa interaction, and subsequent serine phosphorylation and activation of FLNa. Phosphorylated FLNa stimulates the kinase activity of PAK1 and has increased actin-regulating activity. FLNa directly binds to adapter protein SH2B1β (which is tyrosyl phosphorylated by JAK2 in response to PRL), relocates SH2B1β to the JAK2-PAK1-FLNa complex. Since SH2B1β is the enhancer of the kinase activity of JAK2, the formation of the complex results in enhancement of JAK2 activation and further activation of the JAK2-PAK1-FLNa complex that leads to actin cytoskeleton reorganization via actin-regulating proteins PAK1, FLNa, and SH2B1β, which has two actin-binding sites and cross-links actin filaments [169, 180].
PRL-induced pTyr-PAK1 also activates MAPK pathways, leading to the expression and secretion of MMP-1 and MMP-3 in response to 3D collagen IV microenvironment. MMP-1 degrades type I collagen, which is a major component of the ECM and MMP-3 degrades collagen IV which is a main component of basement membrane resulting in increased invasion of breast cancer cells in response to PRL.
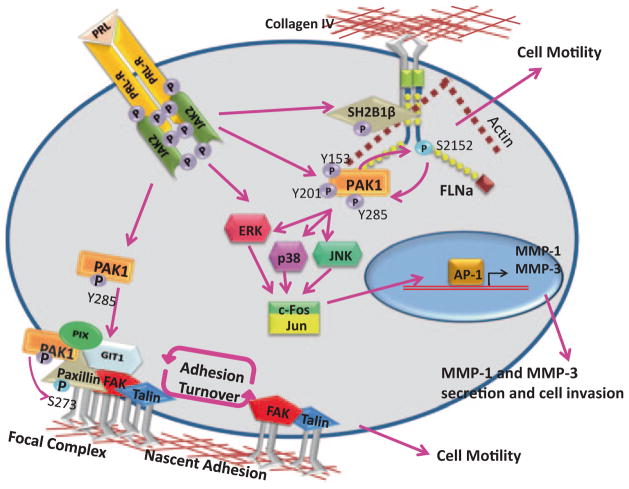
PAK1 is important for a variety of fundamentally different cellular processes therefore it is critical to understand how PAK1 functions are controlled. The role of PAK1 tyrosyl phosphorylation is incompletely understood and there are only a few publications in this field although PAK1 is ubiquitously expressed, subject to growth factors, cytokine and hormone regulation and participates in various cellular functions. Fundamental questions whether PRL-dependent regulation of PAK1 also plays a critical role in normal mammary gland development, growth, and differentiation also remain (Fig. 5.11).
Fig. 5.11.
PRL-dependent tyrosyl phosphorylated PAK1 regulates breast cancer cell motility and invasion
Acknowledgments
This work was supported by a Grant from the National Institutes of Health (R01 DK88127 to MD).
References
- 1.Stricker P, Grueter R. Action of the anterior lobe of the pituitary gland on lactation. Compt Rend Soc Biol. 1928;99:1978–1980. [Google Scholar]
- 2.Riddle O, Bates R, Dykshorn S. The presentation, identification and assay of Prolactin-A hormone of the anterior pituitary. Am J Physiol. 1933;105:191–216. [Google Scholar]
- 3.Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. Embo J. 1997;16:6926–6935. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- 5.Lyons WR. Hormonal synergism in mammary growth. Proc R Soc Lond B Biol Sci. 1958;149:303–325. doi: 10.1098/rspb.1958.0071. [DOI] [PubMed] [Google Scholar]
- 6.Reece R, Leathem J. Growth of mammary glands of hypophysectomized rats following estrogen and lactogen administration. Exp Biol Med. 1945;59:122–124. [Google Scholar]
- 7.Boutin JM, Jolicoeur C, Okamura H, Gagnon J, Edery M, Shirota M, Banville D, Dusanter-Fourt I, Djiane J, Kelly PA. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988;53:69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- 8.Bazan JF. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor beta-chain. Biochem Biophys Res Commun. 1989;164:788–795. doi: 10.1016/0006-291x(89)91528-3. [DOI] [PubMed] [Google Scholar]
- 9.Rui H, Kirken RA, Farrar WL. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem. 1994;269:5364–5368. [PubMed] [Google Scholar]
- 10.Campbell GS, Argetsinger LS, Ihle JN, Kelly PA, Rillema JA, Carter-Su C. Activation of JAK2 tyrosine kinase by prolactin receptors in Nb2 cells and mouse mammary gland explants. Proc Natl Acad Sci U S A. 1994;91:5232–5236. doi: 10.1073/pnas.91.12.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ihle JN, Witthuhn B, Tang B, Yi T, Quelle FW. Cytokine receptors and signal transduction. Baillieres Clin Haematol. 1994;7:17–48. doi: 10.1016/s0950-3536(05)80005-8. [DOI] [PubMed] [Google Scholar]
- 12.Rui H, Lebrun JJ, Kirken RA, Kelly PA, Farrar WL. JAK2 activation and cell proliferation induced by antibody-mediated prolactin receptor dimerization. Endocrinology. 1994;135:1299–1306. doi: 10.1210/endo.135.4.7925093. [DOI] [PubMed] [Google Scholar]
- 13.Lebrun JJ, Ali S, Sofer L, Ullrich A, Kelly PA. Prolactin-induced proliferation of Nb2 cells involves tyrosine phosphorylation of the prolactin receptor and its associated tyrosine kinase JAK2. J Biol Chem. 1994;269:14021–14026. [PubMed] [Google Scholar]
- 14.Feng J, Witthuhn BA, Matsuda T, Kohlhuber F, Kerr IM, Ihle JN. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol Cell Biol. 1997;17:2497–2501. doi: 10.1128/mcb.17.5.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouilleux F, Wakao H, Mundt M, Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. Embo J. 1994;13:4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakao H, Gouilleux F, Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. Embo J. 1994;13:2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DaSilva L, Rui H, Erwin RA, Howard OM, Kirken RA, Malabarba MG, Hackett RH, Larner AC, Farrar WL. Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515 and TYR580. Mol Cell Endocrinol. 1996;117:131–140. doi: 10.1016/0303-7207(95)03738-1. [DOI] [PubMed] [Google Scholar]
- 18.Das R, Vonderhaar BK. Involvement of SHC, GRB2, SOS and RAS in prolactin signal transduction in mammary epithelial cells. Oncogene. 1996;13:1139–1145. [PubMed] [Google Scholar]
- 19.Das R, Vonderhaar BK. Activation of raf-1, MEK, and MAP kinase in prolactin responsive mammary cells. Breast Cancer Res Treat. 1996;40:141–149. doi: 10.1007/BF01806209. [DOI] [PubMed] [Google Scholar]
- 20.Waters SB, Rillema JA. Role of protein kinase C in the prolactin-induced responses in mouse mammary gland explants. Mol Cell Endocrinol. 1989;63:159–166. doi: 10.1016/0303-7207(89)90092-0. [DOI] [PubMed] [Google Scholar]
- 21.Berlanga JJ, Gualillo O, Buteau H, Applanat M, Kelly PA, Edery M. Prolactin activates tyrosyl phosphorylation of insulin receptor substrate 1 and phosphatidylinositol-3-OH kinase. J Biol Chem. 1997;272:2050–2052. doi: 10.1074/jbc.272.4.2050. [DOI] [PubMed] [Google Scholar]
- 22.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 23.Maus MV, Reilly SC, Clevenger CV. Prolactin as a chemoattractant for human breast carcinoma. Endocrinology. 1999;140:5447–5450. doi: 10.1210/endo.140.11.7245. [DOI] [PubMed] [Google Scholar]
- 24.Kline JB, Moore DJ, Clevenger CV. Activation and association of the Tec tyrosine kinase with the human prolactin receptor: mapping of a Tec/Vav1-receptor binding site. Mol Endocrinol. 2001;15:832–841. doi: 10.1210/mend.15.5.0631. [DOI] [PubMed] [Google Scholar]
- 25.Akhtar N, Streuli CH. Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. J Cell Biol. 2006;173:781–793. doi: 10.1083/jcb.200601059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aksamitiene E, Achanta S, Kolch W, Kholodenko BN, Hoek JB, Kiyatkin A. Prolactin-stimulated activation of ERK1/2 mitogen-activated protein kinases is controlled by PI3-kinase/Rac/PAK signaling pathway in breast cancer cells. Cell Signal. 2011;23:1794–1805. doi: 10.1016/j.cellsig.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller SL, DeMaria JE, Freier DO, Riegel AM, Clevenger CV. Novel association of Vav2 and Nek3 modulates signaling through the human prolactin receptor. Mol Endocrinol. 2005;19:939–949. doi: 10.1210/me.2004-0443. [DOI] [PubMed] [Google Scholar]
- 28.Miller SL, Antico G, Raghunath PN, Tomaszewski JE, Clevenger CV. Nek3 kinase regulates prolactin-mediated cytoskeletal reorganization and motility of breast cancer cells. Oncogene. 2007;26:4668–4678. doi: 10.1038/sj.onc.1210264. [DOI] [PubMed] [Google Scholar]
- 29.Hammer A, Rider L, Oladimeji P, Cook L, Li Q, Mattingly RR, Diakonova M. Tyrosyl phosphorylated PAK1 regulates breast cancer cell motility in response to prolactin through filamin A. Mol Endocrinol. 2013;27:455–465. doi: 10.1210/me.2012-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaidel-Bar R, Geiger B. The switchable integrin adhesome. J Cell Sci. 2010;123:1385–1388. doi: 10.1242/jcs.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J. 2009;418:491–506. doi: 10.1042/BJ20081948. [DOI] [PubMed] [Google Scholar]
- 32.Muschler J, Streuli CH. Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harb Perspect Biol. 2010;2:a003202. doi: 10.1101/cshperspect.a003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rooney N, Streuli CH. How integrins control mammary epithelial differentiation: a possible role for the ILK-PINCH-Parvin complex. FEBS Lett. 2011;585:1663–1672. doi: 10.1016/j.febslet.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Shiu RP, Paterson JA. Alteration of cell shape, adhesion, and lipid accumulation in human breast cancer cells (T-47D) by human prolactin and growth hormone. Cancer Res. 1984;44:1178–1186. [PubMed] [Google Scholar]
- 35.Canbay E, Norman M, Kilic E, Goffin V, Zachary I. Prolactin stimulates the JAK2 and focal adhesion kinase pathways in human breast carcinoma T47-D cells. Biochem J. 1997;324(Pt 1):231–236. doi: 10.1042/bj3240231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123:1007–1013. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- 37.Acosta JJ, Munoz RM, Gonzalez L, Subtil-Rodriguez A, Dominguez-Caceres MA, Garcia-Martinez JM, Calcabrini A, Lazaro-Trueba I, Martin-Perez J. Src mediates prolactin-dependent proliferation of T47D and MCF7 cells via the activation of focal adhesion kinase/Erk1/2 and phosphatidylinositol 3-kinase pathways. Mol Endocrinol. 2003;17:2268–2282. doi: 10.1210/me.2002-0422. [DOI] [PubMed] [Google Scholar]
- 38.Galbaugh T, Feeney YB, Clevenger CV. Prolactin receptor-integrin cross-talk mediated by SIRPalpha in breast cancer cells. Mol Cancer Res. 2010;8:1413–1424. doi: 10.1158/1541-7786.MCR-10-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holtkamp W, Nagel GA, Wander HE, Rauschecker HF, von Heyden D. Hyperprolactinemia is an indicator of progressive disease and poor prognosis in advanced breast cancer. Int J Cancer. 1984;34:323–328. doi: 10.1002/ijc.2910340307. [DOI] [PubMed] [Google Scholar]
- 40.Bhatavdekar JM, Shah NG, Balar DB, Patel DD, Bhaduri A, Trivedi SN, Karelia NH, Ghosh N, Shukla MK, Giri DD. Plasma prolactin as an indicator of disease progression in advanced breast cancer. Cancer. 1990;65:2028–2032. doi: 10.1002/1097-0142(19900501)65:9<2028::aid-cncr2820650924>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Mujagic Z, Mujagic H. Importance of serum prolactin determination in metastatic breast cancer patients. Croat Med J. 2004;45:176–180. [PubMed] [Google Scholar]
- 42.Harbaum L, Pollheimer MJ, Bauernhofer T, Kornprat P, Lindtner RA, Schlemmer A, Rehak P, Langner C. Clinicopathological significance of prolactin receptor expression in colorectal carcinoma and corresponding metastases. Mod Pathol. 2010;23:961–971. doi: 10.1038/modpathol.2010.83. [DOI] [PubMed] [Google Scholar]
- 43.Liby K, Neltner B, Mohamet L, Menchen L, Ben-Jonathan N. Prolactin overexpression by MDA-MB-435 human breast cancer cells accelerates tumor growth. Breast Cancer Res Treat. 2003;79:241–252. doi: 10.1023/a:1023956223037. [DOI] [PubMed] [Google Scholar]
- 44.Rider L, Shatrova A, Feener EP, Webb L, Diakonova M. JAK2 tyrosine kinase phosphorylates PAK1 and regulates PAK1 activity and functions. J Biol Chem. 2007;282:30985–30996. doi: 10.1074/jbc.M701794200. [DOI] [PubMed] [Google Scholar]
- 45.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 46.Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 47.Pirruccello M, Sondermann H, Pelton JG, Pellicena P, Hoelz A, Chernoff J, Wemmer DE, Kuriyan J. A dimeric kinase assembly underlying autophosphorylation in the p21 activated kinases. J Mol Biol. 2006;361:312–326. doi: 10.1016/j.jmb.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Parrini MC, Camonis J, Matsuda M, de Gunzburg J. Dissecting activation of the PAK1 kinase at protrusions in living cells. J Biol Chem. 2009;284:24133–24143. doi: 10.1074/jbc.M109.015271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King CC, Gardiner EM, Zenke FT, Bohl BP, Newton AC, Hemmings BA, Bokoch GM. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1) J Biol Chem. 2000;275:41201–41209. doi: 10.1074/jbc.M006553200. [DOI] [PubMed] [Google Scholar]
- 50.Bokoch GM, Reilly AM, Daniels RH, King CC, Olivera A, Spiegel S, Knaus UG. A GTPase-independent mechanism of p21-activated kinase activation. Regulation by sphingosine and other biologically active lipids. J Biol Chem. 1998;273:8137–8144. doi: 10.1074/jbc.273.14.8137. [DOI] [PubMed] [Google Scholar]
- 51.Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol. 2002;4:681–690. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- 52.Maceyka M, Alvarez SE, Milstien S, Spiegel S. Filamin A links sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 at lamellipodia to orchestrate cell migration. Mol Cell Biol. 2008;28:5687–5697. doi: 10.1128/MCB.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang Y, Zhou H, Chen A, Pittman RN, Field J. The Akt proto-oncogene links Ras to Pak and cell survival signals. J Biol Chem. 2000;275:9106–9109. doi: 10.1074/jbc.275.13.9106. [DOI] [PubMed] [Google Scholar]
- 54.Zhou GL, Zhuo Y, King CC, Fryer BH, Bokoch GM, Field J. Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol Cell Biol. 2003;23:8058–8069. doi: 10.1128/MCB.23.22.8058-8069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 56.Daniels RH, Hall PS, Bokoch GM. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. Embo J. 1998;17:754–764. doi: 10.1093/emboj/17.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagheri-Yarmand R, Mandal M, Taludker AH, Wang RA, Vadlamudi RK, Kung HJ, Kumar R. Etk/Bmx tyrosine kinase activates Pak1 and regulates tumorigenicity of breast cancer cells. J Biol Chem. 2001;276:29403–29409. doi: 10.1074/jbc.M103129200. [DOI] [PubMed] [Google Scholar]
- 58.Wang RA, Zhang H, Balasenthil S, Medina D, Kumar R. PAK1 hyperactivation is sufficient for mammary gland tumor formation. Oncogene. 2006;25:2931–2936. doi: 10.1038/sj.onc.1209309. [DOI] [PubMed] [Google Scholar]
- 59.Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003;12:841–849. doi: 10.1016/s1097-2765(03)00382-4. [DOI] [PubMed] [Google Scholar]
- 60.Alahari SK, Reddig PJ, Juliano RL. The integrin-binding protein Nischarin regulates cell migration by inhibiting PAK. EMBO J. 2004;23:2777–2788. doi: 10.1038/sj.emboj.7600291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Talukder AH, Meng Q, Kumar R. CRIPak, a novel endogenous Pak1 inhibitor. Oncogene. 2006;25:1311–1319. doi: 10.1038/sj.onc.1209172. [DOI] [PubMed] [Google Scholar]
- 62.Xia C, Ma W, Stafford LJ, Marcus S, Xiong WC, Liu M. Regulation of the p21-activated kinase (PAK) by a human Gbeta -like WD-repeat protein, hPIP1. Proc Natl Acad Sci U S A. 2001;98:6174–6179. doi: 10.1073/pnas.101137298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- 64.Koh CG, Tan EJ, Manser E, Lim L. The p21-activated kinase PAK is negatively regulated by POPX1 and POPX2, a pair of serine/threonine phosphatases of the PP2 C family. Curr Biol. 2002;12:317–321. doi: 10.1016/s0960-9822(02)00652-8. [DOI] [PubMed] [Google Scholar]
- 65.Chen S, Yin X, Zhu X, Yan J, Ji S, Chen C, Cai M, Zhang S, Zong H, Hu Y, Yuan Z, Shen Z, Gu J. The C-terminal kinase domain of the p34cdc2-related PITSLRE protein kinase (p110 C) associates with p21-activated kinase 1 and inhibits its activity during anoikis. J Biol Chem. 2003;278:20029–20036. doi: 10.1074/jbc.M300818200. [DOI] [PubMed] [Google Scholar]
- 66.Frost JA, Khokhlatchev A, Stippec S, White MA, Cobb MH. Differential effects of PAK1-activating mutations reveal activity-dependent and -independent effects on cytoskeletal regulation. J Biol Chem. 1998;273:28191–28198. doi: 10.1074/jbc.273.43.28191. [DOI] [PubMed] [Google Scholar]
- 67.Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol. 1999;145:837–849. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adam L, Vadlamudi R, Mandal M, Chernoff J, Kumar R. Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J Biol Chem. 2000;275:12041–12050. doi: 10.1074/jbc.275.16.12041. [DOI] [PubMed] [Google Scholar]
- 70.Sundberg-Smith LJ, Doherty JT, Mack CP, Taylor JM. Adhesion stimulates direct PAK1/ERK2 association and leads to ERK-dependent PAK1 Thr212 phosphorylation. J Biol Chem. 2005;280:2055–2064. doi: 10.1074/jbc.M406013200. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, Fu M, Wang L, Liu J, Li Y, Brakebusch C, Mei Q. p21-activated kinase 1 (PAK1) can promote ERK activation in a kinase-independent manner. J Biol Chem. 2013;288:20093–20099. doi: 10.1074/jbc.M112.426023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coles LC, Shaw PE. PAK1 primes MEK1 for phosphorylation by Raf-1 kinase during cross-cascade activation of the ERK pathway. Oncogene. 2002;21:2236–2244. doi: 10.1038/sj.onc.1205302. [DOI] [PubMed] [Google Scholar]
- 73.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 74.Kohn AD, Takeuchi F, Roth RA. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem. 1996;271:21920–21926. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- 75.Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol. 2008;10:1356–1364. doi: 10.1038/ncb1795. [DOI] [PubMed] [Google Scholar]
- 76.Thullberg M, Gad A, Beeser A, Chernoff J, Stromblad S. The kinase-inhibitory domain of p21-activated kinase 1 (PAK1) inhibits cell cycle progression independent of PAK1 kinase activity. Oncogene. 2007;26:1820–1828. doi: 10.1038/sj.onc.1209983. [DOI] [PubMed] [Google Scholar]
- 77.Tao J, Oladimeji P, Rider L, Diakonova M. PAK1-Nck regulates cyclin D1 promoter activity in response to prolactin. Mol Endocrinol. 2011;25:1565–1578. doi: 10.1210/me.2011-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mira JP, Benard V, Groffen J, Sanders LC, Knaus UG. Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc Natl Acad Sci U S A. 2000;97:185–189. doi: 10.1073/pnas.97.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang RA, Mazumdar A, Vadlamudi RK, Kumar R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. Embo J. 2002;21:5437–5447. doi: 10.1093/emboj/cdf543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, Chernoff J, Hung MC, Kumar R. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem. 2000;275:36238–36244. doi: 10.1074/jbc.M002138200. [DOI] [PubMed] [Google Scholar]
- 81.Li F, Adam L, Vadlamudi RK, Zhou H, Sen S, Chernoff J, Mandal M, Kumar R. p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells. EMBO Rep. 2002;3:767–773. doi: 10.1093/embo-reports/kvf157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD. Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc Natl Acad Sci U S A. 1998;95:7480–7484. doi: 10.1073/pnas.95.13.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell. 2000;5:905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- 84.Vadlamudi RK, Barnes CJ, Rayala S, Li F, Balasenthil S, Marcus S, Goodson HV, Sahin AA, Kumar R. p21-activated kinase 1 regulates microtubule dynamics by phosphorylating tubulin cofactor B. Mol Cell Biol. 2005;25:3726–3736. doi: 10.1128/MCB.25.9.3726-3736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hannak E, Kirkham M, Hyman AA, Oegema K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J Cell Biol. 2001;155:1109–1116. doi: 10.1083/jcb.200108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell. 2005;20:237–249. doi: 10.1016/j.molcel.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 87.Balasenthil S, Sahin AA, Barnes CJ, Wang RA, Pestell RG, Vadlamudi RK, Kumar R. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J Biol Chem. 2004;279:1422–1428. doi: 10.1074/jbc.M309937200. [DOI] [PubMed] [Google Scholar]
- 88.Balasenthil S, Barnes CJ, Rayala SK, Kumar R. Estrogen receptor activation at serine 305 is sufficient to upregulate cyclin D1 in breast cancer cells. FEBS Lett. 2004;567:243–247. doi: 10.1016/j.febslet.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 89.Mazumdar A, Kumar R. Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS Lett. 2003;535:6–10. doi: 10.1016/s0014-5793(02)03846-2. [DOI] [PubMed] [Google Scholar]
- 90.Frost JA, Steen H, Shapiro P, Lewis T, Ahn N, Shaw PE, Cobb MH. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. Embo J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang Y, Chen Z, Ambrose D, Liu J, Gibbs JB, Chernoff J, Field J. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol Cell Biol. 1997;17:4454–4464. doi: 10.1128/mcb.17.8.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beeser A, Jaffer ZM, Hofmann C, Chernoff J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J Biol Chem. 2005;280:36609–36615. doi: 10.1074/jbc.M502306200. [DOI] [PubMed] [Google Scholar]
- 93.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 94.Schurmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, Bokoch GM. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol. 2000;20:453–461. doi: 10.1128/mcb.20.2.453-461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jin S, Zhuo Y, Guo W, Field J. p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. J Biol Chem. 2005;280:24698–24705. doi: 10.1074/jbc.M413374200. [DOI] [PubMed] [Google Scholar]
- 96.Wolf D, Witte V, Laffert B, Blume K, Stromer E, Trapp S, d’Aloja P, Schurmann A, Baur AS. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat Med. 2001;7:1217–1224. doi: 10.1038/nm1101-1217. [DOI] [PubMed] [Google Scholar]
- 97.Vadlamudi RK, Bagheri-Yarmand R, Yang Z, Balasenthil S, Nguyen D, Sahin AA, den Hollander P, Kumar R. Dynein light chain 1, a p21-activated kinase 1-interacting substrate, promotes cancerous phenotypes. Cancer Cell. 2004;5:575–585. doi: 10.1016/j.ccr.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 98.Puthalakath H, Huang DC, O’Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 99.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 100.Frost JA, Swantek JL, Stippec S, Yin MJ, Gaynor R, Cobb MH. Stimulation of NFkappa B activity by multiple signaling pathways requires PAK1. J Biol Chem. 2000;275:19693–19699. doi: 10.1074/jbc.M909860199. [DOI] [PubMed] [Google Scholar]
- 101.Dadke D, Fryer BH, Golemis EA, Field J. Activation of p21-activated kinase 1-nuclear factor kappaB signaling by Kaposi’s sarcoma-associated herpes virus G protein-coupled receptor during cellular transformation. Cancer Res. 2003;63:8837–8847. [PubMed] [Google Scholar]
- 102.Friedland JC, Lakins JN, Kazanietz MG, Chernoff J, Boettiger D, Weaver VM. alpha6beta4 integrin activates Rac-dependent p21-activated kinase 1 to drive NF-kappaB-dependent resistance to apoptosis in 3D mammary acini. J Cell Sci. 2007;120:3700–3712. doi: 10.1242/jcs.03484. [DOI] [PubMed] [Google Scholar]
- 103.Foryst-Ludwig A, Naumann M. p21-activated kinase 1 activates the nuclear factor kappa B (NF-kappa B)-inducing kinase-Ikappa B kinases NF-kappa B pathway and proinflammatory cytokines in Helicobacter pylori infection. J Biol Chem. 2000;275:39779–39785. doi: 10.1074/jbc.M007617200. [DOI] [PubMed] [Google Scholar]
- 104.Sells MA, Pfaff A, Chernoff J. Temporal and spatial distribution of activated Pak1 in fibroblasts. J Cell Biol. 2000;151:1449–1458. doi: 10.1083/jcb.151.7.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J Cell Biol. 1997;138:1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lappalainen P, Drubin DG. Cofilin promotes rapid actin filament turnover in vivo. Nature. 1997;388:78–82. doi: 10.1038/40418. [DOI] [PubMed] [Google Scholar]
- 107.Aizawa H, Sutoh K, Yahara I. Overexpression of cofilin stimulates bundling of actin filaments, membrane ruffling, and cell movement in Dictyostelium. J Cell Biol. 1996;132:335–344. doi: 10.1083/jcb.132.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 109.Delorme V, Machacek M, DerMardirossian C, Anderson KL, Wittmann T, Hanein D, Waterman-Storer C, Danuser G, Bokoch GM. Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev Cell. 2007;13:646–662. doi: 10.1016/j.devcel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep. 2004;5:154–160. doi: 10.1038/sj.embor.7400079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ikebe M, Hartshorne DJ. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J Biol Chem. 1985;260:10027–10031. [PubMed] [Google Scholar]
- 112.Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 113.Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK) J Muscle Res Cell Motil. 1998;19:839–854. doi: 10.1023/a:1005417926585. [DOI] [PubMed] [Google Scholar]
- 114.Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kiosses WB, Daniels RH, Otey C, Bokoch GM, Schwartz MA. A role for p21-activated kinase in endothelial cell migration. J Cell Biol. 1999;147:831–844. doi: 10.1083/jcb.147.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.del Pozo MA, Price LS, Alderson NB, Ren XD, Schwartz MA. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 2000;19:2008–2014. doi: 10.1093/emboj/19.9.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Manser E, Huang HY, Loo TH, Chen XQ, Dong JM, Leung T, Lim L. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao ZS, Manser E, Chen XQ, Chong C, Leung T, Lim L. A conserved negative regulatory region in alphaPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Delorme-Walker VD, Peterson JR, Chernoff J, Waterman CM, Danuser G, DerMardirossian C, Bokoch GM. Pak1 regulates focal adhesion strength, myosin IIA distribution, and actin dynamics to optimize cell migration. J Cell Biol. 2011;193:1289–1303. doi: 10.1083/jcb.201010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu F, Jia L, Thompson-Baine AM, Puglise JM, Ter Beest MB, Zegers MM. Cadherins and Pak1 control contact inhibition of proliferation by Pak1-betaPIX-GIT complex-dependent regulation of cell-matrix signaling. Mol Cell Biol. 2010;30:1971–1983. doi: 10.1128/MCB.01247-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith SD, Jaffer ZM, Chernoff J, Ridley AJ. PAK1-mediated activation of ERK1/2 regulates lamellipodial dynamics. J Cell Sci. 2008;121:3729–3736. doi: 10.1242/jcs.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol. 2006;172:759–769. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 124.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 125.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 126.Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- 127.Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail’s subcellular localization and functions. Cancer Res. 2005;65:3179–3184. doi: 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
- 128.Lozano E, Frasa MA, Smolarczyk K, Knaus UG, Braga VM. PAK is required for the disruption of E-cadherin adhesion by the small GTPase Rac. J Cell Sci. 2008;121:933–938. doi: 10.1242/jcs.016121. [DOI] [PubMed] [Google Scholar]
- 129.Nola S, Daigaku R, Smolarczyk K, Carstens M, Martin-Martin B, Longmore G, Bailly M, Braga VM. Ajuba is required for Rac activation and maintenance of E-cadherin adhesion. J Cell Biol. 2011;195:855–871. doi: 10.1083/jcb.201107162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tay HG, Ng YW, Manser E. A vertebrate-specific Chp-PAK-PIX pathway maintains E-cadherin at adherens junctions during zebrafish epiboly. PLoS One. 2010;5:e10125. doi: 10.1371/journal.pone.0010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barnes CJ, Bagheri-Yarmand R, Mandal M, Yang Z, Clayman GL, Hong WK, Kumar R. Suppression of epidermal growth factor receptor, mitogen-activated protein kinase, and Pak1 pathways and invasiveness of human cutaneous squamous cancer cells by the tyrosine kinase inhibitor ZD1839 (Iressa) Mol Cancer Ther. 2003;2:345–351. [PubMed] [Google Scholar]
- 132.He H, Shulkes A, Baldwin GS. PAK1 interacts with beta-catenin and is required for the regulation of the beta-catenin signalling pathway by gastrins. Biochim Biophys Acta. 2008;1783:1943–1954. doi: 10.1016/j.bbamcr.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 133.Zhu G, Wang Y, Huang B, Liang J, Ding Y, Xu A, Wu W. A Rac1/PAK1 cascade controls beta-catenin activation in colon cancer cells. Oncogene. 2012;31:1001–1012. doi: 10.1038/onc.2011.294. [DOI] [PubMed] [Google Scholar]
- 134.He H, Huynh N, Liu KH, Malcontenti-Wilson C, Zhu J, Christophi C, Shulkes A, Baldwin GS. P-21 activated kinase 1 knockdown inhibits beta-catenin signalling and blocks colorectal cancer growth. Cancer Lett. 2012;317:65–71. doi: 10.1016/j.canlet.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 135.Lv Z, Hu M, Zhen J, Lin J, Wang Q, Wang R. Rac1/PAK1 signaling promotes epithelial-mesenchymal transition of podocytes in vitro via triggering beta-catenin transcriptional activity under high glucose conditions. Int J Biochem Cell Biol. 2013;45:255–264. doi: 10.1016/j.biocel.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 136.Chen L, Necela BM, Su W, Yanagisawa M, Anastasiadis PZ, Fields AP, Thompson EA. Peroxisome proliferator-activated receptor gamma promotes epithelial to mesenchymal transformation by Rho GTPase-dependent activation of ERK1/2. J Biol Chem. 2006;281:24575–24587. doi: 10.1074/jbc.M604147200. [DOI] [PubMed] [Google Scholar]
- 137.Yuan L, Santi M, Rushing EJ, Cornelison R, MacDonald TJ. ERK activation of p21 activated kinase-1 (Pak1) is critical for medulloblastoma cell migration. Clin Exp Metastasis. 2010;27:481–491. doi: 10.1007/s10585-010-9337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aoki H, Yokoyama T, Fujiwara K, Tari AM, Sawaya R, Suki D, Hess KR, Aldape KD, Kondo S, Kumar R, Kondo Y. Phosphorylated Pak1 level in the cytoplasm correlates with shorter survival time in patients with glioblastoma. Clin Cancer Res. 2007;13:6603–6609. doi: 10.1158/1078-0432.CCR-07-0145. [DOI] [PubMed] [Google Scholar]
- 139.Carter JH, Douglass LE, Deddens JA, Colligan BM, Bhatt TR, Pemberton JO, Konicek S, Hom J, Marshall M, Graff JR. Pak-1 expression increases with progression of colorectal carcinomas to metastasis. Clin Cancer Res. 2004;10:3448–3456. doi: 10.1158/1078-0432.CCR-03-0210. [DOI] [PubMed] [Google Scholar]
- 140.Ito M, Nishiyama H, Kawanishi H, Matsui S, Guilford P, Reeve A, Ogawa O. P21-activated kinase 1: a new molecular marker for intravesical recurrence after transurethral resection of bladder cancer. J Urol. 2007;178:1073–1079. doi: 10.1016/j.juro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 141.Schraml P, Schwerdtfeger G, Burkhalter F, Raggi A, Schmidt D, Ruffalo T, King W, Wilber K, Mihatsch MJ, Moch H. Combined array comparative genomic hybridization and tissue microarray analysis suggest PAK1 at 11q13.5-q14 as a critical oncogene target in ovarian carcinoma. Am J Pathol. 2003;163:985–992. doi: 10.1016/S0002-9440(10)63458-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bekri S, Adelaide J, Merscher S, Grosgeorge J, Caroli-Bosc F, Perucca-Lostanlen D, Kelley PM, Pebusque MJ, Theillet C, Birnbaum D, Gaudray P. Detailed map of a region commonly amplified at 11q13 → q14 in human breast carcinoma. Cytogenet Cell Genet. 1997;79:125–131. doi: 10.1159/000134699. [DOI] [PubMed] [Google Scholar]
- 143.Ching YP, Leong VY, Lee MF, Xu HT, Jin DY, Ng IO. P21-activated protein kinase is overexpressed in hepatocellular carcinoma and enhances cancer metastasis involving c-Jun NH2-terminal kinase activation and paxillin phosphorylation. Cancer Res. 2007;67:3601–3608. doi: 10.1158/0008-5472.CAN-06-3994. [DOI] [PubMed] [Google Scholar]
- 144.Kamai T, Shirataki H, Nakanishi K, Furuya N, Kambara T, Abe H, Oyama T, Yoshida K. Increased Rac1 activity and Pak1 overexpression are associated with lymphovascular invasion and lymph node metastasis of upper urinary tract cancer. BMC Cancer. 2010;10:164. doi: 10.1186/1471-2407-10-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.O’Sullivan GC, Tangney M, Casey G, Ambrose M, Houston A, Barry OP. Modulation of p21-activated kinase 1 alters the behavior of renal cell carcinoma. Int J Cancer. 2007;121:1930–1940. doi: 10.1002/ijc.22893. [DOI] [PubMed] [Google Scholar]
- 146.McCarty SK, Saji M, Zhang X, Jarjoura D, Fusco A, Vasko VV, Ringel MD. Group I p21-activated kinases regulate thyroid cancer cell migration and are overexpressed and activated in thyroid cancer invasion. Endocr Relat Cancer. 2010;17:989–999. doi: 10.1677/ERC-10-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 148.Rayala SK, Talukder AH, Balasenthil S, Tharakan R, Barnes CJ, Wang RA, Aldaz M, Khan S, Kumar R. P21-activated kinase 1 regulation of estrogen receptor-alpha activation involves serine 305 activation linked with serine 118 phosphorylation. Cancer Res. 2006;66:1694–1701. doi: 10.1158/0008-5472.CAN-05-2922. [DOI] [PubMed] [Google Scholar]
- 149.Bostner J, Skoog L, Fornander T, Nordenskjold B, Stal O. Estrogen receptor-alpha phosphorylation at serine 305, nuclear p21-activated kinase 1 expression, and response to tamoxifen in postmenopausal breast cancer. Clin Cancer Res. 2010;16:1624–1633. doi: 10.1158/1078-0432.CCR-09-1733. [DOI] [PubMed] [Google Scholar]
- 150.Holm C, Rayala S, Jirstrom K, Stal O, Kumar R, Landberg G. Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst. 2006;98:671–680. doi: 10.1093/jnci/djj185. [DOI] [PubMed] [Google Scholar]
- 151.Arias-Romero LE, Villamar-Cruz O, Pacheco A, Kosoff R, Huang M, Muthuswamy SK, Chernoff J. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene. 2010;29:5839–5849. doi: 10.1038/onc.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shrestha Y, Schafer EJ, Boehm JS, Thomas SR, He F, Du J, Wang S, Barretina J, Weir BA, Zhao JJ, Polyak K, Golub TR, Beroukhim R, Hahn WC. PAK1 is a breast cancer oncogene that coordinately activates MAPK and MET signaling. Oncogene. 2012;31:3397–3408. doi: 10.1038/onc.2011.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R. PAK signaling in oncogenesis. Oncogene. 2009;28:2545–2555. doi: 10.1038/onc.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bekri S, Adelaide J, Merscher S, Grosgeorge J, Caroli-Bosc F, Perucca-Lostanlen D, Kelley PM, Pebusque MJ, Theillet C, Birnbaum D, Gaudray P. Detailed map of a region commonly amplified at 11q13 → q14 in human breast carcinoma. Cytogenet Cell Genet. 1997;79:125–131. doi: 10.1159/000134699. [DOI] [PubMed] [Google Scholar]
- 155.Ong CC, Jubb AM, Haverty PM, Zhou W, Tran V, Truong T, Turley H, O’Brien T, Vucic D, Harris AL, Belvin M, Friedman LS, Blackwood EM, Koeppen H, Hoeflich KP. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci USA. 2011;108:7177–7182. doi: 10.1073/pnas.1103350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Salh B, Marotta A, Wagey R, Sayed M, Pelech S. Dysregulation of phosphatidylinositol 3-kinase and downstream effectors in human breast cancer. Int J Cancer. 2002;98:148–154. doi: 10.1002/ijc.10147. [DOI] [PubMed] [Google Scholar]
- 157.Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–3020. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 158.Li Q, Mullins SR, Sloane BF, Mattingly RR. p21-Activated kinase 1 coordinates aberrant cell survival and pericellular proteolysis in a three-dimensional culture model for premalignant progression of human breast cancer. Neoplasia. 2008;10:314–329. doi: 10.1593/neo.07970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol. 2008;28:4162–4172. doi: 10.1128/MCB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rider L, Oladimeji P, Diakonova M. PAK1 regulates breast cancer cell invasion through secretion of matrix metalloproteinases in response to prolactin and three-dimensional collagen IV. Mol Endocrinol. 2013;27:1048–1064. doi: 10.1210/me.2012-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Premont RT, Perry SJ, Schmalzigaug R, Roseman JT, Xing Y, Claing A. The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell Signal. 2004;16:1001–1011. doi: 10.1016/j.cellsig.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 162.Paris S, Longhi R, Santambrogio P, de Curtis I. Leucine-zipper-mediated homo- and hetero-dimerization of GIT family p95-ARF GTPase-activating protein, PIX-, paxillin-interacting proteins 1 and 2. Biochem J. 2003;372:391–398. doi: 10.1042/BJ20030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim S, Lee SH, Park D. Leucine zipper-mediated homodimerization of the p21-activated kinase-interacting factor, beta Pix. Implication for a role in cytoskeletal reorganization. J Biol Chem. 2001;276:10581–10584. doi: 10.1074/jbc.C000806200. [DOI] [PubMed] [Google Scholar]
- 164.Loo TH, Ng YW, Lim L, Manser E. GIT1 activates p21-activated kinase through a mechanism independent of p21 binding. Mol Cell Biol. 2004;24:3849–3859. doi: 10.1128/MCB.24.9.3849-3859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhao ZS, Manser E, Loo TH, Lim L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol. 2000;20:6354–6363. doi: 10.1128/mcb.20.17.6354-6363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhao ZS, Manser E. PAK and other Rho-associated kinases-effectors with surprisingly diverse mechanisms of regulation. Biochem J. 2005;386:201–214. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Manabe R, Kovalenko M, Webb DJ, Horwitz AR. GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J Cell Sci. 2002;115:1497–1510. doi: 10.1242/jcs.115.7.1497. [DOI] [PubMed] [Google Scholar]
- 168.Brown MC, West KA, Turner CE. Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol Biol Cell. 2002;13:1550–1565. doi: 10.1091/mbc.02-02-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rider L, Diakonova M. Adapter protein SH2B1beta binds filamin A to regulate prolactin-dependent cytoskeletal reorganization and cell motility. Mol Endocrinol. 2011;25:1231–1243. doi: 10.1210/me.2011-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Borm B, Requardt RP, Herzog V, Kirfel G. Membrane ruffles in cell migration: indicators of inefficient lamellipodia adhesion and compartments of actin filament reorganization. Exp Cell Res. 2005;302:83–95. doi: 10.1016/j.yexcr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 171.Ridley AJ. Membrane ruffling and signal transduction. Bioessays. 1994;16:321–327. doi: 10.1002/bies.950160506. [DOI] [PubMed] [Google Scholar]
- 172.Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–145. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- 173.Nakamura F, Stossel TP, Hartwig JH. The filamins: organizers of cell structure and function. Cell Adh Migr. 2011;5:160–169. doi: 10.4161/cam.5.2.14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cunningham CC, Gorlin JB, Kwiatkowski DJ, Hartwig JH, Janmey PA, Byers HR, Stossel TP. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992;255:325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- 175.Woo MS, Ohta Y, Rabinovitz I, Stossel TP, Blenis J. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol. 2004;24:3025–3035. doi: 10.1128/MCB.24.7.3025-3035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Feng Y, Chen MH, Moskowitz IP, Mendonza AM, Vidali L, Nakamura F, Kwiatkowski DJ, Walsh CA. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci U S A. 2006;103:19836–19841. doi: 10.1073/pnas.0609628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Johansen LD, Naumanen T, Knudsen A, Westerlund N, Gromova I, Junttila M, Nielsen C, Bottzauw T, Tolkovsky A, Westermarck J, Coffey ET, Jaattela M, Kallunki T. IKAP localizes to membrane ruffles with filamin A and regulates actin cytoskeleton organization and cell migration. J Cell Sci. 2008;121:854–864. doi: 10.1242/jcs.013722. [DOI] [PubMed] [Google Scholar]
- 178.Sarkisian MR, Bartley CM, Chi H, Nakamura F, Hashimoto-Torii K, Torii M, Flavell RA, Rakic P. MEKK4 signaling regulates filamin expression and neuronal migration. Neuron. 2006;52:789–801. doi: 10.1016/j.neuron.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xu Y, Bismar TA, Su J, Xu B, Kristiansen G, Varga Z, Teng L, Ingber DE, Mammoto A, Kumar R, Alaoui-Jamali MA. Filamin A regulates focal adhesion disassembly and suppresses breast cancer cell migration and invasion. J Exp Med. 2010;207:2421–2437. doi: 10.1084/jem.20100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rider L, Tao J, Snyder S, Brinley B, Lu J, Diakonova M. Adapter protein SH2B1beta cross-links actin filaments and regulates actin cytoskeleton. Mol Endocrinol. 2009;23:1065–1076. doi: 10.1210/me.2008-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rui L, Carter-Su C. Identification of SH2-bbeta as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc Natl Acad Sci U S A. 1999;96:7172–7177. doi: 10.1073/pnas.96.13.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427–1440. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci. 2008;121:2435–2444. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–148. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- 186.Birge RB, Fajardo JE, Reichman C, Shoelson SE, Songyang Z, Cantley LC, Hanafusa H. Identification and characterization of a high-affinity interaction between v-Crk and tyrosine-phosphorylated paxillin in CT10-transformed fibroblasts. Mol Cell Biol. 1993;13:4648–4656. doi: 10.1128/mcb.13.8.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Petit V, Boyer B, Lentz D, Turner CE, Thiery JP, Valles AM. Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J Cell Biol. 2000;148:957–970. doi: 10.1083/jcb.148.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Stofega MR, Sanders LC, Gardiner EM, Bokoch GM. Constitutive p21-activated kinase (PAK) activation in breast cancer cells as a result of mislocalization of PAK to focal adhesions. Mol Biol Cell. 2004;15:2965–2977. doi: 10.1091/mbc.E03-08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol. 2006;173:587–589. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Daniels RH, Zenke FT, Bokoch GM. alphaPix stimulates p21-activated kinase activity through exchange factor-dependent and -independent mechanisms. J Biol Chem. 1999;274:6047–6050. doi: 10.1074/jbc.274.10.6047. [DOI] [PubMed] [Google Scholar]
- 191.Rennefahrt UE, Deacon SW, Parker SA, Devarajan K, Beeser A, Chernoff J, Knapp S, Turk BE, Peterson JR. Specificity profiling of Pak kinases allows identification of novel phosphorylation sites. J Biol Chem. 2007;282:15667–15678. doi: 10.1074/jbc.M700253200. [DOI] [PubMed] [Google Scholar]
- 192.Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol. 2011;3(5):1–2. doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Boyd NF, Lockwood GA, Martin LJ, Knight JA, Byng JW, Yaffe MJ, Tritchler DL. Mammographic densities and breast cancer risk. Breast Dis. 1998;10:113–126. doi: 10.3233/bd-1998-103-412. [DOI] [PubMed] [Google Scholar]
- 194.Colpaert C, Vermeulen P, Van Marck E, Dirix L. The presence of a fibrotic focus is an independent predictor of early metastasis in lymph node-negative breast cancer patients. Am J Surg Pathol. 2001;25:1557–1558. doi: 10.1097/00000478-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 195.Vachon CM, Brandt KR, Ghosh K, Scott CG, Maloney SD, Carston MJ, Pankratz VS, Sellers TA. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:43–49. doi: 10.1158/1055-9965.EPI-06-0738. [DOI] [PubMed] [Google Scholar]
- 196.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 197.Vachon CM, van Gils CH, Sellers TA, Ghosh K, Pruthi S, Brandt KR, Pankratz VS. Mammographic density, breast cancer risk and risk prediction. Breast Cancer Res. 2007;9:217. doi: 10.1186/bcr1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 200.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10 A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 201.Adriance MC, Inman JL, Petersen OW, Bissell MJ. Myoepithelial cells: good fences make good neighbors. Breast Cancer Res. 2005;7:190–197. doi: 10.1186/bcr1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 204.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 206.Ziober BL, Turner MA, Palefsky JM, Banda MJ, Kramer RH. Type I collagen degradation by invasive oral squamous cell carcinoma. Oral Oncol. 2000;36:365–372. doi: 10.1016/s1368-8375(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 207.Kerkela E, Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp Dermatol. 2003;12:109–125. doi: 10.1034/j.1600-0625.2003.120201.x. [DOI] [PubMed] [Google Scholar]
- 208.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12):1–24. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vincenti MP, White LA, Schroen DJ, Benbow U, Brinckerhoff CE. Regulating expression of the gene for matrix metalloproteinase-1 (collagenase): mechanisms that control enzyme activity, transcription, and mRNA stability. Crit Rev Eukaryot Gene Expr. 1996;6:391–411. doi: 10.1615/critreveukargeneexpr.v6.i4.40. [DOI] [PubMed] [Google Scholar]
- 210.Murray GI, Duncan ME, O’Neil P, McKay JA, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J Pathol. 1998;185:256–261. doi: 10.1002/(SICI)1096-9896(199807)185:3<256::AID-PATH115>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 211.Murray GI, Duncan ME, O’Neil P, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996;2:461–462. doi: 10.1038/nm0496–461. [DOI] [PubMed] [Google Scholar]
- 212.Airola K, Karonen T, Vaalamo M, Lehti K, Lohi J, Kariniemi AL, Keski-Oja J, Saarialho-Kere UK. Expression of collagenases-1 and -3 and their inhibitors TIMP-1 and -3 correlates with the level of invasion in malignant melanomas. Br J Cancer. 1999;80:733–743. doi: 10.1038/sj.bjc.6690417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ito T, Ito M, Shiozawa J, Naito S, Kanematsu T, Sekine I. Expression of the MMP-1 in human pancreatic carcinoma: relationship with prognostic factor. Mod Pathol. 1999;12:669–674. [PubMed] [Google Scholar]
- 214.Inoue T, Yashiro M, Nishimura S, Maeda K, Sawada T, Ogawa Y, Sowa M, Chung KH. Matrix metalloproteinase-1 expression is a prognostic factor for patients with advanced gastric cancer. Int J Mol Med. 1999;4:73–77. doi: 10.3892/ijmm.4.1.73. [DOI] [PubMed] [Google Scholar]
- 215.Nakopoulou L, Giannopoulou I, Gakiopoulou H, Liapis H, Tzonou A, Davaris PS. Matrix metalloproteinase-1 and -3 in breast cancer: correlation with progesterone receptors and other clinicopathologic features. Hum Pathol. 1999;30:436–442. doi: 10.1016/s0046-8177(99)90120-x. [DOI] [PubMed] [Google Scholar]
- 216.Poola I, DeWitty RL, Marshalleck JJ, Bhatnagar R, Abraham J, Leffall LD. Identification of MMP-1 as a putative breast cancer predictive marker by global gene expression analysis. Nat Med. 2005;11:481–483. doi: 10.1038/nm1243. [DOI] [PubMed] [Google Scholar]
- 217.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lochter A, Srebrow A, Sympson CJ, Terracio N, Werb Z, Bissell MJ. Misregulation of stromelysin-1 expression in mouse mammary tumor cells accompanies acquisition of stromelysin-1-dependent invasive properties. J Biol Chem. 1997;272:5007–5015. doi: 10.1074/jbc.272.8.5007. [DOI] [PubMed] [Google Scholar]
- 220.Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene. 2000;19:1102–1113. doi: 10.1038/sj.onc.1203347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stetler-Stevenson WG, Liotta LA, Kleiner DE., Jr Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993;7:1434–1441. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- 225.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 226.Ballin M, Gomez DE, Sinha CC, Thorgeirsson UP. Ras oncogene mediated induction of a 92 kDa metalloproteinase; strong correlation with the malignant phenotype. Biochem Biophys Res Commun. 1988;154:832–838. doi: 10.1016/0006-291x(88)90215-x. [DOI] [PubMed] [Google Scholar]
- 227.Zucker S, Lysik RM, Zarrabi MH, Moll U. M(r) 92,000 type IV collagenase is increased in plasma of patients with colon cancer and breast cancer. Cancer Res. 1993;53:140–146. [PubMed] [Google Scholar]
- 228.Radisky ES, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:201–212. doi: 10.1007/s10911-010-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2:252–257. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Duffy MJ, McGowan PM, Gallagher WM. Cancer invasion and metastasis: changing views. J Pathol. 2008;214:283–293. doi: 10.1002/path.2282. [DOI] [PubMed] [Google Scholar]
- 231.Folgueras AR, Pendas AM, Sanchez LM, Lopez-Otin C. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol. 2004;48:411–424. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 232.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 233.Barcus CE, Keely PJ, Eliceiri KW, Schuler LA. Stiff collagen matrices increase tumorigenic prolactin signaling in breast cancer cells. J Biol Chem. 2013;288:12722–12732. doi: 10.1074/jbc.M112.447631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Arendt LM, Rugowski DE, Grafwallner-Huseth TA, Garcia-Barchino MJ, Rui H, Schuler LA. Prolactin-induced mouse mammary carcinomas model estrogen resistant luminal breast cancer. Breast Cancer Res. 2011;13:R11. doi: 10.1186/bcr2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gutzman JH, Rugowski DE, Nikolai SE, Schuler LA. Stat5 activation inhibits prolactin-induced AP-1 activity: distinct prolactin-initiated signals in tumorigenesis dependent on cell context. Oncogene. 2007;26:6341–6348. doi: 10.1038/sj.onc.1210454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Carver KC, Schuler LA. Prolactin does not require insulin-like growth factor intermediates but synergizes with insulin-like growth factor I in human breast cancer cells. Mol Cancer Res. 2008;6:634–643. doi: 10.1158/1541-7786.MCR-07-2069. [DOI] [PubMed] [Google Scholar]
- 237.Sultan AS, Xie J, LeBaron MJ, Ealley EL, Nevalainen MT, Rui H. Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene. 2005;24:746–760. doi: 10.1038/sj.onc.1208203. [DOI] [PubMed] [Google Scholar]
- 238.Plotnikov A, Varghese B, Tran TH, Liu C, Rui H, Fuchs SY. Impaired turnover of prolactin receptor contributes to transformation of human breast cells. Cancer Res. 2009;69:3165–3172. doi: 10.1158/0008-5472.CAN-08-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhou L, Yan C, Gieling RG, Kida Y, Garner W, Li W, Han YP. Tumor necrosis factor-alpha induced expression of matrix metalloproteinase-9 through p21-activated kinase-1. BMC Immunol. 2009;10:15. doi: 10.1186/1471-2172-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Fu D, Yang Y, Xiao Y, Lin H, Ye Y, Zhan Z, Liang L, Yang X, Sun L, Xu H. Role of p21-activated kinase 1 in regulating the migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Rheumatology (Oxford) 2012;51:1170–1180. doi: 10.1093/rheumatology/kes031. [DOI] [PubMed] [Google Scholar]
- 241.Elloul S, Vaksman O, Stavnes HT, Trope CG, Davidson B, Reich R. Mesenchymal-to-epithelial transition determinants as characteristics of ovarian carcinoma effusions. Clin Exp Metastasis. 2010;27:161–172. doi: 10.1007/s10585-010-9315-2. [DOI] [PubMed] [Google Scholar]
- 242.Ferri N, Colombo G, Ferrandi C, Raines EW, Levkau B, Corsini A. Simvastatin reduces MMP1 expression in human smooth muscle cells cultured on polymerized collagen by inhibiting Rac1 activation. Arterioscler Thromb Vasc Biol. 2007;27:1043–1049. doi: 10.1161/ATVBAHA.107.139881. [DOI] [PubMed] [Google Scholar]
- 243.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 244.Enjoji M, Kotoh K, Iwamoto H, Nakamuta M, Nawata H. Self-regulation of type I collagen degradation by collagen-induced production of matrix metalloproteinase-1 on cholangiocarcinoma and hepatocellular carcinoma cells. In Vitro Cell Dev Biol Anim. 2000;36:71–73. doi: 10.1290/1071-2690(2000)036<0071:SROTIC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 245.Ferri N, Carragher NO, Raines EW. Role of discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am J Pathol. 2004;164:1575–1585. doi: 10.1016/S0002-9440(10)63716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Langholz O, Rockel D, Mauch C, Kozlowska E, Bank I, Krieg T, Eckes B. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by alpha 1 beta 1 and alpha 2 beta 1 integrins. J Cell Biol. 1995;131:1903–1915. doi: 10.1083/jcb.131.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cortes-Reynosa P, Robledo T, Macias-Silva M, Wu SV, Salazar EP. Src kinase regulates metalloproteinase-9 secretion induced by type IV collagen in MCF-7 human breast cancer cells. Matrix Biol. 2008;27:220–231. doi: 10.1016/j.matbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 248.Castro-Sanchez L, Soto-Guzman A, Guaderrama-Diaz M, Cortes-Reynosa P, Salazar EP. Role of DDR1 in the gelatinases secretion induced by native type IV collagen in MDA-MB-231 breast cancer cells. Clin Exp Metastasis. 2011;28:463–477. doi: 10.1007/s10585-011-9385-9. [DOI] [PubMed] [Google Scholar]
- 249.Haas TL, Davis SJ, Madri JA. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. J Biol Chem. 1998;273:3604–3610. doi: 10.1074/jbc.273.6.3604. [DOI] [PubMed] [Google Scholar]
- 250.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 251.Nguyen M, Arkell J, Jackson CJ. Three-dimensional collagen matrices induce delayed but sustained activation of gelatinase A in human endothelial cells via MT1-MMP. Int J Biochem Cell Biol. 2000;32:621–631. doi: 10.1016/s1357-2725(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 252.Han YP, Tuan TL, Wu H, Hughes M, Garner WL. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci. 2001;114:131–139. doi: 10.1242/jcs.114.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Seltzer JL, Lee AY, Akers KT, Sudbeck B, Southon EA, Wayner EA, Eisen AZ. Activation of 72-kDa type IV collagenase/gelatinase by normal fibroblasts in collagen lattices is mediated by integrin receptors but is not related to lattice contraction. Exp Cell Res. 1994;213:365–374. doi: 10.1006/excr.1994.1211. [DOI] [PubMed] [Google Scholar]
- 254.Ruangpanit N, Chan D, Holmbeck K, Birkedal-Hansen H, Polarek J, Yang C, Bateman JF, Thompson EW. Gelatinase A (MMP-2) activation by skin fibroblasts: dependence on MT1-MMP expression and fibrillar collagen form. Matrix Biol. 2001;20:193–203. doi: 10.1016/s0945-053x(01)00135-4. [DOI] [PubMed] [Google Scholar]
- 255.Ellerbroek SM, Fishman DA, Kearns AS, Bafetti LM, Stack MS. Ovarian carcinoma regulation of matrix metalloproteinase-2 and membrane type 1 matrix metalloproteinase through beta1 integrin. Cancer Res. 1999;59:1635–1641. [PubMed] [Google Scholar]
- 256.Gilles C, Polette M, Seiki M, Birembaut P, Thompson EW. Implication of collagen type I-induced membrane-type 1-matrix metalloproteinase expression and matrix metal-loproteinase-2 activation in the metastatic progression of breast carcinoma. Lab Invest. 1997;76:651–660. [PubMed] [Google Scholar]
- 257.Kurschat P, Zigrino P, Nischt R, Breitkopf K, Steurer P, Klein CE, Krieg T, Mauch C. Tissue inhibitor of matrix metalloproteinase-2 regulates matrix metalloproteinase-2 activation by modulation of membrane-type 1 matrix metalloproteinase activity in high and low invasive melanoma cell lines. J Biol Chem. 1999;274:21056–21062. doi: 10.1074/jbc.274.30.21056. [DOI] [PubMed] [Google Scholar]
- 258.Maquoi E, Frankenne F, Noel A, Krell HW, Grams F, Foidart JM. Type IV collagen induces matrix metalloproteinase 2 activation in HT1080 fibrosarcoma cells. Exp Cell Res. 2000;261:348–359. doi: 10.1006/excr.2000.5063. [DOI] [PubMed] [Google Scholar]
- 259.Borrirukwanit K, Lafleur MA, Mercuri FA, Blick T, Price JT, Fridman R, Pereira JJ, Leard-kamonkarn V, Thompson EW. The type I collagen induction of MT1-MMP-mediated MMP-2 activation is repressed by alphaVbeta3 integrin in human breast cancer cells. Matrix Biol. 2007;26:291–305. doi: 10.1016/j.matbio.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 260.Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–223. doi: 10.1016/j.tcb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 261.Vogel V, Sheetz MP. Cell fate regulation by coupling mechanical cycles to biochemical signaling pathways. Curr Opin Cell Biol. 2009;21:38–46. doi: 10.1016/j.ceb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]