Abstract
Both radiation and stresses cause detrimental effects on humans. Besides possible health effects resulting directly from radiation exposure, the nuclear plant accident is a cause of social psychological stresses. A recent study showed that chronic restraint-induced stresses (CRIS) attenuated Trp53 functions and increased carcinogenesis susceptibility of Trp53-heterozygous mice to total-body X-irradiation (TBXI), having a big impact on the academic world and a sensational effect on the public, especially the residents living in radioactively contaminated areas. It is important to investigate the possible modification effects from CRIS on radiation-induced health consequences in Trp53 wild-type (Trp53wt) animals. Prior to a carcinogenesis study, effects of TBXI on the hematopoietic system under CRIS were investigated in terms of hematological abnormality in the peripheral blood and residual damage in the bone marrow erythrocytes using a mouse restraint model. Five-week-old male Trp53wt C57BL/6J mice were restrained 6 h per day for 28 consecutive days, and TBXI (4 Gy) was given on the 8th day. Results showed that CRIS alone induced a marked decrease in the red blood cell (RBC) and the white blood cell (WBC) count, while TBXI caused significantly lower counts of RBCs, WBCs and blood platelets, and a lower concentration of hemoglobin regardless of CRIS. CRIS alone did not show any significant effect on erythrocyte proliferation and on induction of micronucleated erythrocytes, whereas TBXI markedly inhibited erythrocyte proliferation and induced a significant increase in the incidences of micronucleated erythrocytes, regardless of CRIS. These findings suggest that CRIS does not have a significant impact on radiation-induced detrimental effects on the hematopoietic system in Trp53wt mice.
Keywords: chronic restraint-induced stress, total-body irradiation, peripheral blood hemogram, bone marrow micronucleated erythrocytes, mouse restraint model
INTRODUCTION
Nuclear power plant accidents (NPPAs) often result in the release of many different radioisotopes, leading to radioactive contamination. Ionizing radiation (IR) can induce deleterious effects, such as carcinogenicity, mutagenicity, teratogenicity and organ system toxicity. For the public, disasters involving radiation are thought to be particularly pernicious due to the fact that exposure is invisible and universally dreaded. Exposure to IR due to NPPAs is a significant threat and a major health concern. On the other hand, NPPAs cause social psychological stresses. In addition, radioactive contamination often restricts people's outdoor activities, causing further physiological and psychological stresses (PPSs) [1–6]. Thus NPPAs may pose a long-term threat to health, resulting in directly and indirectly adverse health outcomes.
Humans are exposed to a multitude of PPSs that contribute to varied adverse health consequences, such as vision disorders, hypertension, cardiovascular disease, diabetes, metabolic syndrome, reproductive disorders and development of cancer [7–13]. Recent studies show that for children, growing up in disadvantaged social environments (i.e. poverty or unstable family) was associated with adverse health outcomes [14]. Genetics moderated the magnitude of the health consequence, but stresses determined the direction [15]. In animals, stresses generally affected cytokine, endocrine and stress hormone (i.e. corticosterone) levels, and immune parameters [16–19]. Chronic restraint-induced stresses (CRISs) significantly impacted upon the hypothalamo–pituitary–adrenocortical axis, causing apical dendritic atrophy [20, 21]. Prenatal exposure to maternal stress altered physiological and immune functions in the offspring [22–24].
Stress increased susceptibility to a number of diseases, including Alzheimer's disease, and was a risk factor for cancer in humans [9, 25]. In mouse models, stresses altered responsiveness to carcinogens, accelerated tumor onset, and altered tumor type and location [26]. CRIS promoted progression of lymphomas [27] and growth of carcinoma xenografts [28]. Behavioral stress accelerated prostate cancer development [29]. Notably, a recent study showed that CRIS increased susceptibility of Trp53-heterozygous mice to radiation (4 Gy) carcinogenesis, having a big impact on the academic world and a sensational effect on the public [30]. Although mechanisms remain largely elusive, altered metabolism, and degraded physiological and immune functions were critical for increasing susceptibility to pathogens and toxicological assaults, including IR [31–37].
Psychosocial consequences of disasters have been studied for more than 100 years; however, investigations after NPPAs are neither complete nor comprehensive [38], and the importance of the psychological impact is underscored [39, 40]. In fact, following large-scale disasters (i.e. the Chernobyl and Fukushima accidents), psychosocial sequelae were intense and long lasting, and occurred independently of the actual exposure received, and mental health effects were the most significant health consequence [1–6]. It is noted that the evacuee mothers rated their evacuated children's wellbeing as significantly worse, and the most important risk factors of this health consequence were maternal somatization and Chernobyl-related stress [41]. This work highlights the stress effects on health at a young age. However, such studies are still rare, and the documented works have limited information [5].
Effects from stress exposure on radiation-induced health consequences and the mechanisms underlying these outcomes remain largely unknown, constraining our capacity to ascertain the potential human relevance of the health effects observed in animal models. In a series of investigations, possible modification effects from CRIS on the biological responses of and subsequent consequences for young mice with normal genotype exposed to total-body X-irradiation (TBXI) were studied with multidisciplinary analyses. Measurements included changes of body weight gain and weight of immune organs (such as thymus and spleen), alterations in the levels of blood cytokines and stress hormones, changes in the peripheral blood hemogram and the anti-oxidative activity of blood cells, chromosome aberrations in splenocytes and micronuclei in bone marrow erythrocytes, and epigenetic variations (DNA methylation and miRNA expression) and protein expression profiles in the liver. This paper describes investigation of the possible modification effect from CRIS on TBXI-induced hematopoietic toxicity.
It should be pointed out that this work did not simulate the residents in the contaminated environment, who were exposed to a very low dose at a very low dose rate. Although the hematopoietic system is highly sensitive to radiation, it is not clear that if the very low dose received by the residents in the contaminated environment could cause any detectable effect on the hematopoietic system in humans or in mice. A dose of 4 Gy TBI was delivered to the mice at a high dose rate. This was the same dose as that used in Feng et al.'s work, which promoted tumorigenesis in Trp53-heterozygous mice [24]. This dose could increase incidences of micronucleus bone marrow erythrocytes and cause abnormality in the peripheral blood hemogram in Trp53 wild-type (Trp53wt) mice, according to our preliminary study. Before investigating the effect of exposure to very low dose irradiation and stress, we first determined whether the mouse response to a high dose of irradiation (capable of inducing a detectable effect on the hematopoietic system in Trp53wt mice) could be modulated under chronic restraint-induced stress in Trp53wt mice.
It should be noticed that in the field of radiation biology, IR is considered one type of stress. The term ‘stress’ used in the present work (studying the modifying effect of chronic restraint on radiation hematopoietic toxicity) refers to chronic restraint-induced psychological stress.
MATERIALS AND METHODS
Study subjects and experimental group design
C57BL/6J Jms strain male mice were used in the present study. Based on our preliminary two trials, mice aged 4 weeks old were purchased from SLC, Inc. (Japan) and maintained in a clean conventional temperature- and humidity-controlled animal facility under a 12 h light – 12 h dark photoperiod (lights on from 7:00 A.M. to 7:00 P.M.). Animals housed in autoclaved cages (three mice per cage) with sterilized wood chips, were allowed free access to standard laboratory chow (MB-1, Funabashi Farm Co., Japan) and acidified water (pH = 3.0 ± 0.2). Animals were acclimatized to the laboratory conditions for 1 week before use. The 5-week-old mice were randomly assigned to four experimental groups with 9 to 12 mice in each group, namely, the ‘control group (C-Gr)’ (receiving neither restraint nor TBXI), the ‘restraint group (R-Gr)’ (receiving only restraint), the ‘TBXI group (IR-Gr)’ (receiving only TBXI), and the ‘restraint and TBXI group ((R+IR)-Gr)’ (receiving both restraint and TBXI). All experimental protocols involving mice were reviewed and approved (Experimental Animal Research Plan and Protocol No. 12-1026) by The Institutional Animal Care and Use Committee of the National Institute of Radiological Sciences (NIRS). The experiments were performed in strict accordance with the NIRS Guidelines for the Care and Use of Laboratory Animals.
Mouse model for chronic restraint-induced stresses
Chronic restraint, a well-established typical mouse model [30] to induce psychological stresses, was adopted and applied to the present work with minor modification. In brief, the mouse restraint system (Flat Bottom Rodent Holder, RSTR541, Kent Scientific Co., USA) was used for chronic periodic restraint on a daily basis of 6 h for 28 consecutive days. Individual mice aged 5 weeks were placed in the strainer, and the restrained mice were maintained horizontally in their home cage during the 6-h restraint session (9:30 A.M. to 3:30 P.M.) daily, then the animals were released into the same cage and allowed to access food and water during the free session (3:30 P.M. to 9:30 A.M.). The animals in C-Gr and IR-Gr received no restraint but their food and water was withheld while the R-Gr and (R+IR)-Gr animals underwent the 6-h restraint session each day.
Total body X-irradiation of the mice
X-rays were generated with an X-ray machine (Pantak-320S, Shimadzu, Japan) operated at 200 kVp and 20 mA, using a 0.50-mm Al + 0.50-mm Cu filter. An exposure rate meter (AE-1321M, Applied Engineering Inc., Japan) was used for the dosimetry. The dose of TBXI was 4.0 Gy, and it was delivered at a dose rate of 0.25 Gy/min to the animals in IR-Gr and (R+IR)-Gr on the 8th day of the 28-day restraint regimen. The mice held in acryl containers were exposed to TBXI at room temperature without anesthesia.
Measurement of body weight gain
The body weight gain of the animals in each experiment group was recorded daily. At the end of the restraint regimen, the animals were anesthetized by inhalation of gaseous isoflurane (2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane) (CDS019936, Sigma-Aldrich, Japan). Then the animals were euthanized by cervical dislocation. Significant decrease in body weight gain, together with decrease in spleen weight and marked alteration in the corticosterone concentration in the peripheral blood plasma, were used as indicators for evaluating the success of CRIS in the mouse restraint model.
Assessment of the peripheral blood hemogram
As peripheral blood is the only tissue routinely available from human subjects, the peripheral blood hemogram was also assessed in the present work in order to provide information for possible comparative study in the future. The peripheral blood collected from the right femoral artery with a heparinized syringe in vacutainer blood collection tubes containing EDTA (Venoject II, Terumo Co., Japan) were immediately subjected to differential blood cell counts (red blood cells (RBCs), white blood cells (WBCs), and blood platelets (PLTs)) and measurement of blood hemoglobin concentration using a blood cell differential automatic analyzer (SYSMEX KX-21NV, Sysmex Corporation, Japan). The data for each experimental group were obtained from three to five mice.
Micronucleus test
The bone marrow micronucleus test was carried out according to Schmid [42] with minor modifications [43, 44]. Bone marrow smears prepared from both femurs were processed for the enumeration of micronucleated polychromatic erythrocytes (MNPCEs) and micronucleated normochromatic erythrocytes (MNNCEs). The slides were coded to avoid any observer bias. The micronuclei were scored using a light microscope at a magnification of ×1000. At least 5000 cells per mouse were counted, and the data for each experimental point were from five to six mice.
Statistical analysis
Statistical evaluation of the body weight data was done by 2-way ANOVA; all other evaluation of the data was done using Student's t-test, except for the micronucleus data on which the χ2 test was performed. Statistical significance was assigned to P < 0.05.
RESULTS
Verification of the CRIS Model
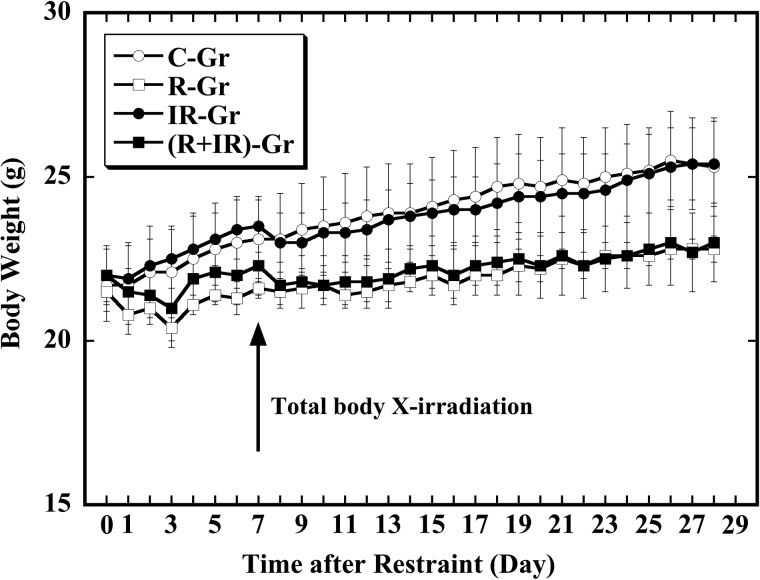
The treatments used in this study have been previously shown to induce a stress response sufficient to significantly affect several immunological parameters [30]. Under the experimental setup in the present study, reproducibility of this CRIS model in young male mice with normal genotype was verified using endpoints: namely, significant decrease in body weight gain and in the weight of immune organs (such as thymus and spleen), and alterations in the concentration of corticosterone in peripheral blood plasma. Significantly reduced body weight gain induced by CRIS appeared one day after onset of the restraint and this phenomenon was observed throughout the experiment in the animals that received the restraint (R-Gr and (R+IR)-Gr) (Fig. 1). For the animals that received the restraint, a marked decrease in weight of spleen and increase in concentrations of stress-related hormones and cytokines in the blood plasma were also detected (data not shown as they are to be published elsewhere in a paper on a hormone and cytokine study). These results clearly indicated the successful establishment of the CRIS model of restraint under our experimental setup.
Fig. 1.
Effect of CRIS and TBXI on body weight gain of mice. Group mean ± SD levels of the control group (C-Gr, open circle), the restraint group (R-Gr, open square), the TBI group (IR-Gr, solid circle), and the restraint and TBXI group ((R+IR)-Gr, solid square).
Change in body weight gain
The effects from restraint and TBXI on the body weight gain of mice are shown in Fig. 1. As mentioned, significant decrease in body weight gain was observed one day after onset of the restraint (P < 0.05), and the restraint resulted in the lowest body weight on the third day after onset (P < 0.0001): on the day of restraint onset, the body weight (g) of C-Gr, R-Gr, IR-Gr and (R+IR)-Gr was 21.7 ± 1.1, 21.5 ± 1.1, 22.0 ± 0.8 and 22.0 ± 0.9, respectively; on the following day, the body weight was 21.7 ± 1.2, 20.8 ± 0.6, 21.9 ± 1.1, and 21.9 ± 1.1, respectively. After a single exposure to TBXI with 4 Gy of X-rays, significant reduction in body weight gain was observed on the following day in both IR-Gr and (R+IR)-Gr (P < 0.05), while no interaction (namely, neither synergistic nor antagonistic effect) between the restraint and TBXI was observed (P > 0.05): on the day just before the animals were given TBXI, the body weight (g) of C-Gr, R-Gr, IR-Gr and (R+IR)-Gr was 23.1 ± 1.3, 21.6 ± 0.3, 23.5 ± 0.8 and 22.3 ± 0.9, respectively; on the following day, the body weight was 23.1 ± 1.4, 21.5 ± 0.4, 23.0 ± 0.9 and 21.7 ± 0.9, respectively. The recovery of body weight gain appeared late in the animals receiving the restraint (R-Gr and (R+IR)-Gr). In general, there was a statistically significant difference in the mean body weight between the groups that received the restraint (R-Gr and (R+IR)-Gr) and the groups that received no restraint (C-Gr and IR-Gr) one day after the onset of restraint, regardless of the TBXI: the mean body weight was markedly higher in groups that received no restraint compared with those groups that received the restraint. At the end of the experiment, the body weight in gram for C-Gr, R-Gr, IR-Gr and (R+IR)-Gr was 25.3 ± 1.5, 22.8 ± 0.4, 25.4 ± 1.3 and 23.0 ± 1.2, respectively. These results indicated that a single TBXI with 4 Gy of X-rays could induce a transient reduction in the body weight gain; however, CRIS for 28 days was more effective in terms of causing a continuous reduction in body weight gain in mice.
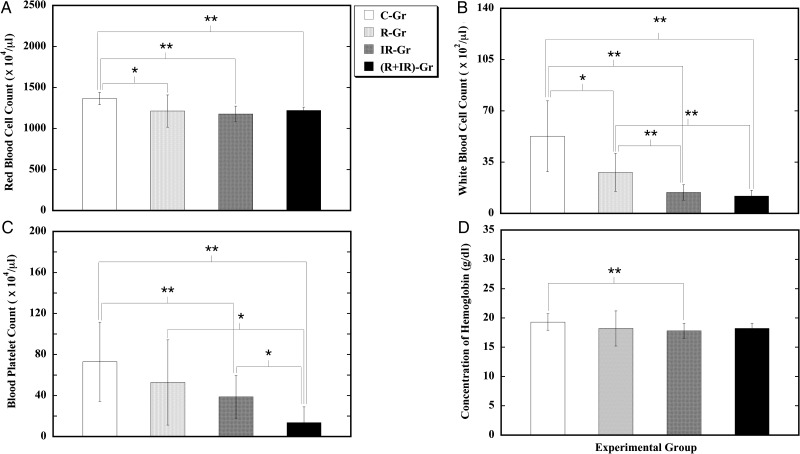
Hematological abnormality in the peripheral blood hemogram
Alterations in the hematopoietic system were studied in the peripheral blood hemogram (Fig. 2). Mice subjected to CRIS alone (R-Gr) displayed a significant decrease in both RBC count and WBC count when compared with the control (C-Gr). On the other hand, when compared with the control (C-Gr), mice exposed to TBXI alone (IR-Gr) showed a significant reduction in total peripheral blood cell count, manifesting as decreased RBC count, decreased WBC count, decreased PLT count, and low hemoglobin concentration. In addition, the effect from TBXI alone on induction of a decreased WBC count was significantly higher than that from CRIS alone. In animals subjected to both CRIS and TBXI ((R+IR)-Gr), a decreased RBC count, decreased WBC count, and decreased PLT count were observed. Combination of CRIS and TBXI caused a further marked decrease in the PLT count, and the severity of the decrease in the PLT count was significantly greater than that induced by either CRIS or TBXI alone. These results indicated that CRIS alone could cause, to a certain extent, detrimental effects on the hematopoietic system; however, this effect was not as strong as that caused by TBXI. Except for worsening the decreased PLT count, the combination of CRIS and TBXI seemed to show little effect on TBXI alone–induced hematological abnormality in the peripheral blood hemogram.
Fig. 2.
Effect of CRIS and TBXI on the peripheral blood hemogram of mice. Group mean ± SD levels of RBC count (A), WBC count (B), PLT count (C), and hemoglobin concentration (D). ‘*’ and ‘**’ stand for significant difference between two groups compared at P < 0.05 and P < 0.01, respectively.
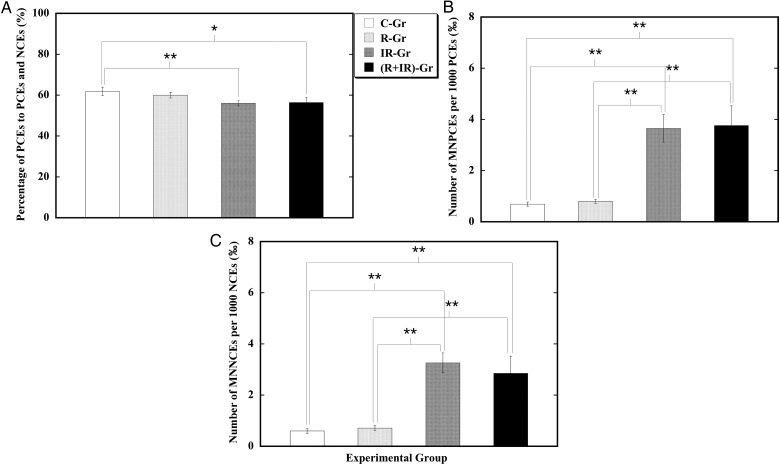
Residual damage in bone marrow erythrocytes
Cytotoxicity and genotoxicity of CRIS and TBXI were evaluated by measuring the residual damage in the bone marrow cells of the animals (Fig. 3). The number of polychromatic erythrocytes (PCEs) expressed as a percentage of the sum of PCEs and normochromatic erythrocytes (NCEs) is an indicator of bone marrow proliferation in the erythroid lineage, and its decrease is an indicator of mutagen-induced cytotoxicity [45]. The micronucleus test is a tool for genotoxic assessment. CRIS alone (R-Gr) showed no significant effect on the percentage of PCEs with respect to the sum of PCEs and NCEs, or on the occurrence of MNPCEs in PCEs or the occurrence of MNNCEs in NCEs in the femur bone marrow when respectively compared with that in the control (C-Gr). On the other hand, TBXI results in a marked reduction in the number of PCEs expressed as a percentage of the sum of PCEs and NCEs, and a significant increase in the occurrences of MNPCEs and MNNCEs when respectively compared with that in the control (C-Gr). Exposure to both CRIS and TBXI ((R+IR)-Gr) led to a decreased number of PCEs expressed as a percentage of the sum of PCEs and NCEs, and increased occurrences of MNPCEs and MNNCEs when respectively compared with that in the control (C-Gr). The effects of exposure to both CRIS and TBXI was comparable with that from exposure to TBXI alone. These results indicated that CRIS alone did not have a significant cytotoxic or genotoxic effect on the bone marrow erythrocytes. CRIS did not markedly modulate the cytotoxic and genotoxic effects from TBXI on the bone marrow erythrocytes.
Fig. 3.
Effect of CRIS and TBXI on the femur bone marrow erythrocytes of mice. Group mean ± SD of the percentage of PCEs to the sum of PCEs and NCEs (A), the number of MNPCEs per 1000 PCEs (B), and the number of MNNCEs per 1000 NCEs (C). ‘*’ and ‘**’ stand for significant difference between two groups compared at P < 0.05 and P < 0.01, respectively.
DISCUSSION
Humans are exposed to a multitude of PPSs that have a dramatic adverse impact on health, such as inhibition of the immune system, increased susceptibility to infections, and altered disease risk later in life [46–48]. In fact, it could be that stress can induce genetic, epigenetic and genotoxical changes in humans and animals alike. On the other hand, exposure to IR can result in varied health effects on humans. However, little is known about the combined health consequences from exposure to both stresses and IR. Notably, the contamination of environments with radionuclides resulting from NPPAs could give rise to consequences that encompass far more than health risks from exposure to IR [49]. As the experience of the Chernobyl nuclear disaster demonstrated, the long-term psychosocial consequences are serious. There is a wide range of mental and behavioral sequelae in children following exposure to stress that can last a long time [14] and even cause shortening of telomere length [50]. Poor mental health status due to anxiety about IR exposure has been reported, even in the younger generation born after the accident in the region around Chernobyl [51]. In animal models, traumatic stress in early life was found to alter mouse microRNA expression, and behavioral and metabolic responses in the progeny [52]. CRIS was able to reduce significant body weight gain from 1 week after onset of restraint in rats [53]. CRIS promotes immune suppression, inducing lymphocyte reduction [35, 54], and exposure of rats to continuous stress from photoperiod, temperature and noise was observed to cause an increase in micronuclei incidence in peripheral RBCs [55]. A recent study showed that CRIS increased the susceptibility of Trp53-heterozygous mice to radiation (4 Gy) carcinogenesis [30]. The regulation of multiple Trp53 stress responses was mediated through MDM2 [56], and attenuation of Trp53 function was an important part of the mechanism underlying promotion by CRIS of Trp53-heterozygous mice to radiation carcinogenesis [30]. Stress-induced instability of cellular mechanisms may play an important role in increasing cell division disorders. Exposure to acute restraint stress could enhance the damaging actions of an aneugenic agent (vinblastine) on mouse bone marrow erythrocytes, inducing increased frequency of micronuclei [57].
Both the academic world and the public recognize the importance of psychological consequences arising from a catastrophic accident and its aftermath, and seek strategies for mitigating the serious consequences [6]. Thus, there is great concern about whether the psychological stress could cause any alterations in the response of human beings to radiation and whether the combined effects of multi-stresses and radiation would be fundamentally additive, synergistic or antagonistic. It is expected that study of the possible effects of psychological stress on responses to radiation exposure and the subsequent consequences could have important implications for the health risk of humans living with exposure to irradiation and psychological stresses. Since the Fukushima nuclear accident in Japan, children living in radioactively contaminated areas are often restricted or even prohibited from doing outdoor activities, and this is thought to cause additional strong psychological stresses. IR is genotoxic to the highly radiosensitive hematopoietic system, inducing cell injury and causing profound effects [58–60]. The mouse hematopoietic system provides a suitable model for study of the potential modifying effects of CRIS on radiosensitivity, functional recovery after IR, and IR-induced late effect [59]. Psychological stress affects a range of physiological processes, including hematopoiesis. In mice, CRIS decreased the concentration of hemoglobin in the blood, elevated circulating levels of erythropoietin and corticosterone, and resulted in a markedly increased number of erythroid progenitors and precursors in the spleen, leading to the prolonged activation of stress erythropoiesis pathways, and resulting in excessive production of immature erythroid cells, which may predispose chronically stressed subjects to a higher risk of leukaemic transformation [61]. On the other hand, it was reported that chronic stress could also influence hematopoietic stem cells (HSC) and lineages, causing increased proliferation of HSCs in humans and mice, leading to increased numbers of myeloid and lymphoid progenitors in bone marrow, and resulting in an increased output of neutrophils and inflammatory monocytes [62]. In the present study, we set out to identify the effects of radiation on the hematopoietic system that may be altered in a CRIS mouse model using alterations in hematology in peripheral blood and incidence of micronuclei in bone marrow erythrocytes as the endpoints. The reproducibility of this model was verified by decreased body weight gain, decreased immune organ weight, and an increased stress hormone level in the blood plasma. Results of the PLT count showed a significant reduction in animals that received both CRIS and TBXI when compared with that in animals receiving either CRIS or TBXI alone. The biological consequences of a severely decreased PLT count are bleeding and loss of body fluid. This result may suggest a health problem requiring further study. However, except for the results for the PLT count, the results for most of the endpoints did not reveal a significant synergistic or antagonistic effect from CRIS on TBXI-induced cytotoxicity and genotoxicity in the hematopoietic system. In mice receiving CRIS alone, no marked change in erythrocyte proliferation was observed in the bone marrow; however, a significantly decreased RBC count was found in the peripheral blood. As the lifespan of RBCs in peripheral blood is ∼40 days, and 18–21 days are required to produce a mature RBC from the burst-forming unit-erythroid [63, 64], results may suggest that RBCs in the peripheral blood may undergo excessive eryptosis, possibly resulting from CRIS-induced oxidative stress [65], and young RBCs produced under CRIS conditions [66] may be predisposed to CRIS and have a short lifespan in the peripheral blood.
Though different endpoints were used, the results obtained in the present study are not inconsistent with the report on increased susceptibility induced by CRIS for Trp53-heterozygous mice to radiation carcinogenesis [30]. It should be noticed that, although the mice used in these two studies were of the same strain, Trp53wt animals were used in the present study. It is known that chronic stresses induce susceptibility to pathogens, and that toxicological assaults (including IR) on health are dependent on the genetics of the exposed organism [14, 31–34, 37, 67]. Based on these previous studies and the results obtained in the present work, we suggest that CRIS would have little influence on the sensitivity of Trp53wt mice to radiation effects on the hematopoietic system, including genotoxic effects. It is possible that the methodology of our experimental system was not sensitive enough to detect the influence of CRIS on the genotoxic effect. To improve the sensitivity for detection of genomic damage, further study using the fluorescence in situ hybridization technique for detection of chromosome aberrations in splenic cells is in progress. As investigations in both humans and laboratory animals have demonstrated the existence of sex differences in response to stresses [68–72] (a recent study even showed that chronic prenatal restraint stress could induce memory impairment in a sex-specific manner in post weaning rats [73]), further study using different strains of mice and both genders is recommended.
In summary, our results suggest that CRIS does not have a significant modifying impact, either synergistic or antagonistic, on radiation-induced detrimental effects on the hematopoietic system in young Trp53tw mice under the present experimental setup. For most people, especially those living in radioactively contaminated areas, the present work may partially allay their concern that stresses could increase their susceptibility to cancer as a result of radiation.
FUNDING
This work was supported by both the Ministry of the Environment, Japan, and National Institute of Radiological Sciences, Japan. It was conducted as part of the Study of the Health Effects of Radiation organized by the Ministry of the Environment, Japan. Funding to pay the Open Access publication charges for this article was provided by National Institute of Radiological Sciences, Japan.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest. Grantors had no role in the study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
ACKNOWLEDGEMENTS
The authors would like to thank Ms Yasuko Morimoto, Ms Kyoko Sakuma, Ms Taeko Iwai, Mr Sadao Hirobe, Mr Ikutarou Saito, Ms Mikiko Nakajima and Ms Hiromi Arai for their expert technical assistance and administrative support. Critical and constructive comments on the data analysis and manuscript preparation from Dr Yi Shang are gratefully acknowledged. We particularly thank the anonymous peer reviewers for providing constructive comments that strengthened the presentation of this work.
REFERENCES
- 1.Leon GR. Overview of the psychosocial impact of disasters. Prehosp Disaster Med 2004;19:4–9. [DOI] [PubMed] [Google Scholar]
- 2.Stephan V. Chernobyl: poverty and stress pose ‘bigger threat’ than radiation. Nature 2005;437:181. [DOI] [PubMed] [Google Scholar]
- 3.Bromet EJ, Havenaar JM. Psychological and perceived health effects of the Chernobyl disaster: a 20-year review. Health Phys 2007;93:516–21. [DOI] [PubMed] [Google Scholar]
- 4.Bromet EJ, Havenaar JM, Guey LT. A 25 year retrospective review of the psychological consequences of the Chernobyl accident. Clin Oncol 2011;23:297–305. [DOI] [PubMed] [Google Scholar]
- 5.Boice JD., Jr Radiation epidemiology: a perspective on Fukushima. J Radiol Prot 2012;32:N33–40. [DOI] [PubMed] [Google Scholar]
- 6.González AJ, Akashi M, Boice J, et al. Radiological protection issues arising during and after the Fukushima nuclear reactor accident. J Radiol Prot 2013;33:497–571. [DOI] [PubMed] [Google Scholar]
- 7.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999;99:2192–217. [DOI] [PubMed] [Google Scholar]
- 8.Trachtman JN. Post-traumatic stress disorder and vision. Optometry 2010;81:240–52. [DOI] [PubMed] [Google Scholar]
- 9.Hyland ME, Alkhalaf AM, Whalley B. Beating and insulting children as a risk for adult cancer, cardiac disease and asthma. J Behav Med 2013;36:632–40. [DOI] [PubMed] [Google Scholar]
- 10.Floras JS. Blood pressure variability: a novel and important risk factor. Can J Cardiol 2013;29:557–63. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann N, Gyntelberg F, Faber J. The appraisal of chronic stress and the development of the metabolic syndrome: a systematic review of prospective cohort studies. Endocr Connect 2014;3:R55–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toufexis D, Rivarola MA, Lara H, et al. Stress and the reproductive axis. J Neuroendocrinol 2014;26:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Bonsdorff MB, von Bonsdorff M, Kulmala J, et al. Job strain in the public sector and hospital in-patient care use in old age: a 28-year prospective follow-up. Age Ageing 2014;43:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kar N. Psychological impact of disasters on children: review of assessment and interventions. World J Pediatr 2009;5:5–11. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell C, Hobcraft J, McLanahan SS, et al. Social disadvantage, genetic sensitivity, and children's telomere length. Proc Natl Acad Sci U S A 2014;111:5944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song C, Kelly JP, Leonard BE. The effect of stressful behavioural exposure on endocrine and immune parameters in the rat. Stress Med 1994;10:239–45. [Google Scholar]
- 17.Vogel WH, Jensh R. Chronic stress and plasma catecholamine and corticosterone levels in male rats. Neurosci Lett 1988;8:183–8. [DOI] [PubMed] [Google Scholar]
- 18.Himmerich H, Fischer J, Bauer K, et al. Stress-induced cytokine changes in rats. Eur Cytokine Netw 2013;24:97–103. [DOI] [PubMed] [Google Scholar]
- 19.Kim JG, Jung HS, Kim K, et al. Basal blood corticosterone level is correlated with susceptibility to chronic restraint stress in mice. Neurosci Lett 2013;555:137–42. [DOI] [PubMed] [Google Scholar]
- 20.Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A 2008;105:359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gądek-Michalska A, Spyrka J, Rachwalska P, et al. Influence of chronic stress on brain corticosteroid receptors and HPA axis activity. Pharmacol Rep 2013;65:1163–75. [DOI] [PubMed] [Google Scholar]
- 22.Mayer N, Greco C, Bertuzzi M, et al. Immobilization stress responses in adult rats exposed in utero to immobilization. Stress Health 2011;27:e1–10. [DOI] [PubMed] [Google Scholar]
- 23.Soberanes-Chávez P, López-Rubalcava C, de Gortari P, et al. Exposure to toluene and stress during pregnancy impairs pups' growth and dams' lactation. Neurotoxicol Teratol 2013;40:9–16. [DOI] [PubMed] [Google Scholar]
- 24.Veru F, David P, Laplante DP, et al. Prenatal maternal stress exposure and immune function in the offspring. Stress 2014;17:133–48. [DOI] [PubMed] [Google Scholar]
- 25.Johansson L, Guo X, Hällström T, et al. Common psychosocial stressors in middle-aged women related to longstanding distress and increased risk of Alzheimer's disease: a 38-year longitudinal population study. BMJ Open 2013;3:e003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flint M, McCarty K, Jenkins F, et al. Psychological stress accelerates the onset of tumour formation and alters the type and location of tumours in a DMBA mouse carcinogenesis model. Stress Health 2011;27:e129–38. [Google Scholar]
- 27.Frick LR, Arcos ML, Rapanelli M, et al. Chronic restraint stress impairs T-cell immunity and promotes tumor progression in mice. Stress 2009;12:134–43. [DOI] [PubMed] [Google Scholar]
- 28.Lin Q, Wang F, Yang R, et al. Effect of chronic restraint stress on human colorectal carcinoma growth in mice. PLoS One 2013;8:e61435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan S, Karpova Y, Baiz D, et al. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest 2013;123:874–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Z, Liu L, Zhang C, et al. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci U S A 2012;109:7013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matamoros RA, Levine BS. Stress response and drug metabolism in mice. Fundam Appl Toxicol 1996;30:255–63. [DOI] [PubMed] [Google Scholar]
- 32.Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don't. Ann NY Acad Sci 1999;896:3–15. [DOI] [PubMed] [Google Scholar]
- 33.Friedman EM, Lawrence DA. Environmental stress mediates changes in neuroimmunological interactions. Toxicol Sci 2002;67:4–10. [DOI] [PubMed] [Google Scholar]
- 34.Cao L, Hudson CA, Lawrence DA. Immune changes during acute cold/restraint stress-induced inhibition of host resistance to Listeria. Toxicol Sci 2003;74:325–34. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Foster R, Sun X, et al. Restraint stress induces lymphocyte reduction through p53 and PI3 K/NF-kappaB pathways. J Neuroimmunol 2008;200:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin J, Wang X, Wang Q, et al. Chronic psychological stress induces the accumulation of myeloid-derived suppressor cells in mice. PLoS One 2013;8:e74497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson SB, Riley AW, Granger DA, et al. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics 2013;131:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saenko V, Ivanov V, Tsyb A, et al. The Chernobyl accident and its consequences. Clin Oncol 2011;23:234–43. [DOI] [PubMed] [Google Scholar]
- 39.Fushiki S. Radiation hazards in children – lessons from Chernobyl, Three Mile Island and Fukushima. Brain Dev 2013;35:220–7. [DOI] [PubMed] [Google Scholar]
- 40.Bromet EJ. Emotional consequences of nuclear power plant disasters. Health Phys 2014;106:206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bromet EJ, Goldgabe D, Carlson G, et al. Children's well-being 11 years after the Chernobyl catastrophe. Arch Gen Psychiatry 2000;57:563–71. [DOI] [PubMed] [Google Scholar]
- 42.Schmid M. The micronucleus test. Mutat Res 1975;31:9–15. [DOI] [PubMed] [Google Scholar]
- 43.Chaubey RC, Bhilwade HN, Joshi BN, et al. Studies on the migration of micronucleated erythrocytes from bone marrow to the peripheral blood in irradiated Swiss mice. Int J Radiat Biol 1993;63:239–45. [DOI] [PubMed] [Google Scholar]
- 44.Wang B, Tanaka K, Ninomiya Y, et al. Relieved residual damage in the hematopoietic system of mice rescued by radiation-induced adaptive response (Yonezawa Effect). J Radiat Res 2013;54:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki Y, Nagae Y, Li J, et al. The micronucleus test and erythropoiesis. Effects of erythropoietin and a mutagen on the ratio of polychromatic to normochromatic erythrocytes (P/N ratio) . Mutagenesis 1989;4:420–4. [DOI] [PubMed] [Google Scholar]
- 46.Dhabhar FS, Miller AH, Stein M, et al. Diurnal and acute stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav Immun 1994;8:66–79. [DOI] [PubMed] [Google Scholar]
- 47.Vanhoudt N, Vandenhove H, Real A, et al. A review of multiple stressor studies that include ionising radiation. Environ Pollut 2012;168:177–92. [DOI] [PubMed] [Google Scholar]
- 48.Zieziulewicz TJ, Mondal TK, Gao D, et al. Stress-induced effects, which inhibit host defenses, alter leukocyte trafficking. Cell Stress Chaperones 2013;18:279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oughton DH. Social and ethical issues in environmental remediation projects. J Environ Radioact 2013;119:21–5. [DOI] [PubMed] [Google Scholar]
- 50.Drury SS, Mabile E, Brett ZH, et al. The association of telomere length with family violence and disruption. Pediatr 2014;134:e128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masunaga T, Kozlovsky A, Lyzikov A, et al. Mental health status among younger generation around Chernobyl. Arch Med Sci 2013;9:1114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gapp K, Jawaid A, Sarkies P, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 2014;17:667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiba S, Numakawa T, Ninomiya M, et al. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Bio Psychiatry 2012;39:112–9. [DOI] [PubMed] [Google Scholar]
- 54.Shi Y, Devadas S, Greeneltch KM, et al. Stressed to death: implication of lymphocyte apoptosis for psychoneuroimmunology. Brain Behav Immun 2003;17 Suppl 1:S18–26. [DOI] [PubMed] [Google Scholar]
- 55.Adam ML, Torres MFP, Franci AC, et al. On the stress by photoperiod, temperature and noise as possible causes of genomic damaging in an animal model. Stress Health 2011;27:e152–6. [Google Scholar]
- 56.Hu W, Feng Z, Levine AJ. The regulation of multiple p53 stress responses is mediated through MDM2. Genes Cancer 2012;3:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malvandi AM, Haddad F, Moghimi A. Acute restraint stress increases the frequency of vinblastine-induced micronuclei in mouse bone marrow cells. Stress 2010;13:276–80. [DOI] [PubMed] [Google Scholar]
- 58.Kulkarni S, Ghosh SP, Hauer-Jensen M, et al. Hematological targets of radiation damage. Curr Drug Targets 2000;11:1375–85. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Schulte BA, LaRue AC, et al. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 2006;107:358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shao L, Luo Y, Zhou D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid Redox Signal 2014;20:1447–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vignjević S, Budeč M, Marković D, et al. Chronic psychological stress activates BMP4-dependent extramedullary erythropoiesis. J Cell Mol Med 2014;18:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heidt T, Sager HB, Courties G, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med 2014;20:754–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Putten LM. The life span of red cells in the rat and the mouse as determined by labeling with DFP32 in vivo. Blood 1958;13:789–94. [PubMed] [Google Scholar]
- 64.Doig K. Erythrocyte production and destruction. In: Rodak BF, Fritsma GA, Keohane EM. (eds). Hematology: Clinical Principles and Applications. Elsevier Saunders, 2012, 86–102. [Google Scholar]
- 65.Lang F, Abed M, Lang E, et al. Oxidative stress and suicidal erythrocyte death. Antioxid Redox Signal 2014;21:138–53. [DOI] [PubMed] [Google Scholar]
- 66.Voorhees JL, Powell ND, Moldovan L, et al. Chronic restraint stress upregulates erythropoiesis through glucocorticoid stimulation. PLoS One 2013;8:e77935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tannenbaum B, Anisman H. Impact of chronic intermittent challenges in stressor-susceptible and resilient strains of mice. Biol Psychiatry 2003;53:292–303. [DOI] [PubMed] [Google Scholar]
- 68.Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav 2002;75:661–73. [DOI] [PubMed] [Google Scholar]
- 69.Elliott BM, Grunberg NE. Effects of social and physical enrichment on open field activity differ in male and female Sprague-Dawley rats. Behav Brain Res 2005;165:187–96. [DOI] [PubMed] [Google Scholar]
- 70.Kallai J, Makany T, Karadi K, et al. Spatial orientation strategies in Morris-type virtual water task for humans. Behav Brain Res 2005;159:187–96. [DOI] [PubMed] [Google Scholar]
- 71.van Well S, Kolk AM, Klugkis IG. Effects of sex, gender role identification, and gender relevance of two types of stressors on cardiovascular and subjective responses: sex and gender match and mismatch effects. Behav Modif 2008;32:427–49. [DOI] [PubMed] [Google Scholar]
- 72.Woolley DG, Vermaercke B, Op de Beeck H, et al. Sex differences in human virtual water maze performance: novel measures reveal the relative contribution of directional responding and spatial knowledge. Behav Brain Res 2010;208:408–14. [DOI] [PubMed] [Google Scholar]
- 73.Cherian SB, Bairy KL, Rao MS. Chronic prenatal restraint stress induced memory impairment in passive avoidance task in post weaned male and female Wistar rats. Indian J Exp Biol 2009;47:893–9. [PubMed] [Google Scholar]