Abstract
Radioactive copper (II) (diacetyl-bis N4-methylthiosemicarbazone) (Cu-ATSM) isotopes were originally developed for the imaging of hypoxia in tumors. Because the decay of a 64Cu atom is emitting not only positrons but also Auger electrons, this radionuclide has great potential as a theranostic agent. However, the success of 64Cu-ATSM internal radiation therapy would depend on the contribution of Auger electrons to tumor cell killing. Therefore, we designed a cell culture system to define the contributions to cell death from Auger electrons to support or refute our hypothesis that the majority of cell death from 64Cu-ATSM is a result of high-LET Auger electrons and not positrons or other low-LET radiation. Chinese hamster ovary (CHO) wild type and DNA repair–deficient xrs5 cells were exposed to 64Cu-ATSM during hypoxic conditions. Surviving fractions were compared with those surviving gamma-radiation, low-LET hadron radiation, and high-LET heavy ion exposure. The ratio of the D10 values (doses required to achieve 10% cell survival) between CHO wild type and xrs5 cells suggested that 64Cu-ATSM toxicity is similar to that of high-LET Carbon ion radiation (70 keV/μm). γH2AX foci assays confirmed DNA double-strand breaks and cluster damage by high-LET Auger electrons from 64Cu decay, and complex types of chromosomal aberrations typical of high-LET radiation were observed after 64Cu-ATSM exposure. The majority of cell death was caused by high-LET radiation. This work provides strong evidence that 64Cu-ATSM damages DNA via high-LET Auger electrons, supporting further study and consideration of 64Cu-ATSM as a cancer treatment modality for hypoxic tumors.
Keywords: auger electron, high LET, 64Cu-ATSM, DNA double strand break, CHO
INTRODUCTION
Radiolabeled copper (II) (diacetyl-bis N4-methylthiosemicarbazone) (Cu-ATSM) was originally designed as a positron emission tomography (PET) agent [1]. Later, researchers found Cu-ATSM preferentially accumulates in hypoxic tumor regions, enabling non-invasive identification and quantification of hypoxic tissue [2] and providing a clinically appealing method for assessing a tumor's oxygenation state. Hypoxic tumors are resistant to conventional radiation therapy and chemotherapy due to less oxygen tension and poor drug delivery. Therefore, the tumor's hypoxic state has been known as a useful prognostic factor for many years. Recently, it has been discovered that Cu-ATSM-positive but fluorodeoxyglucose (FDG)-negative tumor regions are rich in cancer stem cells [3]. Cancer stem cells are a subpopulation within tumors that may be involved in tumor progression and resistance to cancer treatments, including radiation therapy and chemotherapy. Therefore, radiolabeled Cu-ATSM is a promising agent for targeting the regions related to therapeutic resistance.
The design of Cu-ATSM with a variety of radioactive Cu nuclides allows for the development of a new Cu-ATSM as a theranostic: namely 64Cu-ATSM for internal radiation therapy with simultaneous monitoring of the radiation dose. Use of 64Cu-ATSM for internal radiation therapy with simultaneous monitoring of the radiation dose is attractive based on a number of critical factors [4]. It decays by emission of β+ (0.656 MeV, 19%), β– (0.573 MeV, 40%), γ (1.346 MeV, 0.5%) and electron capture (41%), with a half-life of 12.7 h [5]. Electron capture results in cascades of high-LET Auger electrons, which can overcome a low oxygen enhancement ratio (OER) in hypoxic tumors [6] and kill tumor cells efficiently with higher relative biological effectiveness (RBE) [7] than low-LET radiation. There are several indications that high-LET radiation can efficiently kill radioresistant cells, including cancer stem cells [8, 9]. Auger electrons from 64Cu decay have an average of 2 keV of energy, ∼126 nm range in tissue [10], and are considered high-LET radiation. Therefore, when a 64Cu atom is taken into a hypoxic cell and decays within range of tumor cell DNA, the emitted Auger electrons can kill tumor cells. If the high-LET Auger electrons are the primary cause of cell death, 64Cu-ATSM would be ideal to not only target the radioresistant hypoxic tumor region but also the radioresistant cancer stem cells. Although previous studies have clearly shown the cytotoxicity of 64Cu-ATSM in tumor cells from DNA damage [11], there is no direct evidence that Auger electrons are the primary cause of cell death after 64Cu-ATSM treatment.
This study evaluated the hypothesis that the majority of cell death from 64Cu-ATSM exposure is from high-LET Auger electrons. To characterize cell death from high-LET radiation damage, DNA repair–proficient and –deficient Chinese hamster ovary (CHO) cell lines were used in this study. We exposed those cells to 64Cu-ATSM and other types of ionizing radiation with a variety of LET qualities to determine the similarity of the cell death trends. DNA double-strand breaks and chromosomal aberrations after 64Cu-ATSM exposure were analyzed to determine the characteristics of DNA damage produced by radiation originating from 64Cu.
MATERIALS AND METHODS
64Cu-ATSM preparation
64Cu, generated by a 64Ni(p,n)64Cu reaction [12], was purchased from the University of Wisconsin–Madison Cyclotron Research Group (Madison, WI). The preparation of 64Cu-ATSM was performed as previously reported [13, 14]. The radiolabeling efficiency of 64Cu-ATSM was determined using silica gel thin-layer chromatography with ethyl acetate as the mobile phase [15]. The radiolabeling efficiency was an average of 93.1%, with a standard deviation of 4.7 (n = 8).
Cell culture
Wild-type CHO cells (CHO10B2) and the non-homologous end-joining (NHEJ) repair-deficient xrs5 cells (ku80 deficient) were graciously supplied by Dr Joel Bedford (Colorado State University, Fort Collins, CO) [16]. Cell cultures were maintained in Eagle's Minimal Essential Medium Alpha (MEM-α) (Gibco, Indianapolis, IN) augmented with 10% heat inactivated fetal bovine serum (FBS, Sigma, St Louis, MO), 1% Penicillin and Streptomycin anti-microbial and 0.1% Fungizone antimycotic (Gibco). Cells were maintained in 5% CO2 at 37°C in a humidified incubator. Cell doubling time is ∼12.3 h for CHO wild type and ∼15.0 h for xrs5 cells. Exponential growth cells were used for 64Cu-ATSM uptake measurement and all cell survival experiments. G1 cell synchronization was carried out for γH2AX foci analysis and chromosomal aberration assays. G1 cell synchronization was confirmed with a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ).
Hypoxic treatments and 64Cu-ATSM treatment
Cells to be incubated with 64Cu-ATSM in hypoxic conditions were pretreated in anoxic conditions 1 h prior to the addition of 64Cu-ATSM. Anoxic conditions were maintained in a manner similar to previous studies [11]. Briefly, cell cultures were kept in a 37°C incubator with a continuous flow of 100% nitrogen gas for 1 h [17]. Hypoxia was achieved using the AnaeroPack system (Mitsubishi Gas Chemical, Tokyo, Japan) [18]. The cell cultures were placed into an airtight container with AnaeroPack oxygen-absorbing and CO2-generating agents to reduce the O2 concentration to <1%. The cell cultures were treated with64Cu-ATSM in this hypoxic chamber for 3 h at 37°C.
64Cu-ATSM uptake
Cells were plated at 200 000 cells per 60 mm cell culture dish and incubated overnight. A total of 185 000 Bq (5 μCi) of 64Cu-ATSM was then added to each dish. After incubation for 3 h, cells were washed with PBS and trypsinized. Media, PBS and cells with trypsin were individually retained and mixed with Ultima Gold liquid scintillation cocktail (PerkinElmer, Waltham, MA) to analyze radioactivity in each phase. Vials were shaken and counted on a Beckman LS-5801 liquid scintillation counter (Beckman Coulter, Brea, CA) using the full channel window. Quench differences between the vial compositions were accounted for to determine the relative activity in the vials containing media, PBS and trypsinized cells. Activity present in trypsinized cells was assumed to solely result from cellular incorporation of 64Cu.
External irradiation
Exponentially growing cells were exposed to radiation at room temperature. Gamma-ray irradiations (LET 0.3 keV/µm) were carried out at a dose rate of 2.5 Gy/min using a Model Mark I-68A nominal 222TBq (6,000 Ci) 137Cesium sealed source (J.L. Shepherd, Carlsbad, CA). Hadron radiation experiments were carried out at the National Institute of Radiological Sciences (NIRS) in Chiba, Japan [19]. Protons were accelerated to 70 MeV (LET 1.1 keV/µm) using the NIRS-930 cyclotron at NIRS [20]. Carbon-ions and iron-ions were accelerated to 290 MeV/n and 500 MeV/n, respectively, using the heavy ion medical accelerator (HIMAC) at NIRS. The LET of the entrance region for monoenergetic carbon ions and monoenergetic iron ions were 13 and 200 keV/μm, respectively. Monoenergetic carbon ions with a LET of 70 keV/μm were obtained by Lucite attenuation. The dose-averaged LET of the carbon ions at the middle of the 6-cm spread-out Bragg peak (SOBP) is ∼50 keV/µm at a distance of 119 mm from the entrance [21]. Dose rates for carbon-ions, iron-ions and protons were set at 3 Gy/min. All irradiation was carried out at room temperature. LET values of the various radiation types are summarized in Table 1.
Table 1.
Ratios of D10 doses for CHO wild-type and xrs5 cells exposed to various radiations
| Radiation | LET keV/μm | (A) D10 of CHO10B2 | (B) D10 of xrs5 | Ratio (A)/(B) |
|---|---|---|---|---|
| Gamma rays | 0.3 | 6.37 Gy | 1.18 Gy | 5.40 |
| Proton Mono | 1.1 | 5.31Gy | 1.16 Gy | 4.58 |
| Carbon Mono | 13 | 3.79 Gy | 0.91 Gy | 4.16 |
| Carbon SOBP | 50 | 3.16 Gy | 1.05 Gy | 3.01 |
| Carbon Mono | 70 | 2.49 Gy | 0.94 Gy | 2.65 |
| Iron Mono | 200 | 1.89 Gy | 1.00 Gy | 1.89 |
| 64Cu-ATSM | NA | 0.97 Bq/cell | 0.40 Bq/cell | 2.43 |
Colony formation assay
For the external irradiation, exponentially growing cells were exposed to the radiation sources described above. Cells were then trypsinized and immediately plated in dishes at an appropriate cell density for colony forming. For the internal irradiation by 64Cu-ATSM, single cells were plated onto culture flasks and allowed to adhere for 2 h. After various doses of 64Cu-ATSM incubation, all cells were incubated under normoxic conditions for colony formation. Colonies were fixed and stained 7–10 days later using 100% ethanol followed by 0.1% crystal violet. Macroscopic colonies containing >50 cells were marked as survivors [22]. Each experiment was performed at least three times. Cell-survival curves were drawn from cell-surviving fractions in GraphPad Prism 6 (GraphPad, La Jolla, CA) using a linear quadratic regression model. D10 values (radiation doses required to achieve 10% cell survival) were obtained from the cell survival curves using a linear quadratic regression model. The ratios of D10 doses between CHO wild type and xrs5 were used as a relative metric for the LET of the radiation.
γH2AX foci formation assay
Cells were synchronized on chamber slides in the G1 phase by mitotic shake off and 2 h of incubation [23]. Following 64Cu-ATSM incubation for 3 h, cells were fixed in 4% paraformaldehyde and permeabilized for 5 min in 0.2% Triton X-100 (Sigma, St Louis, MO) in PBS, and blocked overnight at 4°C in 10% goat serum solution. Immunostaining was carried out with anti-γH2AX mouse monoclonal antibody (Millipore, Billerica, MA) and Alexa Fluor488-conjugated goat anti-mouse IgG antibody (Invitrogen, Grand Island, NY). Cells were mounted with ProLong Gold Antifade Reagent with DAPI (Invitrogen). Fluorescence images were captured using a Zeiss Axioskop motorized z-stage fluorescent microscope (Zeiss, Jena, Germany) equipped with CoolSNAP HQ2 (Photometrics, Tucson AZ). One-micron-thick z-stack images were obtained by Metamorph software (Molecular Devices, Sunnyvale, CA). Three independent experiments were performed. Manual counting was performed for foci analysis.
Chromosomal aberration assay
Cells were synchronized into the G1 phase by mitotic shake off and 2 h of incubation. After 64Cu-ATSM was added, cells were incubated for 3 h under hypoxic conditions, then 0.1 μg/ml Colcemid (KaryoMAX® Colcemid™ Solution in PBS, Gibco) was added and incubated for 12–16 h under normoxic conditions. Since we observed a wide variety in a degree of chromosome aberrations after 64Cu-ATSM exposure, we assumed faster entry of less damaged cells and slower entry of heavily damaged cells to mitosis. This prolonged Colcemid treatment was aimed to collect a maximum population in metaphase cells. Harvested cells were treated with hypotonic solution (75 mM KCl) for 20 min at 37°C and fixed with methanol:acetic acid (3:1) solution three times before being dropped onto slides. Samples were stained with 5% (v/v) Giemsa solution in Gurr's buffer (Gibco). At least 50 metaphase cells were scored in at least two separate experiments. Chromosomal aberrations were quantified and classified as various chromosome and chromatid type aberrations.
Statistical methods
Analysis of variance was used to determine statistical significance with GraphPad Prism 6. Standard errors of the means for data were calculated and were depicted in each figure.
RESULTS
64Cu-ATSM uptake
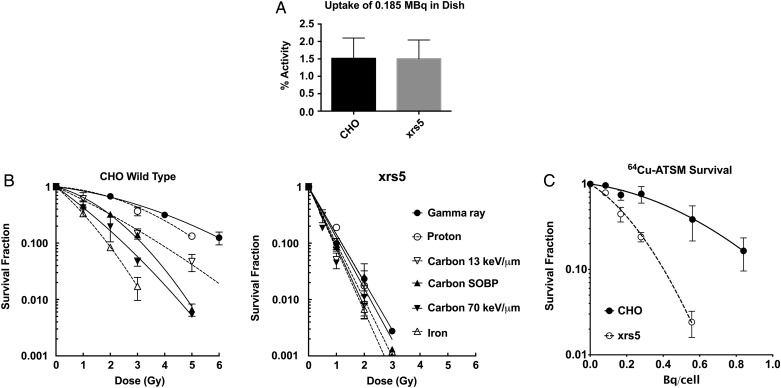
The amount of cellular uptake of 64Cu was compared at 0.185 MBq in P60 dishes under hypoxic conditions (Fig. 1A). Both DNA repair–proficient CHO wild type and –deficient xrs5 cells showed an uptake of ∼1.4% of total activity. There were no significant differences in uptake between cell lines. Therefore, mutation of Ku80 in xrs5 did not affect cellular uptake of 64Cu for CHO cells.
Fig. 1.
64Cu-ATSM uptake and survival curves. (A) 64Cu-ATSM uptake in CHO wild-type and xrs5 cells. (B) Survival curves of CHO wild-type and xrs5 cells exposed to radiation of varying LETs. (C) Cell-survival curves of CHO wild-type and xrs5 cells exposed to 64Cu-ATSM and incubated in hypoxia. Error bars indicate standard errors of the mean of at least three independent experiments.
Colony formation assay
Fig. 1B shows the cell survival curves of CHO wild-type and xrs5 cells after various types of ionizing radiation exposure. CHO wild-type cells showed higher radiosensitivity to higher-LET radiation such as carbon ion LET 70 keV/μm and iron ion LET 200 keV/μm. xrs5 cells were more sensitive than CHO wild-type cells for all types of ionizing radiation. Radiosensitivity of xrs5 was dependent on absorbed dose but not LET.
Figure 1C shows the cell-survival curves of CHO wild-type and xrs5 cells exposed to 64Cu-ATSM. CHO wild-type cells were more resistant to 64Cu-ATSM than xrs5 cells. The cell-survival curves against 64Cu-ATSM exposure fit well with the linear quadratic regression curves.
D10 values were obtained from linear quadratic regression curves (Table 1). D10 values for CHO wild-type cells ranged from 1.89 to 6.37 Gy, while values for xrs5 deviated very little from 1.00 to 1.18 Gy (iron and gamma irradiation, respectively) (Table 1). Ratios of D10 doses (CHO WT/xrs5) were evaluated and used as a relative metric for the LET of the radiation with lower ratios, closer to the high-LET carbon ions or iron ions, indicating higher LET. Survival curves for cells exposed to 64Cu-ATSM showed a D10 ratio of 2.43, just smaller than that of the carbon ion LET 70 keV/μm (ratio = 2.65).
γH2AX foci formation assay
Cells exposed to 64Cu-ATSM under hypoxic conditions showed the formation of γH2AX foci, and it was 64Cu dose dependent (Fig. 2A). This directly supports the hypothesis that 64Cu-ATSM treatments can produce DNA double-strand breaks. The number of induced foci per cell was twice as much in xrs5 compared with that in CHO wild-type cells. For every Bq per cell of uptake, the foci count increased by 11.6 for CHO wild-type cells and by 23.9 for xrs5 cells with the 3 h incubation of 64Cu-ATSM. We also observed several clusters of foci in both CHO wild-type and xrs5 cells after 64Cu-ATSM exposures (Fig. 2B). The difference in the number of foci between CHO wild-type and xrs5 cells may result from the differences in repair capacity during the 3 h of exposure time to 64Cu-ATSM.
Fig. 2.
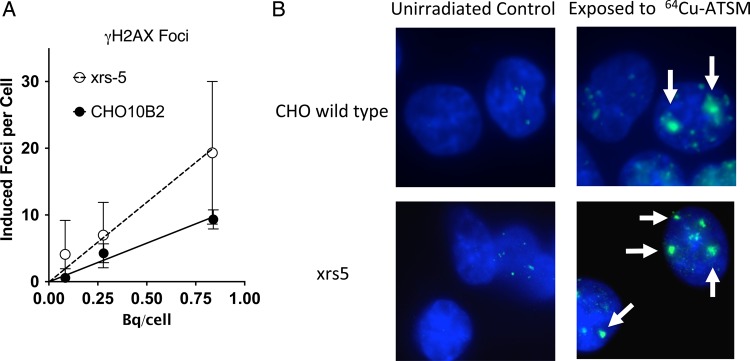
γH2AX foci formation after 64Cu-ATSM exposures. (A) Dose response of γH2AX foci formation after 64Cu-ATSM exposures. Foci response of cells incubated with varied activities normalized to background. Data points are the mean of at least three experiments. Error bars represent the standard error of the mean. (B) Examples of γH2AX foci formation in CHO wild-type and xrs5 cells without irradiation and after 0.84 and 0.83 Bq/cell of 64Cu-ATSM exposure. Arrows indicate cluster foci.
Chromosomal aberrations assay
In the chromosomal aberrations assay, we observed both chromatid- and chromosome-type aberrations in CHO wild-type cells after 64Cu-ATSM treatment, and only chromatid-type aberrations in xrs5 cells (Table 2). Cells were initially synchronized into G1 and exposed to 64Cu-ATSM. When DNA double-strand breaks are produced in G1, chromosome-type aberrations are formed. In S and G2, chromatid-type aberrations are formed instead. 64Cu decays during cell cycle progression and damages DNA in G1, S and G2 phases before cells reach mitosis. Therefore, both chromatid- and chromosome-type aberrations were observed in our experimental conditions.
Table 2.
Chromosomal aberrations of CHO10B2 and xrs5 cells exposed to 64Cu-ATSM
| Dose (Bq/cell) | Total number of cell scored | Total chromosomal aberrations per cell | Total chromatid type per cell | Chromatid breaks per cell | Chromatid exchange per cell | Total chromosome type per cell | Interstitial & terminal deletion per cell | Dicentric & ring per cell |
|---|---|---|---|---|---|---|---|---|
| CHO10B2 | ||||||||
| 0 | 197 | 0.036 | 0.015 | 0.015 | 0 | 0.02 | 0.02 | 0 |
| 0.084 | 200 | 0.105 | 0.050 | 0.05 | 0 | 0.055 | 0.04 | 0.015 |
| 0.28 | 188 | 0.500 | 0.388 | 0.272 | 0.117 | 0.112 | 0.09 | 0.021 |
| 0.84 | 208 | 0.707 | 0.591 | 0.438 | 0.154 | 0.115 | 0.072 | 0.044 |
| xrs5 | ||||||||
| 0 | 139 | 0.309 | 0.266 | 0.244 | 0.022 | 0.043 | 0.022 | 0.021 |
| 0.083 | 145 | 1.694 | 1.660 | 1.34 | 0.319 | 0.035 | 0.007 | 0.028 |
CHO wild-type and xrs5 cells showed more chromatid-type aberrations than chromosome-type aberrations. Dose-dependent increases of both chromatid- and chromosome-type aberrations, up to 0.84 Bq per cell of 64Cu, were observed in CHO wild-type cells. On the other hand, xrs5 cells showed more noticeable increases in chromatid breaks at 0.083 Bq per cell of 64Cu. The background yields of chromosomal aberrations in xrs5 cells were relatively higher than previously reported values [24]. It is possible that radiosensitive xrs5 cells were affected by gamma rays from 64Cu decay in adjacent flasks in the incubator.
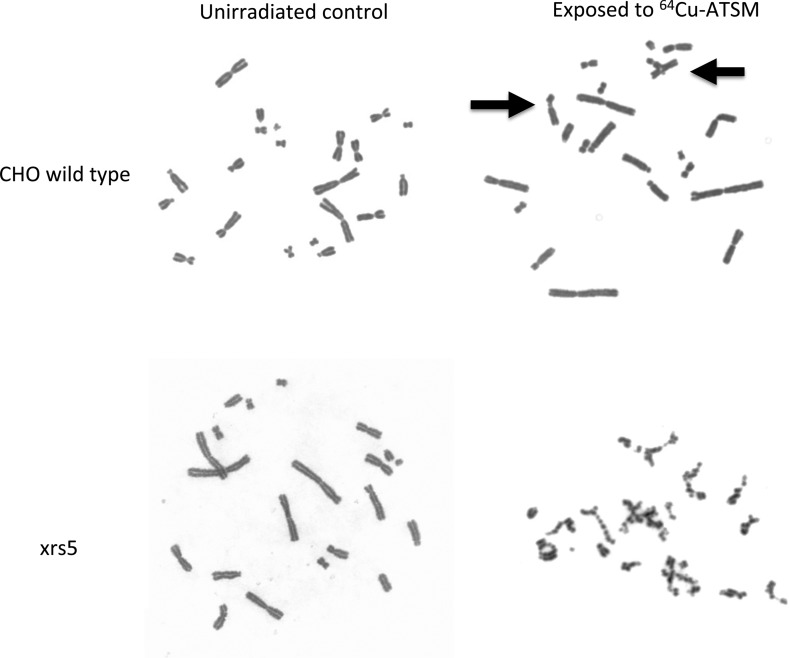
We could not determine the yield of chromosomal aberrations above 0.27 Bq per cell of 64Cu for xrs5 cells because of the severity of the chromatid-type aberrations (Fig. 3). The severe damage to chromosomes observed in the two highest activity experiments for xrs5 cells still yielded metaphase spreads, but the damages were extensive enough to prohibit chromosome aberration qualification and quantification.
Fig. 3.
Representative chromosome spreads for CHO wild-type and xrs5 cells in control group and exposed to 0.84 Bq/cell and 0.83 Bq/cell of 64Cu-ATSM, respectively. Arrows indicate chromatid-type aberrations in CHO wild-type cells after exposure to 64Cu-ATSM. On the other hand, damage in xrs5 was extensive enough to prohibit evaluation of definitive chromosomal aberrations after exposure to 0.83 Bq/cell of 64Cu-ATSM.
Chromatid-type aberrations were dominant in both cell lines for 64Cu-ATSM-induced chromosomal aberrations in our experimental conditions. The half-life of 64Cu is 12.7 h, and the cells were constantly exposed to radiation from 64Cu during the 3-h incubation period. Heavily damaged cells with chromosome-type aberrations produced in G1 stopped the cell cycle by checkpoint activation and did not reach metaphase.
DISCUSSION
The object of this study was to evaluate the role of Auger electron–induced cell killing as a mechanism of cell death after 64Cu-ATSM treatment. The complex pattern of decay modes of 64Cu, including Auger electron emissions, led to difficulties for physics studies. The largest problem was the estimation of absorbed dose from 64Cu. Monte Carlo simulation of decay of 64Cu and information about 64Cu distribution within the cells is required in order to estimate an accurate dose to the nuclei. We used the activity in the cell as a general measure of activity to estimate the radiation exposure to cells (Fig. 1A). The observed uptake in both of the cell lines was lower than in other reported tumor cell lines [11]. This may result from the relatively normal metabolic activity in the CHO cell lines.
Even with this relatively low uptake of 64Cu into the cells, cell-survival curves for CHO wild-type cells were comparable with the previous report of cell killing [11]. Radiosensitive xrs5 cells showed higher sensitivity to 64Cu-ATSM than CHO wild-type cells. Because there were no statistical differences in uptake of 64Cu activities in both the cell lines (Fig. 1A), the higher sensitivity to 64Cu-ATSM in xrs5 cells can be contributed to the DNA repair deficiency in xrs5 cells.
D10 values of repair-proficient cells decrease with LET because there is more cell death in high-LET radiation. On the other hand, it is known that NHEJ repair–deficient mutants show a smaller decrease in D10 values with LET. Especially, the D10 values of xrs5 were independent of LET [24, 25]. Late S phase of CHO wild-type cells are radioresistant. On the other hand, xrs5 cells do not have cell cycle–dependent cellular radiosensitivity [26]. NHEJ-deficient xrs5 and V3 cells are more sensitive to ionizing radiation than homologous recombination repair–deficient 51D1 and irs1SF cells [27]. This suggests that Ku80 proteins and the NHEJ repair pathway have a central role in radiation-induced damage repair. We compared the ratios between D10 values of wild-type and xrs5 cells to estimate the mean LET of lethal events associated with radiation from 64Cu-ATSM. The exposure to radiation from 64Cu-ATSM can be classified as low-dose-rate radiation because 64Cu's half-life is ∼12 h. However, the dose rate effect is known to be smaller in high LET [28]. The ratio of D10 values after 64Cu-ATSM exposure was in the range of high-LET radiation and were similar to those with 70 keV/μm carbon ions. Therefore, the dose rate effect may be a minor issue for 64Cu-ATSM exposure. This D10 ratio comparison supports the hypothesis that 64Cu-ATSM primarily kills cells with high-LET Auger electrons. However, one concern of our analysis may be that we conducted external irradiation under normoxic conditions. One report showed hypoxic conditions changed D10 values of CHO-K1 cells for X-rays from 3.8 Gy to 10.5 Gy and for carbon ions with LET of 80 keV/μm from 1.8 Gy to 3.3 Gy, normoxic and hypoxic, respectively [29]. In future studies, internal and external irradiation methods will be conducted under identical conditions.
Quantitative results of the γH2AX experiments showed a linear increase in foci formation for both CHO cell lines, as in our previous report with X-rays [30]. The NHEJ mutant xrs5 cells showed a more rapid increase in the γH2AX number compared with CHO wild-type cells. A high degree of variability was observed in the foci analysis due to what appeared to be occasional highly damaged cells and intense cluster foci (Fig. 2A). Since cells were synchronized into G1 phase after mitotic shake off and the additional 3 h treatment time with 64Cu-ATSM in hypoxia, there was not enough time for cells to enter S phase. We could exclude the possibility of any replication-dependent foci formation. Therefore, this is thought to be due to heterogeneous 64Cu-ATSM uptake in the cell population or to the difficulty of manual counting of the intense cluster foci. In addition to the quantitative results, a qualitative observation was that many 64Cu-ATSM-incubated cells, both CHO wild type and xrs5, had what appeared to be large and intense foci, along with many regular foci. These are thought to be clustered γH2AX foci [31], potentially resulting from an Auger cascade. In CHO wild-type cells with 0.8 Bq/cell uptake, ∼10 foci were produced and ∼80% of cells were dead (see Figs 2A and 1C). Based on previous research with CHO cells, 1 Gy of X-rays produced ∼30 foci and killed 10–20% of cells, and 1 Gy of carbon ions with LET 70 keV/μm produced ∼20 foci and killed 50% of cells [32]. According to this, 64Cu-ATSM exposures killed cells efficiently, with a smaller number of γH2AX foci formation (like high-LET radiation). This efficient cell killing may suggest that 64Cu-ATSM-induced γH2AX foci are more like high-LET radiation–induced clustered γH2AX foci. Therefore, these quantitative and qualitative observations of γH2AX foci after 64Cu-ATSM exposures provide further support to our hypothesis.
Finally, 64Cu-ATSM induced severe chromosomal aberrations in xrs5 cells, suggesting high-LET radiation exposure. Chromosomal aberrations, especially chromatid-type aberrations, were increased with 64Cu-ATSM treatment in both of the cell types. Radiosensitive xrs5 cells showed a number of massive multi-chromosome-associated chromosomal aberrations in high-activity 64Cu exposure (Fig. 3). Chromatid-type aberrations can be formed from (i) non-double-strand breaks in G1 phase and converted into double-strand breaks during replication collapses and (ii) post-replication double-strand breaks. High-LET radiation often produces severe DNA damage in exposed cells [32]. It is assumed that the massive damage in G1 phase tends to stop or slow down the cell cycle. This may be a reason why S/G2 damage–originated chromatid-type aberrations were dominant in both CHO wild-type and xrs5 cells after 64Cu-ATSM treatment.
125I is the well-studied Auger electron–emitting radionuclide often used as a thymidine analog in the form of 125Iodine-5-iodo-2′-deoxyuridine (125IUdR). 125IUdR is incorporated into DNA and efficiently produces DNA damage leading to high lethality. A level of 90% cell killing can be achieved with <0.01 Bq/cell [33]. 64Cu-ATSM produced 90% cell killing with 1 Bq/cell (Fig. 2B). This 100-times lower efficiency for cell killing can be explained by the cellular localization of 64Cu. Previous reports have shown that 64Cu distributes to cellular organelles, including nuclei, mitochondria and S2 fractions [11]. Therefore, the lower efficiency for cell killing can be explained with the lower accumulation of 64Cu in or near DNA compared with 125IUdR.
Auger electrons from 64Cu decay are considered as high-LET radiation [10]. When 64Cu reaches cellular nuclei, cells will be killed by Auger electron exposure but not by other types of radiation. We showed many reasons why 64Cu-ATSM-induced cell death is due to high-LET Auger electron exposure. Based on our results, future experimental and clinical research should focus on the application of 64Cu-ATSM high-LET theranostic therapy in the management of radioresistant tumors.
FUNDING
This study is partially supported by the National Institute of Radiological Sciences President's Special Grant (YF), the International Open Laboratory (AF), the Colorado Clinical and Translational Sciences Institute (TAK), the Dr Akiko Ueno Radiobiology Research Fund (TAK), the Nuclear Regulatory Commission (NRC-38-10-951 for DM) and the Colorado State University Department of Environmental Radiological Health Sciences. Funding to pay the Open Access publication charges for this article was provided by Dr Akiko Ueno Radiobiology Research Fund.
ACKNOWLEDGEMENTS
We thank NIRS cyclotron and HIMAC for support with the hadron radiation experiments.
REFERENCES
- 1.Fujibayashi Y, Taniuchi H, Yonekura Y, et al. Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med 1997;38:1155–60. [PubMed] [Google Scholar]
- 2.Lewis JS, McCarthy DW, McCarthy TJ, et al. Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J Nucl Med 1999;40:177–83. [PubMed] [Google Scholar]
- 3.Yoshii Y, Furukawa T, Kiyono Y, et al. Copper-64-diacetyl-bis (N4-methylthiosemicarbazone) accumulates in rich regions of CD133+ highly tumorigenic cells in mouse colon carcinoma. Nucl Med Biol 2010;37:395–404. [DOI] [PubMed] [Google Scholar]
- 4.Zweit J. Radionuclides and carrier molecules for therapy. Phys Med Biol 1996;41:1905–14. [DOI] [PubMed] [Google Scholar]
- 5.Johnson TE, Birky BK. Health Physics and Radiological Health. 4th edn. Lippincott Williams & Wilkins, 2011. [Google Scholar]
- 6.Chapman JD, Urtasun RC, Blakely EA, et al. Hypoxic cell sensitizers and heavy charged-particle radiations. Br J Cancer Suppl 1978;3:184–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Raju MR, Amols HI, Bain E, et al. A heavy particle comparative study. Part III: OER and RBE. Br J Radiol 1978;51:712–9. [DOI] [PubMed] [Google Scholar]
- 8.Bertrand G, Maalouf M, Boivin A, et al. Targeting head and neck cancer stem cells to overcome resistance to photon and carbon ion radiation. Stem Cell Rev 2014;10:114–26. [DOI] [PubMed] [Google Scholar]
- 9.Cui X, Oonishi K, Tsujii H, et al. Effects of carbon ion beam on putative colon cancer stem cells and its comparison with X-rays. Cancer Res 2011;71:3676–87. [DOI] [PubMed] [Google Scholar]
- 10.Howell RW. Radiation spectra for Auger-electron emitting radionuclides: Report No. 2 of AAPM Nuclear Medicine Task Group No. 6. Med Phys 1992;19:1371–83. [DOI] [PubMed] [Google Scholar]
- 11.Obata A, Kasamatsu S, Lewis JS, et al. Basic characterization of 64Cu-ATSM as a radiotherapy agent. Nucl Med Biol 2005;32:21–8. [DOI] [PubMed] [Google Scholar]
- 12.Avila-Rodriguez MA, Nye JA, Nickles RJ. Simultaneous production of high specific activity 64Cu and 61Co with 11.4 MeV protons on enriched 64Ni nuclei. Appl Radiat Isot 2007;65:1115–20. [DOI] [PubMed] [Google Scholar]
- 13.Gingras B, Somorjai R, Bayley C. The preparation of some thiosemicarbazones and their copper complexes. Can J Chem 1961;39:973–85. [Google Scholar]
- 14.Fujibayashi Y, Cutler CS, Anderson CJ, et al. Comparative studies of Cu-64-ATSM and C-11-Acetate in an acute myocardial infarction model: ex vivo imaging of hypoxia in rats. Nucl Med Biol 1999;26:117–21. [DOI] [PubMed] [Google Scholar]
- 15.Jalilian AR, Rostampour N, Rowshanfarzad P, et al. Preclinical studies of [61Cu]ATSM as a PET radiopharmaceutical for fibrosarcoma imaging. Acta Pharm 2009;59:45–55. [DOI] [PubMed] [Google Scholar]
- 16.Jeggo P, Kemp L. X-ray-sensitive mutants of Chinese hamster ovary cell line isolation and cross-sensitivity to other DNA-damaging agents. Mutat Res 1983;112:313–27. [DOI] [PubMed] [Google Scholar]
- 17.Miller DL, Roth MB. C. elegans are protected from lethal hypoxia by an embryonic diapause. Curr Biol 2009;19:1233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seko Y, Tobe K, Ueki K, et al. Hypoxia and hypoxia/reoxygenation activate Raf-1, mitogen-activated protein kinase kinase, mitogen-activated protein kinases, and S6 kinase in cultured rat cardiac myocytes. Circ Res 1996;78:82–90. [DOI] [PubMed] [Google Scholar]
- 19.Kanai T, Endo M, Minohara S, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys 1999;44:201–10. [DOI] [PubMed] [Google Scholar]
- 20.Fujisawa H, Genik PC, Kitamura H, et al. Comparison of human chordoma cell-kill for 290 MeV/n carbon ions versus 70 MeV protons in vitro. Radiat Oncol 2013;8:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belli M, Bettega D, Calzolari P, et al. Effectiveness of monoenergetic and spread-out bragg peak carbon-ions for inactivation of various normal and tumour human cell lines. J Radiat Res 2008;49:597–607. [DOI] [PubMed] [Google Scholar]
- 22.Maeda J, Roybal EJ, Brents CA, et al. Natural and glucosyl flavonoids inhibit poly(ADP-ribose) polymerase activity and induce synthetic lethality in BRCA mutant cells. Oncol Rep 2014;31:551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tobey RA, Ley KD. Regulation of initiation of DNA synthesis in Chinese hamster cells. I. Production of stable, reversible G1-arrested populations in suspension culture. J Cell Biol 1970;46:151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagasawa H, Little JB, Inkret WC, et al. Response of X-ray-sensitive CHO mutant cells (xrs-6c) to radiation. II. Relationship between cell survival and the induction of chromosomal damage with low doses of alpha particles. Radiat Res 1991;126:280–8. [PubMed] [Google Scholar]
- 25.Weyrather WK, Ritter S, Scholz M, et al. RBE for carbon track-segment irradiation in cell lines of differing repair capacity. Int J Radiat Biol 1999;75:1357–64. [DOI] [PubMed] [Google Scholar]
- 26.Iliakis GE, Okayasu R. Radiosensitivity throughout the cell cycle and repair of potentially lethal damage and DNA double-strand breaks in an X-ray-sensitive CHO mutant. Int J Radiat Biol 1990;57:1195–211. [DOI] [PubMed] [Google Scholar]
- 27.Maeda J, Bell JJ, Genet SC, et al. Potentially lethal damage repair in drug arrested G2-phase cells after radiation exposure. Radiat Res 2014;182:448–57. [DOI] [PubMed] [Google Scholar]
- 28.Bedford JS, Mitchell JB. Dose-rate effects in synchronous mammalian cells in culture. Radiat Res 1973;54:316–27. [PubMed] [Google Scholar]
- 29.Hirayama R, Furusawa Y, Fukawa T, et al. Repair kinetics of DNA-DSB induced by X-rays or carbon ions under oxic and hypoxic conditions. J Radiat Res 2005;46:325–32. [DOI] [PubMed] [Google Scholar]
- 30.Fujii Y, Yurkon CR, Maeda J, et al. Comparative study of radioresistance between feline cells and human cells. Radiat Res 2013;180:70–7. [DOI] [PubMed] [Google Scholar]
- 31.Fujii Y, Yurkon CR, Maeda J, et al. Influence of track directions on the biological consequences in cells irradiated with high LET heavy ions. Int J Radiat Biol 2013;89:401–10. [DOI] [PubMed] [Google Scholar]
- 32.Fujii Y, Genet MD, Roybal EJ, et al. Comparison of the bromodeoxyuridine-mediated sensitization effects between low-LET and high-LET ionizing radiation on DNA double-strand breaks. Oncol Rep 2013;29:2133–9. [DOI] [PubMed] [Google Scholar]
- 33.Adelstein SJ, Kassis AI, Bodei L, et al. Radiotoxicity of iodine-125 and other Auger-electron-emitting radionuclides: background to therapy. Cancer Biother Radiopharm 2003;18:301–16. [DOI] [PubMed] [Google Scholar]